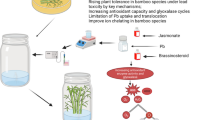
Abstract
Boron is an essential plant micronutrient, but it is phytotoxic if present in excessive amounts in soil for certain plants such as Artemisia annua L. that contains artemisinin (an important antimalarial drug) in its areal parts. Artemisinin is a sesquiterpene lactone with an endoperoxide bridge. It is quite expensive compound because the only commercial source available is A. annua and the compound present in the plant is in very low concentration. Since A. annua is a major source of the antimalarial drug and B stress is a deadly threat to its cultivation, the present research was conducted to determine whether the exogenous application of methyl jasmonate (MeJA) could combat the ill effects of excessive B present in the soil. According to the results obtained, the B toxicity induced oxidative stress and reduced the stem height as well as fresh and dry masses of the plant remarkably. The excessive amounts of soil B also lowered the net photosynthetic rate, stomatal conductance, internal CO2 concentration and total chlorophyll content in the leaves. In contrast, the foliar application of MeJA enhanced the growth and photosynthetic efficiency both in the stressed and non-stressed plants. The excessive B levels also increased the activities of antioxidant enzymes, such as catalase, peroxidase and superoxide dismutase. Endogenous H2O2 and O −2 levels were also high in the stressed plants. However, the MeJA application to the stressed plants reduced the amount of lipid peroxidation and stimulated the synthesis of antioxidant enzymes, enhancing the content and yield of artemisinin as well. Thus, it was concluded that MeJA might be utilized in mitigating the B toxicity and improving the content and yield of artemisinin in A. annua plant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Artemisia annua L. (family Asteraceae) is an antimalarial drug plant that has been used for centuries in Chinese traditional medicines for the treatment of fever and malaria (Klayman 1985). Being the world’s most severe parasitic infection, malaria threatens more than one third of the global population, killing approximately two million people annually (Snow et al. 2005). Despite tremendous efforts regarding the control of malaria, the global morbidity and mortality have not significantly been changed for the last 50 years (WHO 2006). Artemisinin, a sesquiterpene lactone containing an endoperoxide bridge, has been increasingly popular as an effective and safe alternative therapy against malaria (Abdin et al. 2003). Since the chemical synthesis of artemisinin is very costly, the plant remains the only viable source of the artemisinin production. Hence, the enhanced production of artemisinin in the plants is highly desirable (Abdin et al. 2003; Aftab et al. 2010a, c).
B is an essential element required for normal growth of higher plants. It is unique among micronutrients in that the threshold between deficiency and toxicity is narrow (Yau and Ryan 2008). High B concentration might occur naturally in the soil and ground waters or could be added to the soil through mining, fertilizers and irrigation water (Nable et al. 1997). In the recent years, B toxicity has attracted increasing interest owing to the greater demand for desalinated water, in which the B concentrations might be too high for healthy irrigation (Parks and Edwards 2005). In fact, B-rich soils have decreased plant growth and crop yield in different regions of the world (Papadakis et al. 2004). The typical symptoms shown by plants, exposed to high B concentrations in the substrate, are reduced vigour, delayed development, leaf burn and decreased number and size of the fruits (Paull et al. 1992; Nable et al. 1997). Accumulation of reactive oxygen species (ROS), due to B toxicity, has been reported in apple root stock (Molassiotis et al. 2006), wheat (Gunes et al. 2007), barley (Inal et al. 2009) and tomato (Cervilla et al. 2007). Further, lipid peroxidation and hydrogen peroxide (H2O2) accumulation in the cells and tissues caused by high concentration of B has also been reported (Karabal et al. 2003; Gunes et al. 2006; Molassiotis et al. 2006). In addition, altered activities of the antioxidant enzymes due to high B concentration have been used as indicators of oxidative stress in crop plants (Mittler 2002).
Methyl jasmonate (MeJA), a methyl ester of jasmonic acid, is a phytohormone with ubiquitous distribution among the plants. Generally, it is considered to modulate several physiological events in higher plants such as defence responses, flowering and senescence (Cheong and Choi 2003). Also, MeJA mediates plant responses to various biotic and abiotic stresses by triggering a transcriptional reprogramming that allows cells to cope with pathogens and stresses (Wolucka et al. 2005). MeJA has been shown to induce the production of antioxidant defence enzymes and secondary metabolites in some plants and cell cultures (Hampel et al. 2005; Kim et al. 2007). Recently, enhanced artemisinin production has been observed in the whole plant due to MeJA application (Wang et al. 2009; Guo et al. 2010). Such an enhancement in the plant artemisinin content has also been reported as a result of biotic or abiotic stresses like low temperature and mineral deficiency as well as toxicity (Wallaart et al. 2000; Ferreira 2007; Aftab et al. 2010b).
In previous paper, we reported that a mild stress of B accelerated the production of artemisinin content per plant, but it adversely affected the growth of the plants (Aftab et al. 2010b). The main reason behind the poor artemisinin yield was the morphological damage to the stressed plants due to excessive B application. The present research is the outcome of the hypothesis we postulated regarding the effect of exogenous supply of MeJA that led us to think if MeJA supply could combat B toxicity and whether it is involved in the upregulation of artemisinin biosynthesis.
Materials and methods
A pot culture experiment was conducted to analyse the effects of two concentrations of B viz. 1.00 and 2.00 mM applied with or without exogenous MeJA (300 μM) on A. annua. The seeds were initially surface sterilized with 95% ethyl alcohol for 5 min and then washed thoroughly with double distilled water before sowing. Prior to seed sowing, pots were filled with 5.0 kg homogenous mixture of soil and farmyard manure (4:1). Physicochemical characteristics of the soil were texture-sandy loam, pH (1:2) 8.0, E.C. (1:2) 0.48 m mhos/cm and available N, P and K 97.46, 10.21 and 147.0 mg kg−1 soil, respectively. Soil B concentration was 0.05 mM, which is a safe limit for the cultivation of agricultural crops. Seeds were sown at a depth of 2 cm in the earthen pots (25 cm diameter × 25 cm height). B was applied to the soil at the rate of 0, 1.00 and 2.00 mM in the form of boric acid (H3BO3) 60 days after sowing (AS), while MeJA was applied thrice on the foliage of the plants at an interval of 10 days. The first treatment of MeJA was given when the plants were 60 days old, and subsequently, the second and third treatments were applied. The experiment was conducted according to simple randomised block design, and the plants were sampled 120 days AS for various parameters studied.
Analyses of growth and yield parameters
The plants from pots of each treatment were uprooted carefully and then shoot height was recorded. Plants were washed with tap water to remove adhering foreign particles. Roots of the plants were removed and fresh mass of the shoots was recorded after surface drying the shoots. The shoots were dried in a hot air oven at 80°C for 48 h, and then shoot dry mass was recorded. Total dry leaves of the plants were weighed to determine leaf yield.
Photosynthetic parameters
Net photosynthetic rate (P N) was measured on sunny days at 1100 hours using fully expanded leaves with the help of IRGA (Infra Red Gas Analyzer, LI-COR 6400 Portable Photosynthesis System, Lincoln, NE, USA). Before recording the measurements, the IRGA was calibrated and zero was adjusted approximately every 30 min during the measurement period. Each leaf was enclosed in the gas exchange chamber of IRGA for 60 s. All the photosynthetic attributes measured were recorded three times for each treatment.
Total chlorophyll content was estimated in the youngest fully expanded leaves by the method of Lichtenthaler and Buschmann (2001). The fresh tissue from the interveinal leaf area was ground using a mortar and pestle with 80% acetone. The absorbance of the extracted chlorophyll solution was recorded at 662 and 645 nm for chlorophyll estimation using a spectrophotometer (Shimadzu UV-1700, Tokyo, Japan).
Carbonic anhydrase activity
Carbonic anhydrase (CA; E.C. 4.2.1.1) activity was measured in the youngest fully expanded leaves using the method as described by Dwivedi and Randhawa (1974). Two hundred milligrams of fresh leaf pieces was transferred to Petri plates. The leaf pieces were dipped in 10 mL of 0.2 M cysteine hydrochloride solution for 20 min at 4°C. To each test tube, 4 mL of 0.2 M sodium bicarbonate solution and 0.2 mL of 0.022% bromothymol blue were added. The reaction mixture was titrated against 0.05 N HCl using methyl red as indicator. The enzyme was expressed as micromolar CO2 per kilogram leaf FW per second.
Estimation of B
B contents in the roots and leaves were determined by azomethine-H method (Wolf 1971). The plant samples were reduced to dry ash in a muffle furnace at 500°C for 6 h, and the carbon-free residue was then dissolved in 0.1 M HCl. Root and leaf B contents were determined in terms of milligrams per kilogram.
Lipid peroxidation rate (TBARS content)
Oxidative damage to leaf lipids was estimated by the content of total 2-thiobarbituric acid reactive substances (TBARS) expressed as equivalents of malondialdehyde (MDA). TBARS content was estimated by the method of Cakmak and Horst (1991). TBARS were extracted from 0.5 g chopped fresh leaves, ground with 5 mL of 0.1% (w/v) trichloroacetic acid (TCA). Following the centrifugation at 12,000×g for 5 min, an aliquot of 1 mL of the supernatant was added to 4 mL of 0.5% (w/v) TBA in 20% (w/v) TCA. Samples were incubated at 90°C for 30 min. Thereafter, the reaction was stopped using an ice bath. Centrifugation was performed at 10,000×g for 5 min, and the absorbance of the supernatant was recorded at 532 nm with the help of a spectrophotometer and the values were corrected for non-specific turbidity by subtracting the absorbance at 600 nm. TBARS content was expressed as nanomoles per gram FW.
Antioxidant enzymes assay
Catalase (CAT) activity was measured according to the method given by Chandlee and Scandalios (1984) with a minor modification. The assay mixture contained 2.6 mL of 50 mM potassium phosphate buffer (pH 7.0), 0.4 mL of 15 mM H2O2 and 0.04 mL of the enzyme extract. The extract was centrifuged at 4°C for 20 min at 12,500×g. The supernatant was used for enzyme assay. The assay mixture contained 2.6 mL of 50 mM potassium phosphate buffer (pH 7.0), 400 μL of 15 mM H2O2 and 40 μL of enzyme extract. The decomposition of H2O2 was followed by the decline in absorbance at 240 nm. The enzyme activity was expressed in units per milligram protein (U = 1 mM of H2O2 reduced per minute per milligram protein).
Peroxidase (POX) activity was assayed by the method of Kumar and Khan (1982). Assay mixture of POX contained 2 mL of 0.1 M phosphate buffer (pH 6.8), 1 mL of 0.01 M pyrogallol, 1 mL of 0.005 M H2O2 and 0.5 mL of the enzyme extract. The solution was incubated for 5 min at 25°C, after which the reaction was terminated by adding 1 mL of 2.5 N H2SO4. The amount of purpurogallin formed was determined by measuring the absorbance at 420 nm against a reagent blank prepared by adding the extract after the addition of 2.5 N H2SO4 at zero time. The activity was expressed in units per milligram protein. One unit of the enzyme activity corresponded to an amount of the enzyme that caused an increase in the absorbance by 0.1 min−1 mg−1 protein.
Superoxide dismutase (SOD) activity was assayed as described by Beauchamp and Fridovich (1971). The reaction mixture contained 1.17 × 10−6 M of riboflavin, 0.1 of M methionine, 2 × 10−5 M of KCN and 5.6 × 10−5 M of nitroblue tetrazolium salt dissolved in 3 mL of 0.05 M of sodium phosphate buffer (pH 7.8). A 3-mL volume of the reaction medium was added to 1 mL of the enzyme extract. The mixtures were illuminated in glass test tubes by two sets of Philips 40 W fluorescent tubes arranged in a single row. Illumination of the reaction mixture initiated the reaction that was maintained at 30°C for 1 h. Identical solutions were kept under dark served as blanks. The absorbance was measured at 560 nm against the blank using the spectrophotometer. SOD activity was expressed as U mg−1 protein. One unit is defined as the amount of change in the absorbance by 0.1 h−1 mg−1 protein.
Determination of ROS
ROS were estimated as the total content of H2O2 and O −2 in the leaves. The leaf H2O2 content was determined according to the method of Mukherjee and Choudhuri (1983). The youngest fully developed leaves (0.5 g) were homogenized using a cooled mortar and pestle using pre-cooled acetone (5 mL). The homogenate was centrifuged at 12,000×g for 5 min. One millilitre of the supernatant was mixed with 0.1 mL of 5% Ti(SO4)2 and 0.2 mL of 19% ammonia. After a precipitate was formed, the reaction mixture was centrifuged at 12,000×g for 5 min. The resulting pellet was dissolved in 3 mL of 2 M H2SO4, and the absorbance was recorded at 415 nm using the spectrophotometer. The H2O2 concentration was calculated according to the standard curve of H2O2 that was prepared by using known concentrations of H2O2 ranging from 0 to 10 μM.
The production of singlet oxygen (O −2 ) was measured in the fresh leaf tissue according to the method of Able et al. (1998) employing slight modifications. Accordingly, the reduction of XTT [2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carbo-xanilide] was monitored in the presence of O −2 . The leaf samples (0.5 g, FW) were homogenized with 1 mL of 50 mM Tris–HCl buffer (pH 7.5), and then the content was centrifuged at 12,000×g for 15 min. The reaction mixture contained 50 mM of Tris–HCl buffer (pH 7.5), 100 μL of supernatant and 0.5 mM of XTT. The reduction of XTT was determined by measuring the absorbance at 470 nm using the spectrophotometer. The quantity of O −2 was determined using the molar extinction coefficient (ε)—2.16 × 104 M−1 cm−1.
Extraction and estimation of artemisinin
Dry leaf material (1 g) was used for the estimation of artemisinin that was converted to Q260 (Q260 is a UV absorbing compound that can be detected on a UV detector), which was subsequently quantified using the HPLC according to the method of Zhao and Zeng (1986). Stock solution for the standard curve was prepared using 1 mg of standard artemisinin dissolved in 1 mL of HPLC-grade methanol. The artemisinin was extracted with 20 mL of petroleum ether using a shaker maintained at 70 rpm for 24 h. Thereafter, the solvent was decanted and pooled followed by addition of 20 mL of petroleum ether again. This step was repeated thrice. Petroleum ether fractions were pooled and concentrated under reduced pressure, and the residues were defatted with CH3CN (10 mL × 3). The precipitated fat was filtered out and the filtrate was concentrated under reduced pressure. The residues were dissolved in 1 mL of methanol. To a 100-μL aliquot of each sample, 4 mL of 0.3% NaOH was added. The samples were incubated in shaking water bath at 50°C for 30 min; thereafter, they were cooled and neutralized with glacial acetic acid (0.1 M dissolved in 20% MeOH). The pH of the solution was maintained at 6.8. The derivatized artemisinin was analysed and quantified through reverse-phase column (C18; 5 μm; 4.6 mm; 250 mm) using premix methanol/10 mM potassium phosphate buffer (pH 6.5) in the ratio of 60:40 as mobile phase at a constant flow rate of 1 mL/min, with the detector set at 260 nm. Artemisinin was quantified against the standard curve of artemisinin, obtained from Sigma-Aldrich, USA.
Statistical analysis
Each pot was treated as one replicate, and all the treatments were replicated five times. The data were analysed statistically using SPSS-17 statistical software (SPSS Inc., Chicago, IL, USA) according to simple randomized block design. Mean values were statistically compared by Duncan’s multiple range test at P < 0.05 level using different letters.
Results
Growth and yield characteristics
Morphological examination of the treated plants showed that the B, applied to the soil either at 1.00 or 2.00 mM, inhibited the growth of A. annua plants; the most toxic effects were noted at 2.00 mM of B (Table 1). The plants supplied with 300 μM of MeJA showed an improved plant growth compared to the B-treated plants supplied with no MeJA (Table 1). Shoot fresh and dry masses were significantly decreased by either of the concentrations of applied B. The decrease in shoot fresh and dry masses was comparatively higher when 2.00 mM of B was applied through soil, in comparison to the control (Table 1). The toxic effects, generated by excessive B application, were ameliorated when the plants were supplemented with the exogenous MeJA. The fresh and dry leaf yields were also significantly affected by B toxicity, with the MeJA application proving effective in reducing the inhibitory effects of B toxicity (Table 1).
Photosynthetic parameters
Values of all the photosynthetic parameters were significantly reduced by the B concentrations applied. Application of both the levels of B, viz. 1.00 and 2.00 mM, resulted in significant reduction in net photosynthetic rate, stomatal conductance and internal CO2 concentration, the decrease in values being higher at 2.00 mM of B compared to that at 1.00 mM of B and the control (Fig. 1a–c). Contrarily, MeJA alleviated the adverse effect of B toxicity, with the MeJA-treated plants showing significantly improved values of photosynthetic parameters. The chlorophyll content, observed in 2.00 mM of B, was lower in comparison to the plants exposed to 1.00 mM of B and the control. However, when the plants were treated with 300 μM of MeJA, the chlorophyll content in the leaves increased, counteracting the B toxicity at the both the B concentrations applied (Fig. 1d).
Carbonic anhydrase activity
In the present study, there was noted a reduction in the activity of CA in the leaves on account of both the B treatments. At 2.00 mM of B, the activity of CA was the lowest compared to that recorded at 1.00 mM of B and in the control. However, when MeJA was given to the stressed plants, the inhibitory effect of B was nullified (Fig. 2a).
B accumulation in plant tissue
B concentration in plant tissues increased with increasing B concentration in the soil in concentration-dependent manner, with B concentration of root being higher compared to that of leaf. Exogenous application of MeJA, applied to B-stressed plants, also influenced the accumulation of B in roots and leaves by significantly preventing the accumulation of B in the tissues of roots and leaves (Fig. 2b).
Oxidative stress (lipid peroxidation)
Lipid peroxidation was examined by estimating MDA content in the B-treated plants. Since the B is known to induce oxidative stress, the B-treated plants exhibited high rate of lipid peroxidation. At 2.00 mM of B, the lipid peroxidation rate in leaves was higher compared to that at 1.00 mM of B and the control treatment (no B application; Fig. 3a). However, MeJA-treated plants lowered the membrane destruction caused by B application both at 1.00 and 2.00 mM of B (Fig. 3a).
Antioxidant enzymes
The presence of B in the soil accelerated the activities of antioxidant enzymes (CAT, POX, and SOD) in the plants that are a natural mechanism of protection of plants against the B stress. The B applied at 2.00 mM resulted in a rapid stimulation of the activities of CAT, POX and SOD compared to that at 1.00 mM of B. The follow-up exogenous application of MeJA was effective in further inducing the activities of all the antioxidant enzymes measured, suggesting the role of the MeJA regarding protection against the B stress in A. annua plants (Fig. 3b–d).
Endogenous generation of ROS
In the present study, the level of endogenous H2O2 and O −2 was also measured in order to determine the internal status of ROS due to soil applied B, alone and in combination with MeJA. Both H2O2 and O −2 contents were higher in the B-treated plants as compared to the control at both the concentrations of B. At 1.00 mM of B, the increase in internal H2O2 and O −2 was higher compared to that at 2.00 mM of B and in the control treatment (Fig. 4a, b). A slight increase in the levels of ROS was also noted when MeJA was supplied to the plants in combination with B (Fig. 4a, b).
Artemisinin content and yield
The objective behind the present study was to explore whether the exogenously applied MeJA in the presence of B stress can have positive impact on artemisinin production in A. annua. An increase of 58.8% in the artemisinin content was noted when the plants were grown at 1.00 mM concentration of B together with MeJA, while the increase was 31.2% when 2.00 mM of B was applied with MeJA (Figs. 5a and 6). Also, the artemisinin yield was recorded to be the highest when MeJA was applied along with 1.00 mM of B. A significant increase in artemisinin content was also noted when MeJA was applied to the plants alone (Fig. 5b).
Discussion
Plant hormones are the active members of signal cascades involved in the induction of plant stress responses (Pedranzani et al. 2003). Abiotic stresses negatively affect plant growth and development, and to combat with these stresses, the use of exogenous plant growth regulators could be of great importance (Jaleel et al. 2009). B toxicity significantly decreased the values of growth parameters in the present study (Table 1). According to Srivastava and Sharma (1990), A. annua plants, fertilized with either very high (2.50 mg/L) or very low (0.05 mg/L) B levels, were highly sensitive to B deficiency that significantly decreased the plant growth and tissue artemisinin content. Plants that received very low B concentrations exhibited necrosis in growing tips, resulting in delayed flowering (Srivastava and Sharma 1990). Increasing concentrations of B reduced the height of the plant as well as the fresh and dry masses per plant. The observed lower values for fresh and dry masses of the plant due to B treatments are in agreement with the results of several researchers investigating the response of various plant species to B stress (Asad et al. 2003; Molassiotis et al. 2006; Eraslan et al. 2007). B is directly involved in many physiological and biochemical processes during plant growth such as cell elongation and division, cell wall biosynthesis and membrane functions (Blevins and Lukaszewski 1998). Growth reduction under B toxicity is well documented in tomato (Alpaslan and Gunes 2001), cucumber (Cervilla et al. 2007) and barley (Karabal et al. 2003). Therefore, the observed reduction in growth parameters upon exposure of plants to high B levels was quite obvious. Moreover, when MeJA was applied exogenously to non-stressed and stressed plants, it was effective for improvement in plant growth. Also, it proved efficient in reversing the toxic effects of B toxicity. The MeJA is an important regulator of cellular events and is involved in diverse developmental processes such as germination, root growth, fertility and senescence (Wasternack and Hause 2002). In addition, MeJA activates the plant defence mechanism in response to various environmental stresses (Cheong and Choi 2003). In fact, Christov et al. (2001) and Maksymiec and Krupa (2006) showed that MeJA was capable to enhance tolerance in plants against various stresses. Thus, the improved growth of the B-stressed plants by MeJA could be expected in the present investigation.
The net photosynthetic rate in the leaves of B-treated plants decreased in a concentration-dependent manner, showing the lowest photosynthetic activity in the leaves of the plants applied with the highest B level (Fig. 1a). Since the B toxicity also decreased the internal CO2 concentration, less of the photon energy, captured by the light harvesting system, is expected to be used in electron transport system, leading to decrease in the photosynthesis. Further, the decrease in chlorophyll content, leading to reduction in net photosynthesis, could be attributed to oxidation of chlorophyll and chloroplastic membranes, which might be exacerbated by the excess of B (Lee 2006; Ardic et al. 2009). The present results suggested that application of 300 μM of MeJA had a beneficial effect on photosynthesis of control and B-treated A. annua plants. Fedina and Benderliev (2000) showed that the exogenous supply of MeJA improved the CO2 fixation, which gives support to the MeJA-improved CA activity in our study (Fig. 2a). Further, this might also be responsible for the enhanced photosynthetic rate in the present study (Fig. 1a). Earlier reports also suggest that MeJA protects photosynthetic pigments under various stresses (Golovatskaya and Karnachuk 2008; Yoon et al. 2009), supporting the improved photosynthetic response of MeJA-treated plants in this study.
Roots play a number of important roles during plant growth and development and are typically the first part of the plant to encounter several types of environmental stresses (Parra-Lobato et al. 2009). Our results revealed that there was a higher accumulation of B in roots compared to that in leaves in a concentration-dependent manner with increasing B concentrations (Fig. 2b). Exogenous application of MeJA had significant influence on the accumulation of B either in roots or leaves. In this context, our results corroborate the findings of Kaya et al. (2009), who reported comparatively a higher accumulation of B in roots of tomato than in other plant parts. In fact, the ability of the plants to tolerate high B could be due to the capacity of the plants to accumulate more B in roots and a lesser amount of the B being transported to shoots (Paull et al. 1992). However, B uptake responses of the crops might differ significantly with regard to the susceptibility of crops to various stresses (Hu and Brown 1997; Alpaslan and Gunes 2001).
In line with the present results, other researchers have found that excessive B might increase TBARS content in plants (Gunes et al. 2006; Molassiotis et al. 2006). Our results also demonstrated that MeJA was involved in the inhibition of formation of the lipid peroxides, as evident by lower TBARS content in A. annua plants exposed to B stress (Fig. 3a). In Arabidopsis thaliana, the expression of oxidative stress-tolerance genes including those for peroxidases and oxidases has, in fact, been shown to be upregulated by the MeJA (Jung et al. 2007).
Abiotic stresses increase the formation of ROS that oxidize the membrane lipids, proteins and nucleic acids (Gong et al. 2005). Plants with high levels of antioxidants, either constitutive or induced, have been reported to have great resistance to oxidative damage (Sudhakar et al. 2001). The activities of CAT, POX and SOD were increased in the leaves significantly on account of MeJA treatment applied with or without B in respect with the control (Fig. 3b–d) indicate that MeJA triggered this anti-ROS mechanism as well. Enhancement in the activities of antioxidant enzymes has also been documented by several researchers in response to the B toxicity in various plant species (Molassiotis et al. 2006; Cervilla et al. 2007; Gunes et al. 2007; Han et al. 2009; Jaleel et al. 2009). The present results, showing increased activity of antioxidant enzymes in the B-stressed plants, are in agreement with the ROS scavenging capability of A. annua plants (Jaleel et al. 2009). Since the enzymes involved in antioxidant metabolism are usually co-regulated and their activities increase upon exposure to stress, higher activity levels of antioxidant enzymes together with the increased lipid peroxidation is quite justified. The application of MeJA further enhanced the activities of all antioxidant enzymes, both in non-stressed and stressed plants, supplementing the ROS scavenging mechanism in A. annua plants and, thereby, mitigating the B stress. Mitigation of ROS effects by the MeJA has been reported in the case of strawberry under water stress and in maize seedlings subjected to paraquat (Wang 1999; Norastehnia and Nojavan-Asghari 2006). Thus, under B stress, MeJA could induce antioxidant defence activity in plants to remove the possible toxic effects of free radicals, making the plants more resistant to B stress. In the present study, H2O2 and O −2 concentrations were increased significantly by applying 1.00 and 2.00 mM of B. According to our results, when MeJA was applied to the B-stressed plants, it further enhanced the ROS level in the plants, particularly in plants applied with 1.00 mM of B but the ROS level was reduced in plants applied with 2.00 mM of B (Fig. 4a). Thus, it appears that MeJA stimulated the production of ROS when it was applied alone or with mild concentration of B (1.00 mM of B). However, when it was applied with high B concentration (2.00 mM of B), it combated the ROS production more than that it did against the application of 1.00 mM of B by inducing more of the antioxidant enzymes as observed in this investigation (Fig. 3b–d). However, such considerations need further researches to be carried out in this regard.
Like that we observed in the present study (Fig. 5a), in our earlier study also, artemisinin content was enhanced significantly due to 1.00 mM of B (Aftab et al. 2010b). The artemisinin content was further increased due to the exogenous application of MeJA (Fig. 5a). Presumably, a mild B stress (1.00 mM of B) promoted the artemisinin biosynthesis by ROS mediation, which might play an important role of converting dihydroartemisinic acid to artemisinin during artemisinin biosynthesis. In this regard, Wallaart et al. (1999) suggested that dihydroartemisinic acid could act as a scavenger of ROS (H2O2 and O −2 in this case) that might be released in plant cells upon exposure to oxidative stress. During the reaction, dihydroartemisinic acid hydroperoxide (DHAA-OOH) is generated that gets converted into artemisinin. Our results are in agreement with the observations recorded by Pu et al. (2009), Mannan et al. (2010) and Guo et al. (2010), who advocated that relatively high levels of ROS could result in the enhanced the conversion of dihydroartemisinic acid to artemisinin. Further, it has been reported that terpenoid biosynthesis could be significantly induced by MeJA treatment (Zhao et al. 2005). Terpenoids, especially sesquiterpenoids and their oxygenated derivatives, account for the majority of secondary metabolites in A. annua (Li et al. 2006). Studies of Wang et al. (2009) indicated that besides artemisinin, artemisinic acid and dihydroartemisinic acid and other sesquiterpenoids and triterpenoids in A. annua were induced by exogenous application of MeJA. Therefore, the co-regulated compounds, like methyl artemisinic acid, dihydroartemisinin and squalene, might be considered responsible for enhanced artemisinin production on account of MeJA application. In fact, methyl artemisinic acid is highly accumulated in A. annua plants treated by MeJA (Wang et al. 2009). The above discussion can put forth some insight to the overproduction of artemisinin exposed to MeJA under B toxicity; however, the relationship between methyl artemisinic acid, artemisinic acid and artemisinin needs further investigation.
In summary, our study revealed that the B had inhibitory effects on plant growth as evidenced by reduced shoot length and fresh and dry masses of the plants applied with B. The B stress also adversely affected the photosynthetic efficiency; however, a follow-up treatment of MeJA alleviated the toxic effects of B. The B-stressed plants also showed high lipid peroxidation rate and antioxidant enzyme activities in A. annua plants. A mild stress of B (1.00 mM of B), however, stimulated artemisinin biosynthesis. When MeJA was applied exogenously, it alleviated the toxicity of B, improved the plant growth, lowered lipid peroxidation rate and induced the synthesis of several antioxidant enzymes. In the present study, a higher amount of B was accumulated in the roots compared to that in the leaves, indicating the phytoremediation potential of A. annua, which needs serious consideration for removing toxic elements from the soil. Most importantly, artemisinin content and yield was higher in MeJA-treated plants under B toxicity. Therefore, it can be concluded that MeJA had protected A. annua from B toxicity and stimulated artemisinin biosynthesis in presence or absence of B.
References
Abdin MZ, Israr M, Rehman RU, Jain SK (2003) Artemisinin, a novel antimalarial drug: biochemical and molecular approaches for enhanced production. Planta Med 69:1–11
Able AJ, Guest DI, Sutherland MW (1998) Use of a new tetrazolium based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores of Phytophthora parasitica var nicotianae. Plant Physiol 117:491–499
Aftab T, Khan MMA, Idrees M, Naeem M, Singh M, Ram M (2010a) Stimulation of crop productivity, photosynthesis and artemisinin production in Artemisia annua L. by triacontanol and gibberellic acid application. J Plant Interact 5:273–281.
Aftab T, Khan MMA, Idrees M, Naeem M, Ram M (2010b) B induced oxidative stress, antioxidant defense response and changes in artemisinin content in Artemisia annua L. J Agron Crop Sci. doi:10.1111/j.1439-037X.2010.00427.x
Aftab T, Khan MMA, Idrees M, Naeem M, Moinuddin (2010c) Salicylic acid act as potent enhancer of growth, photosynthesis and artemisinin production in Artemisia annua L. J Crop Sci Biotech. doi:10.1007/s12829-010-0040-3
Alpaslan M, Gunes A (2001) Interactive effects of B and salinity stress on the growth, membrane permeability and mineral composition of tomato and cucumber plants. Plant Soil 236:123–128
Ardic M, Sekmen AH, Tokur S, Ozdemir F, Turkan I (2009) Antioxidant responses of chickpea plants subjected to B toxicity. Plant Biol 11:328–338
Asad A, Blamey FPC, Edwards DG (2003) Effects of B foliar applications on vegetative and reproductive growth of sunflower. Ann Bot 92:1–6
Beauchamp CO, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Blevins DG, Lukaszewski KM (1998) B in plant structure and function. Annu Rev Plant Physiol Plant Mol Biol 49:481–500
Cakmak I, Horst J (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Cervilla LM, Blasco B, Rios JJ, Romero L, Ruiz JM (2007) Oxidative stress and antioxidants in tomato (Solanum lycopersicum) plants subjected to B toxicity. Ann Bot 100:747–756
Chandlee JM, Scandalios JG (1984) Analysis of variants affecting the catalase development program in maize scutellum. Theor Appl Genet 69:71–77
Cheong JJ, Choi YD (2003) Methyl jasmonate as a vital substance in plants. Trends Genet 19:409–413
Christov C, Pounieva M, Bozhkova M, Toncheva S, Fournadzieva S, Zafirova T (2001) Influence of temperature and methyl jasmonate on Scenedesmus incrassulatus. Biol Plant 44:367–371
Dwivedi RS, Randhawa NS (1974) Evaluation of rapid test for hidden hunger of zinc in plants. Plant Soil 40:445–451
Eraslan F, Inal A, Gunes A, Apaslan M (2007) B toxicity alters nitrate reductase activity, proline accumulation, membrane permeability, and mineral constituents of tomato and pepper plants. J Plant Nutr 30:981–994
Fedina IS, Benderliev KM (2000) Response of Scenedesmus incrassatulus to salt stress as affected by methyl jasmonate. Biol Plant 43:625–627
Ferreira JFS (2007) Nutrient deficiency in the production of artemisinin, dihydroartemisinic acid, and artemisinic acid in Artemisia annua L. J Agric Food Chem 55:1686–1694
Golovatskaya IF, Karnachuk RA (2008) Effect of jasmonic acid on morphogenesis and photosynthetic pigment level in Arabidopsis seedlings grown under green light. Russ J Plant Physiol 55:240–244
Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci 169:313–321
Gunes A, Soylemezoglu G, Inal A, Bagci EG, Coban S, Sahin O (2006) Antioxidant and stomatal responses of grapevine (Vitis vinifera L) to B toxicity. Sci Hort 110:279–284
Gunes A, Inal A, Bagci EG, Coban S, Sahin O (2007) Silicon increases B tolerance and reduces oxidative damage of wheat grown in soil with excess B. Biol Plant 51:571–574
Guo XX, Yang XQ, Yang RY, Zeng QP (2010) Salicylic acid and methyl jasmonate but not Rose Bengal enhance artemisinin production through invoking burst of endogenous singlet oxygen. Plant Sci 178:390–397
Hampel D, Mosandl A, Wust M (2005) Induction of de novo volatile terpene biosynthesis via cytosolic and plastidial pathways by methyl jasmonate in foliage of Vitis vinifera L. J Agric Food Chem 53:2652–2657
Han S, Tang N, Jiang HX, Yang LT, Li Y, Chen LS (2009) CO2 assimilation, photosystem II photochemistry, carbohydrate metabolism and antioxidant system of citrus leaves in response to B stress. Plant Sci 176:143–153
Hu H, Brown PH (1997) Absorption of B by plant roots. Plant Soil 193:49–58
Inal A, Pilbeam DJ, Gunes A (2009) Silicon increases tolerance to B toxicity and reduces oxidative damage in barley. J Plant Nutr 32:112–128
Jaleel CA, Riadh K, Gopi R, Manivannan P, Inès J, Al-Juburi HJ, Chang-Xing Z, Hong-Bo S, Panneerselvam R (2009) Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant 31:427–436
Jung C, Yeu SY, Koo YJ, Kim M, Choi YD, Cheong JJ (2007) Transcript profile of transgenic Arabidopsis constitutively producing methyl jasmonate. J Plant Biol 50:12–17
Karabal E, Yücel M, Ökte HA (2003) Antioxidants responses of tolerant and sensitive barley cultivars to B toxicity. Plant Sci 164:925–933
Kaya C, Tuna AL, Dikilitas M, Ashraf M, Koskeroglu S, Guneri M (2009) Supplementary phosphorus can alleviate B toxicity in tomato. Sci Hort 121:284–288
Kim YS, Yeung EC, Hahn EJ, Paek KY (2007) Combined effects of phytohormone, indole-3-butyric acid, and methyl jasmonate on root growth and ginsenoside production in adventitious root cultures of Panax ginseng C.A. Meyer. Biotechnol Lett 29:1789–1792
Klayman DL (1985) Qinghaosu (artemisinin): an antimalarial drug from China. Science 228:1049–1055
Kumar KB, Khan PA (1982) Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv. PR 202) leaves during senescence. Indian J Exp Bot 20:412–416
Lee SKD (2006) Hot pepper response to interactive effects of salinity and B. Plant Soil Environ 52:227–233
Li Y, Huang H, Wu YL (2006) Qinghaosu (artemisinin)—a fantastic antimalarial drug from a traditional Chinese medicine. In: Tian X, Liang WSF (eds) Medicinal chemistry of bioactive natural products. Wiley, Hoboken, pp 183–256
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV–VIS spectroscopy. In: Wrolstad RE (ed) Current protocols in food analytical chemistry. Wiley, New York, pp F4.3.1–F4.3.8
Maksymiec W, Krupa Z (2006) The effects of short term exposition to Cd, excess Cu ions and jasmonate on oxidative stress appearing in Arabidopsis thaliana. Environ Exp Bot 57:187–194
Mannan A, Liu C, Arsenault PR, Towler MJ, Vail DR, Lorence A, Weathers PJ (2010) DMSO triggers the generation of ROS leading to an increase in artemisinin and dihydroartemisinic acid in Artemisia annua shoot cultures. Plant Cell Rep 29:143–152
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Molassiotis A, Sotiropoulos T, Tanou G, Diamantidis G, Therios I (2006) B induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM9 (Malus domestica Borkh). Environ Exp Bot 56:54–62
Mukherjee SP, Choudhuri MA (1983) Implications of water stress induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:166–170
Nable RO, Banuelos GS, Paull JG (1997) B toxicity. Plant Soil 193:181–198
Norastehnia A, Nojavan-Asghari M (2006) Effect of methyl jasmonate on the enzymatic antioxidant defense system in Maize seedling subjected to paraquat. Asian J Plant Sci 5:17–23
Papadakis IE, Dimassi KN, Bosabalidis AM, Therios IN, Patakas A, Giannakoula A (2004) Effects of B excess on some physiological and anatomical parameters of ‘Navelina’ orange plants grafted on two rootstocks. Environ Exp Bot 51:247–257
Parks JL, Edwards M (2005) B in the environment. Crit Rev Env Sci Biotechnol 35:81–114
Parra-Lobato MC, Fernandez-Garcia N, Olmos E, Alvarez-Tinaut MC, Gómez-Jiménez MC (2009) Methyl jasmonate-induced antioxidant defence in root apoplast from sunflower seedlings. Environ Exp Bot 66:9–17
Paull JG, Nable RO, Rathjen AJ (1992) Physiological and genetic control of the tolerance of wheat to high concentrations of B and implications for plant breeding. Plant Soil 146:251–260
Pedranzani H, Racagni G, Alemano S, Miersch O, Ramirez I, Pena-Cortes H, Machado-Domenech E, Abdala G (2003) Salt tolerant tomato plants show increased levels of jasmonic acid. Plant Growth Regul 41:149–158
Pu GB, Ma DM, Chen JL, Ma LQ, Wang H, Li GF, Ye HC, Liu BY (2009) Salicylic acid activates artemisinin biosynthesis in Artemisia annua L. Plant Cell Rep 28:1127–1135
Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI (2005) The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214–217
Srivastava NK, Sharma S (1990) Influence of micronutrient imbalance on growth and artemisinin content in Artemisia annua. Indian J Pharm Sci 82:225–227
Sudhakar C, Lakshmi S, Giridarakumar S (2001) Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci 161:613–619
Wallaart TE, van Uden W, Lubberink HG, Woerdenbag HJ, Pras N, Quax WJ (1999) Isolation and identification of dihydroartemisinic acid from Artemisia annua and its possible role in the biosynthesis of artemisinin. J Nat Prod 62:430–433
Wallaart TE, Pras N, Beekman AC, Quax WJ (2000) Seasonal variation of artemisinin and its biosynthetic precursors in plants of Artemisia annua of different geographical origin: proof for the existence of chemotypes. Planta Med 66:57–62
Wang SY (1999) Methyl jasmonate reduces water stress in strawberry. J Plant Growth Regul 18:127–134
Wang H, Ma C, Li Z, Ma L, Wang H, Ye H, Xu G, Liu B (2009) Effects of exogenous methyl jasmonate on artemisinin biosynthesis and secondary metabolites in Artemisia annua L. Ind Crops Prod 31:214–218
Wasternack C, Hause B (2002) Jasmonates and octadecanoids—signals in plant stress responses and development. In: Moldave K (ed) Progress in nucleic acid research and molecular biology. Academic, New York, pp 165–221
WHO (2006) WHO guidelines for the treatment of malaria. World Health Organization, Geneva
Wolf B (1971) The determination of B in soil extracts, plant materials, composts, manures, water and nutrient solutions. Comm Soil Sci Plant Anal 2:363–374
Wolucka BA, Goossens A, Inze D (2005) Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions. J Exp Bot 56:2527–2538
Yau SK, Ryan J (2008) B toxicity tolerance in crops: a viable alternative to soil amelioration. Crop Sci 48:854–865
Yoon JY, Hamayun M, Lee SK, Lee IJ (2009) Methyl jasmonate alleviated salinity stress in soybean. J Crop Sci Biotech 12:63–68
Zhao SS, Zeng MY (1986) Determination of Qinghaosu in Artemisia annua L. by high performance liquid chromatography. Chin J Pharma Anal 6:3–5
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333
Acknowledgements
Authors wish to thank Mr. M. Ram (SRF-CSIR) of Jamia Hamdard (Hamdard University) for his kind help in HPLC analysis regarding artemisinin. The financial support to the first author in the form of Research Assistantship by Council of Science and Technology, UP, Lucknow (CST/D-3539) is also gratefully acknowledged.
Conflict of interest
Authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
An erratum to this article can be found at http://dx.doi.org/10.1007/s00709-010-0238-1
Rights and permissions
About this article
Cite this article
Aftab, T., Khan, M.M.A., Idrees, M. et al. Methyl jasmonate counteracts boron toxicity by preventing oxidative stress and regulating antioxidant enzyme activities and artemisinin biosynthesis in Artemisia annua L.. Protoplasma 248, 601–612 (2011). https://doi.org/10.1007/s00709-010-0218-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-010-0218-5