Abstract
With the humongous boom in the human population with in the last 100 years, there has been an parallel increase in the rate of climate change and prevalence of unfavorable environmental conditions around the globe. These conditions impose stress on the crop plants leading to a huge economic loss of yield. To survive under these stressful conditions, the most prominent way is the endogenous production of various phytohormones. Upon production, these phytohormones exhibit their response by modulating signaling pathways at physiological, biochemical, and molecular levels. They pertain these stresses by controlling the expression of various genes, small RNAs, and transcription factors to mitigate the adverse effect of the stress. Interestingly, Jasmonic acid (JA), a class of polyunsaturated fatty acid-derivative is one such phytohormone that can regulate plant growth and response towards different environmental stresses through interaction with other phytohormones. Various regulatory transcription factors and related genes play a central role in the JA signaling pathway in response to abiotic stresses. For resisting harsh environmental conditions, JA can work both synergistically and antagonistically with different phytohormones. Therefore, this current chapter focuses on the contribution of JAs in plant protection from the various biotic and abiotic stresses and their interaction with different plant hormones.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The current climate change conditions arose due to the unsustainable and erratic use of fossil fuel by humans, which has created a global threat to living systems (Mehta et al. 2020; Mehta et al. 2021; Wuebbles et al. 2017; Singh et al. 2019; Rahman et al. 2019; Mehta et al. 2019a). The adverse conditions like increased atmospheric temperature and carbon dioxide concentration are imposing various stresses on various ecosystems (Bharti et al. 2021). Plants, being sessile, cannot run away from different stresses that prevail in their environment (Sahil et al. 2021). They undergo different stresses caused due to biotic and abiotic factors (Dilawari et al. 2021; Yadav et al. 2021; Rajput et al. 2021; Anamika et al. 2019; Mehta et al. 2019b). Among the two categories, abiotic stresses are the most prevalent that affects the productivity heavily and include temperature, light, salt, water, carbon dioxide, ozone, and nutrient availability (Bharti et al. 2021; Husen 2010; Husen et al. 2014, 2016, 2017; Getnet et al. 2015; Embiale et al. 2016; Hussein et al. 2017; Lal et al. 2018; Isah 2019). All these stresses result in drastic changes in the normal physiological behavior of the plants (Mehta et al. 2020; Sharma et al. 2021). They modify their physiological and developmental conditions to adapt to these adversities by producing certain compounds including phytohormones (Ashraf et al. 2010; Javid et al. 2011; Liu et al. 2019a).
In accordance with multiple studies published in the literature so far, the most prominent way to tackle these stressful conditions is to focus on various endogenously produced phytohormones. These plant hormones like auxins, cytokinins, gibberellins, salicylic acids, jasmonic acids, etc. play a crucial part in sensing the external stresses and also in signal transduction so that the plants can respond to them (Lymperopoulos et al. 2018). Jasmonates, the collection of JA and its derivatives (for example, methyl jasmonate and jasmonate isoleucine conjugate), are the modifications of fatty acids (Viswanath et al. 2020; Ruan et al. 2019). Jasmonates are found in all the higher plants in the reproductive parts and flowers at higher concentrations and in the mature plant parts such as leaves at relatively lower concentration (Wasternack and Hause 2013; Dar et al. 2015). There are different roles played by jasmonates in plants in various processes like seed germination, trichome formation, root growth, gravitropism, sex determination, seedling development, anthocyanin accumulation, chlorophyll degradation, leaf movement, leaf senescence, fertility, and tuber formation (Wasternack and Hause 2002; Wasternack 2014; Wu et al. 2019; Siddiqi and Husen 2019; Viswanath et al. 2020; Fig. 1). Jasmonates also function in providing tolerance against different stresses like salt, heavy metal, temperature, etc. by modifying certain gene expression (Li et al. 2018; Fig. 1). This chapter deals in detail about JA, its biosynthesis, its role in plants’ response to various stresses, and its interaction with other phytohormones involved in the tolerance to these stresses.
2 Biosynthesis of JA
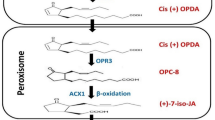
Biosynthesis of JA is not simple straightforward chemistry but actually involves three compartments within a cell, i.e., chloroplast, peroxisome, and cytosol. The α-linolenic acid, an immediate precursor of JA, gets converted into 12-oxo-phytodienoic acid (OPDA) inside the chloroplast with the help of allene oxide synthase along with two more enzymes, lipoxygenase, and allene oxide cyclase. The final product is then transported into peroxisome by an ABC class of transporter COMATOSE (CTS). Oxo-phytodienoate reductase (OPR) is a peroxisomal enzyme that triggers the reduction of cyclopentanone ring of OPDA. Three enzymes of ß-oxidation are required for the final formation of JA from the substrate, i.e., 3-oxo-2(2′[Z]-pentenyl)-cyclopentane-1-octanoate which are l-3-ketoacylCoAthiolase (KAT), Acyl-CoA oxidase (ACX1) and two multifunctional protein (MFP), i.e., 1-3-hydroxy acyl-CoA dehydrogenase, 2-trans-enoyl-CoA hydratase. Then it is exported to the cytosol for further modification (Wasternack and Hause 2002; Wasternack and Hause 2019; Fig. 2).
3 Jasmonic Acid-Mediated Stress Responses
3.1 Biotic Stress
Jasmonic acid is a phytohormone which is well known to protect the plants against different harmful organisms (Mehta et al. 2021; Pieterse et al. 2012). It has also been identified to have a defensive role against wounding, pathogen attack, and herbivory (Rosahl and Feussner 2004). The Arabidopsis mutants with altered JA synthesis have revealed the role of JA in providing resistance to the plants against both the biotrophic and necrotrophic pathogens (Singh et al. 2017).
The JA responsive genes express as a result of E3 ubiquitin ligase mediated proteolysis of JAZ repressor protein involving JA-Ile (JA coupled with isoleucine). This E3 ubiquitin ligase complex is formed when F-box COI 1 protein, a receptor of JA, interacts with Skp1/Cullin counterparts (Thines et al. 2007). Hence, COI 1 is an important protein required for JA responsive gene expression which is also confirmed by studying coi 1 mutants where expression analysis revealed that >80% of JA responsive genes have a decline in expression (Xie et al. 1998; Devoto et al. 2005). In these mutants, this decline resulted in an increase in susceptibility to necrotrophic pathogens. Similarly, in the case of a jar 1-1mutant, the active enzyme involved in JA biosynthesis is mutated making the mutant plant vulnerable to Pythium irregulare (Staswick et al. 1998, 2002). The plants with constitutive expression of JA conferred resistance against fungal pathogens like Erysiphe cichoracearum (Ellis and Turner 2001). These studies explain the involvement of JAs in providing resistance to plants against plant pathogens.
The importance of JA in the regulation of plant response during wounding and insect attack is also reported in tomato. Various studies have revealed that the expression of genes important in wounding and expression of proteinase inhibitors is modulated by exogenous JA (Farmer et al. 1992). The def1 mutant of the tomato plant is found to be defective in the octadecanoid pathway-mediated biosynthesis of wound-induced JA and hence has a lower accumulation of JA (Howe et al. 1996). This mutant also showed reduced resistance to tobacco hornworm, Manduca sexta, which correlates with the decrease in JA accumulation and reduction in proteinase inhibitor gene expression. This demonstrates that the octadecanoid signaling pathway is important for the protection of plants from different types of harmful organisms like chewing insects and harmful fungi. An exogenous application of JA to potato plants resulted in local and systemic defense in those plants against different pathogen attacks (Cohen et al. 1993). JA is found to accumulate in potato infected with Pseudomonas syringae pv. Maculicola, (Landgraf et al. 2002; Halim et al. 2004). However, JA accumulation against P. syringae is not observed in susceptible plants (Weber et al. 1999; Gobel et al. 2002) revealing that the cumulation of JA takes place in response to PAMP recognition and nonhost pathogen interaction.
Another gene JAV1, JA-associated VQ motif gene 1, negatively regulates the JA-mediated defense against insect and pathogen attack (Hu et al. 2013). JA-COI 1 signaling complex is a part of the degradation system responsible for the degradation of this negative regulator, JAV1, through 26S proteasome, thereby contributing to the activation of defense-related genes and protection against pathogens. When there is no such attack by pathogens, active JAV1 is not degraded and interacts with the WRKY transcription factor leading to inactivation of its active functions. Once there is a pathogen attack, JAV1 gets degraded resulting in the activation of a downstream signaling pathway that results in the development of plant defense against these pathogens. This negative regulation by JAV1 helps a damaged plant to mediate their JA-mediated defense response to establish a harmony between growth and defense during stress.
Genetically engineered rice plants with AOS2 expression, allene oxide synthase (plays role in JA synthesis), have induced expression of antipathogenic proteins (like PR3, PR5, etc.) and enhanced resistance against pathogenic fungi (Mei et al. 2006). The two rice osjar1 mutants having lower JA Ile (product of JAR 1) production are susceptible to blast fungi in contrast to the nonmutants (Shimizu et al. 2013), revealing the function of JA Ile in mediating defense mechanisms in rice against blast fungus. Also, the complete silencing of OsCOI 1 enhanced the susceptibility of rice plants against chewing insects that correlated with the downregulation of various genes encoding trypsin protease inhibitor, polyphenol oxidase (converts phenols to toxic quinones), and peroxidase.
The exogenous treatment of MeJA to Arachis hypogea has shown to increase the level of antioxidant enzymes, thereby reducing growth and development of Helicoverpa armigera (War et al. 2015), whereas in Vigna mungo, methyl jasmonate (MeJA) is found to protect the plant from Mungbean yellow mosaic India virus through the restoration of membrane stability and maintenance of reactive oxygen species homeostasis (Chakraborty and Basak 2019). In Solanum lycopersicum, external treatment with methyl jasmonate provides tolerance against Helicoverpa zea by inducing defense gene expression (Tian et al. 2014). The external treatment of MeJA to Phaseolus vulgaris and Panax notoginseng provided resistance against Sclerotinia sclerotiorum and Fusarium solani, respectively (Oliveira et al. 2015; Liu et al. 2019b), as shown in Table 1.
3.2 Abiotic Stress
JA is a crucial plant-hormone that protects against different stresses like cold stress, heat stress, etc. and its role against various abiotic stresses has been studied already in various crops (Acharya and Assmann 2009; Karpets et al. 2014; Wasternack 2014; Siddiqi and Husen 2019). The use of exogenous JA can protect the plants from various abiotic stresses.
3.2.1 Salinity Stress
Salinity stress contributes to huge crop losses every year (Munns and Tester 2008). Na+ and Cl− are the primary salts contributing to salt stress. The prime sources of accumulation of salts in agricultural lands are irrigation water having relatively high salt contents and chemical pesticides/insecticides used for crop protection (Munns and Tester 2008). Salinity hinders various processes such as uptake of nutrients, chlorophyll degradation, etc., and the evolution of ROS is observed under severe salinity conditions. This in turn leads to oxidative damage to various proteins and DNA as well (Farhangi-Abriz and Torabian 2017).
Salt treatment to different plants such as Lycopersicon esculentum, Solanum tuberosum, and Arabidopsis thaliana resulted in enhanced endogenous JA in these plants (Pedranzani et al. 2013; Ellouzi et al. 2013; De Domenico et al. 2019). Sweet potato under salt stress is found to have increased levels of JA as per the transcript profile (Zhang et al. 2017). The immediate increase in JA content is observed in salt-sensitive plants under salt stress whereas this increase is minimal in salt-tolerant plants (De Domenico et al. 2019). In rice, the accumulation of JA resulted in reducing the negative effects of salt stress on biomass production (Kang et al. 2005). The application of JA to safflower leaves growing under salt stress resulted in enhanced yield, and physiological performance (Ghassemi-Golezani and Hosseinzadeh-Mahootchi 2015). The external treatment of JA to salt stress exposed plants has shown better potassium content, lower lipid peroxidation, and higher antioxidant activity (Farhangi-Abriz and Ghassemi-Golezani 2018). The external treatment of JA to different plants like Brassica oleracea, Matricaria chamomilla, Robinia pseudoacacia, Vigna unguiculate, and Brassica napus have shown to increase antioxidant enzyme activity and net photosynthetic rate, thereby alleviating negative effects of salt stress (Wu et al. 2012; Salimi et al. 2016; Jiang et al. 2016; Sadeghipour 2017; Ahmadi et al. 2018).
3.2.2 Drought Stress
Drought stress is responsible for extensive crop losses every year. Various changes in plants are observed during drought, which has serious repercussions on their growth and yield (Pandey et al. 2017). There are various negative impacts of drought stress on different physiological processes of plants including the decrease in turgor pressure, reduction in photosynthetic rates, increase in leaf senescence, and ion toxicity.
As compared to the different stresses, there is little information related to the functions of JA in drought stress. Jasmonic acid can regulate the opening and closing of stomata, thereby reducing the rate of water loss during water-deficient conditions (Savchenko et al. 2014). The endogenous JA levels were increased when different plants such as A. thaliana (Balbi and Devoto 2008) and Citrus spp. (De Ollas et al. 2013) were subjected to water-deficient conditions, but JA concentration was declined to basal level upon prolonged exposure. The different components of JA signaling pathways are involved in making plants tolerant to the shortage of water. The negative regulator or a repressor of the JA signaling pathway termed jasmonate ZIM-domain proteins (JAZ) are found to negatively regulate the rice tolerance to water-deficient conditions (Fu et al. 2017).
The external employment of various plant hormones to plants growing under particular stress has proved to be an effective method to reduce harmful outcomes of the stress on plants, the same is with JA and drought stress. This strategy has been found to increase crop production under drought stress by increasing antioxidant enzyme activities. The exogenous application of JA to maize plants growing under drought stress resulted in an escalated amount of antioxidant enzymes that lower the damage done by the increased ROS (Abdelgawad et al. 2014). Also, the lipid peroxidation decreased in peanut grown under water-deficient conditions by the exogenous supply of JA that resulted in decreased oxidative stress because of the increased antioxidant enzyme production (Kumari et al. 2006). It is found that the synthesis of antioxidant enzymes like glutathione peroxidase, catalase, etc. is enhanced upon foliar application of JA in Brassica that in turn leads to an alleviation of damage caused by increased ROS (Alam et al. 2014). The exact mechanism of induction of these antioxidant enzymes by the JA application is still not known very well. Additionally, the external application of MeJA to different plants like Solanum nigrum, Triticum aestivum, Glycine max, and summer savory has shown to increase growth and decrease oxidative stress when subjected to stress conditions (Yan et al. 2015; Anjum et al. 2016; Mohamed and Latif 2017; Ma et al. 2014; Miranshahi and Sayyari 2018).
3.2.3 Heat Stress
The changes in temperature above and below the limits of ambient temperature decrease crop productivity drastically (Zandalinas et al. 2018). The overall rise in temperature because of the greenhouse effect is termed as heat stress. This type of stress has various adverse effects on plants including the protein denaturation, lowering of enzymatic activity, etc. When exposed to heat stress, plants regulate gene expression, thereby producing essential proteins or controlling signaling pathways. One of the important proteins with excessive production during heat stress is heat-shock protein (HSP). HSPs protect different proteins from heat stress along with some major roles in many other processes.
Jasmonic acid has many protective functions against stress and can also produce different secondary metabolites as well as heat-shock proteins (Creelman and Mullet 1995; Balfagón et al. 2019). JA signaling functions in providing tolerance to Arabidopsis against heat stress (Clarke et al. 2009). The increased concentration of JA in plants growing under heat stress contributes to plant defense against stressful conditions (Hasanuzzaman et al. 2013). The foliar treatment of JA to barley enhances the production of heat-shock proteins by changes in gene expression (Mueller-Uri et al. 1988; Aghdam et al. 2013). A counterview has been proposed by many other scientists stating that the modification in plant phenolic components was responsible for increased production of HSPs, thereby protecting plants from heat stress (Saltveit 2000). The application of JA results in an increase in abscisic acid which led to the induction of stomatal closure and reduced transpiration for maintaining plant temperature (Lehmann et al. 1995; Creelman and Mullet 1997; Acharya and Assmann 2009). JA also regulates water potential in a plant cell resulting in the preservation of water.
3.2.4 Cold Stress
Since a long time, cold stress is considered as harmful for the overall growth of the plant that can be further categorized into freezing stress and chilling stress based on temperature either below 0 °C or above 0 °C, respectively (Huang et al. 2014; Trischuk et al. 2014; Sharma et al. 2020). The major effects of cold stress in plants include ice crystal formation inside the cells and cellular dehydration. Various other changes like the molecular and the biochemical changes along with physiological changes are observed in plants during cold stress that results in the synthesis of cold stress-induced proteins, essential amino acids, and soluble proteins (Hincha and Zuther 2014; Ritonga and Chen 2020).
Different plant hormones are demonstrated to be involved in plant protection against cold stress (Kosova et al. 2012; Wasternack 2014). In plants, during low-temperature stress, an increase in the concentration of JA is observed (Kosova et al. 2012). Also, when the Pinus pinaster plants suffered two different stresses, water stress and cold stress, a rise in JA content of leaves is observed (Pedranzani et al. 2007). The transcription of different genes like allene oxide cyclase (AOC), lipoxygenase 2, and allene oxide synthase 1 (AOS) increased as a result of cold stress and all these genes are found to contribute to JA biosynthesis. Jasmonates can alleviate chilling stress by inducing the production of proteinase inhibitors, antioxidants, abscisic acid, cryoprotectants, and polyamines (Cao et al. 2009; Zhao et al. 2013). The JA application to chilled rice in a low dosage enhances their survival ratio (Lee et al. 1997). Methyl jasmonate has been used on plants like Prunus persica, Punica granatum Solanum lycopersicum, Eriobotrya japonica, and Vigna sinensis, resulting in enhanced antioxidant enzyme activity, decreased oxidative damage, and mitigation of cold stress (Jin et al. 2009; Sayyari et al. 2011; Zhang et al. 2012; Fan et al. 2016).
3.2.5 Heavy Metal Stress
This type of abiotic stress is highly prevalent in agricultural lands because of the usage of chemical fertilizers and pesticides, weathering of rocks, etc. Heavy metal stress has various ill effects on plant growth, which could lead to increased leaf senescence and decreased photosynthetic rates (Maksymiec 2007; Berni et al. 2019).
Heavy metal toxicity like cadmium and copper toxicity can increase the concentration of endogenous JA in Arabidopsis thaliana plants (Xiang and Oliver 1998). The exogenous application of JA to Glycine max seedlings before NiCl2 stress increased plant survival in Ni2+ stress (Sirhindi et al. 2015). Heijari et al. (2008) determined that the external use of methyl jasmonate in lower doses alleviates the adverse effects of aluminum toxicity by elevating the enzymatic and nonenzymatic antioxidants. JA also reduced lipid peroxidation by enhancing the production of glutathione or ascorbate antioxidants (Noriega et al. 2012). The application of jasmonates to soybean leaves growing under cadmium stress alleviated cadmium stress by lowering lipid peroxidation, ROS, and enhancing antioxidant activity (Keramat et al. 2009). The external employment of jasmonates in low dosage to plants under copper toxicity protected plants by modifying photosynthetic pigments (Poonam et al. 2013). The use of MeJA externally can prevent oxidative damage and mitigate heavy metal stress in various plants including Cajanus cajan, Capsicum frutescens, Kandelia obovate, Solanum nigrum, and Brassica napus (Poonam et al. 2013; Yan et al. 2013, 2015; Chen et al. 2014; Farooq et al. 2016). Hence, it can be known from different observations that jasmonates are involved in the reduction of harmful impacts of heavy metal stress.
4 JA Signaling
4.1 In the Absence of JA
The synthesis of JA involves three compartments, chloroplast, peroxisome, and cytosol. JA is then transferred to the cytoplasm/cytosol (Wasternack and Hause 2002; Ruan et al. 2019). In the cytoplasm, JA-Ile was found to be the most bioactive form. In response to different stresses, JA moved to the nucleus and apoplast through a transporter protein known as jasmonic acid transfer protein 1 (JAT1).
Under normal conditions, various transcription factors are unable to activate promoters of jasmonate-responsive genes because of very little concentration of JA-lle. The coronatine insensitive 1 (COI1) and JAZ co-receptors together are a part of the jasmonate receptor. It was reported that the COI1-JAZ co-receptor has >100-fold greater affinity for the ligand than either COI1 or JAZ alone (Sheard et al. 2010). The F-box protein, COI1, associates with Skp/cullin to establish an E3 ubiquitin ligase complex, i.e., SCFCOI1. JAZ proteins are the repressors of jasmonate signaling. JAZ proteins have conserved Jas and ZIM/TIFY domains. Both these domains have different functions like Jas domain interacts with COI1 and MYC2 proteins whereas the ZIM domains cause dimerization and association with JAZ proteins and NINJA (Chung and Howe 2009). The NINJA [having an EAR motif] communicates with TOPLESS (TPL). NINJA, TOPLESS along with JAZ proteins form a complex having a role in the repression of transcription (Fig. 3). Through the participation of histone deacetylase 6 (HDA6) and HDA19, this complex aims at inhibiting the jasmonate-responsive genes expression by the formation of a closed complex from an open one (Pauwels et al. 2010; Causier et al. 2012; Acosta et al. 2013; Chini et al. 2016; Wasternack and Song 2017).
4.2 In the Presence of JA
After the plant perceives any kind of abiotic stress, it starts elevating the production of JA-lle and it is recognized by COI1. In the nucleus, JA-lle promotes interaction between JAZ and COI1 (Xie et al. 1998; Zhai et al. 2015; Ali and Baek 2020). This JA-lle, COI1, and JAZ interaction facilitate the ubiquitin-dependent degradation of JAZ proteins by 26S proteasome which serves as a repressor of jasmonate signaling. Degradation of repressor permits transcriptional activation of different jasmonate-responsive genes by the activity of various transcription factors (Fig. 4). In Arabidopsis, Mediator 25 (MED25) (Bäckström et al. 2007) serves as a communicator between the general transcription factors (GTFs), RNA polymerase II, and the gene-specific TFs (Chen et al. 2012).
5 Interaction of JA with Other Phytohormone Signaling Pathways Under Stress Conditions
5.1 Interaction of JA with Auxins for Root Development
Cross talk can be visualized as two distinct pathways not independent of each other which can be negative or positive and can alter synthesis, transport, or signaling pathway of another hormone (Jang et al. 2020). For example, the growth of plant roots is being regulated by different hormonal pathways. Auxin plays a crucial role among them. It has been already reported that PLT genes that encode AP2 (APETALA2) class of TFs plays a crucial part in the patterning of SCN (Galinha et al. 2007). Auxin signaling pathway was reported by Dharmasiri et al. (2005), Kepinski and Leyser (2005), and Mockaitis and Estelle (2008), where it was demonstrated that TRANSPORT INHIBITOR RESPONSE1 (TIR1) which is an F-box protein serves as auxin receptor. After attachment of auxin with TIR1, it facilitates the association of TIR1 with Aux/IAA substrates which serve as an inhibitor of auxin signaling. TIR1 is a component of SCF ubiquitin ligase complex SCFTIR1 leading to the ubiquitin-dependent proteolysis of Aux/IAA by 26S proteasome (Fig. 5). Degradation of repressor permits transcriptional activation by ARFs that promote the expression of different auxin-responsive genes including PLT1and PLT2.
Chen et al. (2011) observed that the expression levels of PLT1 and PLT2 are downregulated by JA, which is reliant on the MYC2 transcription factor. Dombrecht et al. (2007) have reported that the preferable site of MYC2 binding is 5′-CACATG-3′ motif in the target genes. Chen et al. (2011) also reported the existence of this single motif in P1 region (−1609 to −1614 bp) whereas two motifs in P2 region (−940 to −945 and −1098 to −1103 bp) of PLT 1 promoter and also one same motif in P3 region (+288 to +293) of PLT2 promoter. Their study also revealed MYC2 TFs act upstream of PLT1 and PLT2 TFs in regulating SCN maintenance and root meristem activity. It has already been reported that transcription of PLT1 and PLT2 are being upregulated by auxin. SCN maintenance and meristem activity are also being positively regulated by auxin (Aida et al. 2004). As compared to this, the study of Chen et al. (2011) focused on lowered PLT1 and PLT2 expression by JA and negative regulation of SCN maintenance and meristem activity, therefore, hints at the convergence of different JA and auxin signaling pathways in the modulation of maintenance of root SCN.
5.2 Interaction of JA with Ethylene in the Regulation of Apical Hook Formation
Various epigeal plants have developed an organ known as an apical hook during evolution to protect apical meristem and cotyledons from any sort of damage. It is already reported that formation of ethylene (ET) and gibberellins (GAs) contributes to apical hook formation whereas jasmonate (JA) and brassinosteroids discourage the apical hook formation (Vriezen et al. 2004; Li et al. 2004; De Grauwe et al. 2005). Lehman et al. (1996) reported that HOOKLESS1 (HLS1) is a key regulator of auxin distribution and responses at the time of apical hook formation of Arabidopsis seedlings. HLS1 transcript accumulation is promoted by ET through ETHYLENE INSENSITIVE3 (EIN3) which binds directly to the promoter of HLS1.
Several ET receptors like ERS1, ERS2, ETHYLENE TRIPLE RESPONSE (ETR1), ETR2, and ETHYLENE INSENSITIVE4 (EIN4) have already been identified. In the absence of ET, the ET responses are negatively regulated by its receptors. CONSTITUTIVE TRIPLE RESPONSE1 (CTR1) is present downstream of the ET receptors and negatively regulates the ethylene response in the absence of ethylene. The binding of ethylene to its receptors promotes the deactivation of its receptors. EIN2 works downstream of CTR1 which gets inactive in the absence of ET because of ETP1 and ETP2 that are components of the ubiquitin ligase complex that targets EIN2 for proteolysis. In the presence of ethylene, ETP1 and ETP2 get degraded whereas the degradation of EIN2 gets halted leading to the activation of downstream signaling. EIN2 promotes the stabilization of EIN3 and EIL1 that are TFs binding at a particular site on the promoter of ERF1 termed as ethylene binding site (EBS). Another TF is encoded by ERF1 that modulates the transcription of ethylene-responsive genes including HLS1. During the unavailability of ET, EIN3 and EIL1 are degraded via ubiquitin-mediated proteolysis by F-box proteins, EBF1, and EBF2 (Fig. 6).
The activity of EIN3/EIL1 is repressed by the JA-activated transcription factor (MYC2) in the modulation of HLS1 expression and hook development (Zhang et al. 2014). There are several pieces of evidence available that support the statement that EIN3/EIL1 degradation is being promoted by JA in a SCFEBF1-dependent manner. It can also be concluded that apical hook development is being governed by the antagonistic action of the JA-ET signaling pathway where these two hormones have opposite effects on the stability of EIN3/EIL1 protein. ET stabilizes the cumulation of EIN3/EIL1 whereas JA is responsible to foster their degradation.
5.3 JA and GA Cross talk for Stamen Development
Stamen is a male reproductive organ that produces pollen, crucial for the fertility of a plant. Different development stages of stamen development are governed by various environmental factors and hormonal signaling. JA and GA are the master regulators of stamen development.
GIBBELELLINE INSENSITIVE DWARF1 (GID1) serves as a receptor in the GA signaling pathway. After binding of bioactive GA to GID1, a conformational switch in the GID1 ensures DELLA binding. This interaction in turn promotes transition in the GRAS domain of the DELLA protein for recognition of SKP, Cullin, and F-box proteins, SCFSLY1/GID2 that results in ubiquitin-mediated proteolysis of DELLA protein. Degradation of DELLA promotes the transcription of different GA responsive genes. GA responses begin and SPINDLY (SPY) encodes an N-acetylglucosamine transferase that adds N-acetylglucosamine to DELLA for its activation and therefore serves as GA signaling repressor. EARLY FLOWERING 1 (EL1), a casein kinase in rice, may also phosphorylate and activate DELLA (Sun 2010; Qin et al. 2011).
Various studies revealed that JA promotes the transcription of R2R3 MYB TFs MYB21, MYB24, etc. crucial for stamen development (Cheng et al. 2009; Mandaokar and Browse 2009; Song et al. 2011). JAZ proteins act as a repressor of JA signaling where it inhibits the activity of MYB TFs MYB21, MYB24, etc. (Song et al. 2011). The bHLH-MYB complex formed as a result of an association between lle bHLH and MYB21, MYB24 regulates the stamen development (Qi et al. 2015). At the time plant perceives environmental cues it starts synthesizing JA. Interaction of JA to its co-receptor COI1 and JAZ facilitates JAZ to degrade through 26S proteasome, thereby releasing the bHLH-MYB complex that also modulates the downstream gene expression in mediating stamen development (Fig. 7). It was already reported that DELLA is involved in inhibition of JA biosynthesis gene expression in flowers that lead to altering the process of stamen development. Hong et al. (2012) demonstrated direct interaction of DELLA with MYC2 to repress its activity. Further, it remains an area of investigation to find out whether all the bHLH members (MYC2, MYC3, etc.) and MYB21 and MYB24 are targets of DELLA in inhibiting the transcription function of the bHLH-MYB complex for regulating stamen development.
6 Concluding remarks
While growing in nature, the green plants are often subjected to multiple abiotic and biotic stresses either in single or in combination. To tackle challenging conditions and support their growth, plants initiate a fine-tuning in their signaling networks as well as bring changes in the concentration of endogenous plant hormones. This makes it necessary to further investigate the cross talk of natural plant hormones in the balance warfare of growth and defense. One of such important phytohormone is jasmonic acid (JA). Due to the continuous research and parallel advancements in omics in recent years, the crucial roles of JA and its derivatives have been investigated from seed germination to plant’s defense as well as tolerance. During the challenging various stresses, JAs induce antioxidative enzymes and other defensive compounds as well as modulate the nutrition uptake for combating the stresses.
At present, it has been deduced that the action mechanism of the core JA signaling pathway varies under every stress due to the diversity of both positive and negative interactions with numerous genes, regulatory TFs, small RNAs, hormones, elicitor, and treatments. However, so far, the data still fail to throw light on the highly complex molecular mechanism of JA signaling with changeable networks of other plant hormones in combinatorial stresses at a time. Furthermore, the identified components of hormone signaling are still very low as compared with the unidentified components. Moreover, there are very high chances of the variability in the data recorded in labs from those that actually occur in the farmer fields. Additionally, there is still no single report available in the literature that embarks the complete temporal analysis of plant hormone signaling networks starting from the seedling stage to senescence. So overall, we have to keep in mind that the current knowledge is limited as compared with a big list of related questions. As a result, in the future, systematic omics research on the interaction of JA signaling with each and every interacting factor will be done for broad application prospect, i.e., super tolerance in plants that do not compromise yield. In the future, the exact molecular mechanisms of both vascular and airborne transmission of the JA signal will also be elucidated. The light will be also given on how various environmental signals initiate the synthesis of JA.
Abbreviations
- ACX1:
-
Acyl-CoA oxidase
- AD:
-
Activation domain
- ARF:
-
Auxin response factor
- AUX/IAA:
-
Auxin/indole acetic acid
- BD:
-
DNA binding domain
- EAR:
-
Ethylene-responsive element binding factor-associated amphiphilic repression
- HAD19:
-
Histone deacetylase 19
- HDA6:
-
Histone deacetylase 6
- IAA:
-
Indole acetic acid
- JA:
-
Jasmonic acid
- Jas:
-
C-terminal JA-associated domain
- JAV1:
-
JA-associated VQ motif gene 1
- KAT:
-
l-3-ketoacylCoAthiolase
- NINJA:
-
Novel interactor of JAZ
- OPDA:
-
12-oxo-phytodienoic acid
- OPR:
-
Oxo-phytodienoate reductase
- PLT:
-
PLETHORA
- TPL:
-
TOPLESS
References
Abdelgawad ZA, Khalafaallah AA, Abdallah MM (2014) Impact of methyl jasmonate on antioxidant activity and some biochemical aspects of maize plant grown under water stress condition. Agric Sci 5:1077–1088
Acharya BR, Assmann SM (2009) Hormone interactions in stomatal function. Plant Mol Biol 69:451–462
Acosta IF, Gasperini D, Chételat A, Stolz S, Santuari L, Farmer EE (2013) Role of NINJA in root jasmonate signaling. Proc Natl Acad Sci 110:15473–15478
Aghdam MS, Sevillano L, Flores FB, Bodbodak S (2013) Heat shock proteins as biochemical markers for postharvest chilling stress in fruits and vegetables. Sci Hortic 160:54–64
Ahmadi FI, Karimi K, Struik PC (2018) Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S Afr J Bot 115:5–11
Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Scheres B (2004) The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119:109–120
Alam MM, Nahar K, Hasanuzzaman M, Fujita M (2014) Exogenous jasmonic acid modulates the physiology, antioxidant defense and glyoxalase systems in imparting drought stress tolerance in different Brassica species. Plant Biotechnol Rep 8:279–293
Ali M, Baek KH (2020) Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int J Mol Sci 21:621
Anamika, Mehta S, Singh B, Patra A, Islam MA (2019) Databases: a weapon from the arsenal of bioinformatics for plant abiotic stress research. In: Recent approaches in omics for plant resilience to climate change. Springer, Cham, pp 135–169
Anjum SA, Tanveer M, Hussain S, Tung SA, Samad RA, Wang L, Khan I, ur Rehman N, Shah AN, Shahzad B (2016) Exogenously applied methyl jasmonate improves the drought tolerance in wheat imposed at early and late developmental stages. Acta Physiol Plant 38:25
Ashraf M, Akram NA, Arteca RN, Foolad MR (2010) The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Crit Rev Plant Sci 29:162–190
Bäckström S, Elfving N, Nilsson R, Wingsle G, Björklund S (2007) Purification of a plant mediator from Arabidopsis thaliana identifies PFT1 as the Med25 subunit. Mol Cell 26:717–729
Balbi V, Devoto A (2008) Jasmonate signalling network in Arabidopsis thaliana. Crucial regulatory nodes and new physiological scenarios. New Phytol 177:301–318
Balfagón D, Sengupta S, Gómez-Cadenas A, Fritschi FB, Azad RK, Mittler R, Zandalinas SI (2019) Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiol 181:1668–1682
Berni R, Luyckx M, Xu X, Legay S, Sergeant K, Hausman JF, Lutts S, Cai G, Guerriero G (2019) Reactive oxygen species and heavy metal stress in plants: impact on the cell wall and secondary metabolism. Environ Exp Bot 161:98–106
Bharti J, Sahil, Mehta S, Ahmad S, Singh B, Padhy AK, Srivastava N, Pandey V (2021) Mitogen-Activated Protein Kinase, Plants, and Heat Stress. In Harsh Environment and Plant Resilience: Molecular and Functional Aspects. Springer Nature, Switzerland AG, pp. 323–354
Cao S, Zheng Y, Wang K, Jin P, Rui H (2009) Methyl jasmonate reduces chilling injury and enhances antioxidant enzyme activity in postharvest loquat fruit. Food Chem 115:1458–1463
Causier B, Ashworth M, Guo W, Davies B (2012) The TOPLESS interactome: a framework for gene repression in Arabidopsis. Plant Physiol 158:423–438
Chakraborty N, Basak J (2019) Exogenous application of methyl jasmonate induces defense response and develops tolerance against mungbean yellow mosaic India virus in Vigna mungo. Funct Plant Biol 46:69–81
Chen PQ, Yu SL, Zhan YN, Kang XL (2006) Effects of jasmonate acid on thermotolerance of grape seedlings. Journal of Shihezi University (Natural Science). J Shihezi Univ (Nat Sci) 24:87–91
Chen Q, Sun J, Zhai Q, Zhou W, Qi L, Xu L, Li X (2011) The basic helix-loop-helix transcription factor MYC2 directly represses PLETHORA expression during jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell 23:3335–3352
Chen R, Jiang H, Li L, Zhai Q, Qi L, Zhou W, Li C (2012) The Arabidopsis mediator subunit MED25 differentially regulates jasmonate and abscisic acid signaling through interacting with the MYC2 and ABI5 transcription factors. Plant Cell 24:2898–2916
Chen J, Yan Z, Li X (2014) Effect of methyl jasmonate on cadmium uptake and antioxidative capacity in Kandelia obovata seedlings under cadmium stress. Ecotoxicol Environ Saf 104:349–356
Cheng H, Song S, Xiao L, Soo HM, Cheng Z, Xie D, Peng J (2009) Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS Genet 5:e1000440
Chini A, Gimenez-Ibanez S, Goossens A, Solano R (2016) Redundancy and specificity in jasmonate signalling. Curr Opin Plant Biol 33:147–156
Chung HS, Howe GA (2009) A critical role for the TIFY motif in repression of jasmonate signaling by a stabilized splice variant of the JASMONATE ZIM-domain protein JAZ10 in Arabidopsis. Plant Cell 21:131–145
Clarke SM, Cristescu SM, Miersch O, Harren FJ, Wasternack C, Mur LA (2009) Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol 182:175–187
Cohen Y, Gisi U, Niderman T (1993) Local and systemic protection against Phytophthora infestans induced in potato and tomato plants by jasmonic acid and jasmonic-methylester. Phytopathology 83:1054–1062
Creelman RA, Mullet JE (1995) Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci 92:4114–4119
Creelman RA, Mullet JE (1997) Biosynthesis and action of jasmonates in plants. Annu Rev Plant Biol 48:355–381
Dar TA, Uddin M, Khan MMA, Hakeem KR, Jaleel H (2015) Jasmonates counter plant stress: a review. Environ Exp Bot 115:49–57
De Domenico S, Taurino M, Gallo A, Poltronieri P, Pastor V, Flors V, Santino A (2019) Oxylipin dynamics in Medicago truncatula in response to salt and wounding stresses. Physiol Plant 165:198–208
De Grauwe L, Vandenbussche F, Tietz O, Palme K, Van Der Straeten D (2005) Auxin, ethylene and brassinosteroids: tripartite control of growth in the Arabidopsis hypocotyl. Plant Cell Physiol 46:827–836
De Ollas C, Hernando B, Arbona V, Gómez-Cadenas A (2013) Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol Plant 147:296–306
Devoto A, Ellis C, Magusin A, Chang HS, Chilcott C, Zhu T (2005) Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol Biol 58:497–513
Dharmasiri N, Dharmasiri S, Estelle M (2005) The F-box protein TIR1 is an auxin receptor. Nature 435:441–445
Dilawari R, Kaur N, Priyadarshi N, Kumar B, Abdelmotelb KF, Lal SK, Singh B, Tripathi A, Aggarwal SK, Jat BS, Mehta S (2021) Genome Editing: A Tool from the Vault of Science for Engineering Climate-Resilient Cereals. In: Harsh Environment and Plant Resilience: Molecular and Functional Aspects. Springer Nature, Switzerland AG, pp 45–72
Dombrecht B, Xue GP, Sprague SJ, Kirkegaard JA, Ross JJ, Reid JB, Kazan K (2007) MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 19:2225–2245
Ellis C, Turner JG (2001) The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell 13:1025–1033
Ellouzi H, Ben Hamed K, Cela J, Müller M, Abdelly C, Munné-bosch S (2013) Increased sensitivity to salt stress in tocopherol-deficient Arabidopsis mutants growing in a hydroponic system. Plant Signal Behav 8:1–13
Embiale A, Hussein A, Husen A, Sahile S, Mohammed K (2016) Differential sensitivity of Pisum sativum L. cultivars to water-deficit stress: changes in growth, water status, chlorophyll fluorescence and gas exchange attributes. J Agron 15:45–57
Fan L, Wang Q, Lv J, Gao L, Zuo J, Shi J (2016) Amelioration of postharvest chilling injury in cowpea (Vigna sinensis) by methyl jasmonate (MeJA) treatments. Sci Hortic 203:95–101
Farhangi-Abriz S, Ghassemi-Golezani K (2018) How can salicylic acid and jasmonic acid mitigate salt toxicity in soybean plants? Ecotoxicol Environ Saf 147:1010–1016
Farhangi-Abriz S, Torabian S (2017) Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol Environ Saf 137:64–70
Farmer EE, Johnson RR, Ryan CA (1992) Regulation of proteinase inhibitor gene expression by methyl jasmonate and jasmonic acid. Plant Physiol 98:995–1002
Farooq MA, Gill RA, Islam F, Ali B, Liu H, Xu J (2016) Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front Plant Sci 7:468–483
Fu J, Wu H, Ma SQ, Xiang DH, Liu RY, Xiong LZ (2017) OsJAZ1 attenuates drought resistance by regulating JA and ABA signaling in Rice. Front Plant Sci 8:1–13
Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, Scheres B (2007) PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 449:1053–1057
Getnet Z, Husen A, Fetene M, Yemata G (2015) Growth, water status, physiological, biochemical and yield response of stay green sorghum {Sorghum bicolor (L.) Moench} varieties—a field trial under drought-prone area in Amhara regional state, Ethiopia. J Agron 14:188–202
Ghassemi-Golezani K, Hosseinzadeh-Mahootchi A (2015) Improving physiological performance of safflower under salt stress by application of salicylic acid and jasmonic acid. Walia J 31:104–109
Gobel C, Feussner I, Hamberg M, Rosahl S (2002) Oxylipin profiling in pathogen-infected potato leaves. Biochim Biophys Acta 1584:55–64
Halim VA, Hunger A, Macioszek V, Landgraf P, Nürnberger T, Scheel D, Rosahl S (2004) The oligopeptide elicitor Pep-13 induces salicylic acid-dependent and -independent defense reactions in potato. Physiol Mol Plant Pathol 64:311–318
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684
Heijari J, Nerg AM, Kainulainen P, Vuorinen M, Holopainen JK (2008) Long term effects of exogenous methyl jasmonate application on Scots pine (Pinus sylvestris) needle chemical defence and diprionid sawfly performance. Entomol Exp Appl 128:162–171
Hincha DK, Zuther E (2014) Introduction: plant cold acclimation and freezing tolerance. Methods Mol Biol 1166:1–6
Hong GJ, Xue XY, Mao YB, Wang LJ, Chen XY (2012) Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 24:2635–2648
Howe GA, Lightner J, Browse J, Ryan CA (1996) An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8:2067–2077
Hu P, Zhou W, Cheng Z, Fan M, Wang L, Xie D (2013) JAV1 controls jasmonate-regulated plant defense. Mol Cell 50:504–515
Huang BR, DaCosta M, Jiang YW (2014) Research advances in mechanisms of turfgrass tolerance to abiotic stresses: from physiology to molecular biology. Crit Rev Plant Sci 33:141–189
Husen A (2010) Growth characteristics, physiological and metabolic responses of teak (Tectona grandis Linn. f.) clones differing in rejuvenation capacity subjected to drought stress. Silva Genet 59:124–136
Husen A, Iqbal M, Aref IM (2014) Growth, water status and leaf characteristics of Brassica carinata under drought and rehydration conditions. Braz J Bot 37:217–227
Husen A, Iqbal M, Aref IM (2016) IAA-induced alteration in growth and photosynthesis of pea (Pisum sativum L.) plants grown under salt stress. J Environ Biol 37:421–429
Husen A, Iqbal M, Aref IM (2017) Plant growth and foliar characteristics of faba bean (Vicia faba L.) as affected by indole-acetic acid under water-sufficient and water-deficient conditions. J Environ Biol 38:179–186
Hussein M, Embiale A, Husen A, Aref IM, Iqbal M (2017) Salinity-induced modulation of plant growth and photosynthetic parameters in faba bean (Vicia faba) cultivars. Pak J Bot 49:867–877
Isah T (2019) Stress and defense responses in plant secondary metabolites production. Biol Res 52:1–25
Jang G, Yoon Y, Choi YD (2020) Crosstalk with jasmonic acid integrates multiple responses in plant development. Int J Mol Sci 21:305
Javid MG, Sorooshzadeh A, Moradi F, Sanavy SAMM, Allahdadi I (2011) The role of phytohormones in alleviating salt stress in crop plants. Aust J Crop Sci 5:726–734
Jiang M, Xu F, Peng M, Huang F, Meng F (2016) Methyl jasmonate regulated diploid and tetraploid black locust tolerance to salt stress. Acta Physiol Plant 38:106
Jin P, Zheng Y, Tang S, Rui H, Wang CY (2009) A combination of hot air and methyl jasmonate vapor treatment alleviates chilling injury of peach fruit. Postharvest Biol Technol 52:24–29
Jin P, Duan Y, Wang L, Wang J, Zheng Y (2014) Reducing chilling injury of loquat fruit by combined treatment with hot air and methyl jasmonate. Food Bioprocess Technol 7:2259–2266
Kang DJ, Seo YJ, Lee JD, Ishii R, Kim KU, Shin DH, Lee IJ (2005) Jasmonic acid differentially affects growth, ion uptake and abscisic acid concentration in salt-tolerant and salt-sensitive rice cultivars. J Agron Crop Sci 191:273–282
Karpets YV, Kolupaev YE, Lugovaya AA, Oboznyi AI (2014) Effect of jasmonic acid on the pro-/antioxidant system of wheat coleoptiles as related to hyperthermia tolerance. Russ J Plant Physiol 61:339–346
Kepinski S, Leyser O (2005) The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435:446–451
Keramat B, Kalantari KM, Arvin MJ (2009) Effects of methyl jasmonate in regulating cadmium induced oxidative stress in soybean plant (Glycine max L.). Afr J Microbiol Res 3:240–244
Kosova K, Prášil IT, Vítámvás P, Dobrev P, Motyka V, Floková K, Novák O, Turečková V, Rolčik J, Pešek B, Trávničková A (2012) Complex phytohormone responses during the cold acclimation of two wheat cultivars differing in cold tolerance, winter Samanta and spring Sandra. J Plant Physiol 169:567–576
Kumari GJ, Reddy AM, Naik ST, Kumar SG, Prasanthi J, Sriranganayakulu G, Reddy PC, Sudhakar C (2006) Jasmonic acid induced changes in protein pattern, antioxidative enzyme activities and peroxidase isozymes in peanut seedlings. Biol Plant 50:219–226
Lal SK, Kumar S, Sheri V, Mehta S, Varakumar P, Ram B, Borphukan B, James D, Fartyal D, Reddy MK (2018) Seed priming: an emerging technology to impart abiotic stress tolerance in crop plants. In: Advances in seed priming. Springer, Singapore, pp 41–50
Landgraf P, Feussner I, Hunger A, Scheel D, Rosahl S (2002) Systemic accumulation of 12-oxo-phytodienoic acid in SAR-induced potato plants. Eur J Plant Pathol 108:279–283
Lee TM, Lur HS, Chu C (1997) Role of abscisic acid in chilling tolerance of rice (Oryza sativa L.) seedlings: II. Modulation of free polyamine levels. Plant Sci 126:1–10
Lehman A, Black R, Ecker JR (1996) HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 85:183–194
Lehmann J, Atzorn R, Brückner C, Reinbothe S, Leopold J, Wasternack C, Parthier B (1995) Accumulation of jasmonate, abscisic acid, specific transcripts and proteins in osmotically stressed barley leaf segments. Planta 197:156–162
Li H, Johnson P, Stepanova A, Alonso JM, Ecker JR (2004) Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Dev Cell 7:193–204
Li J, Zhang K, Meng Y, Hu J, Ding M, Bian J, Yan M, Han J, Zhou M (2018) Jasmonic acid/ethylene signaling coordinates hydroxycinnamic acid amides biosynthesis through ORA59 transcription factor. Plant J 95:444–457
Liu K, Zou W, Gao X, Wang X, Yu Q, Ge L (2019a) Young seedlings adapt to stress by retaining starch and retarding growth through ABA-Dependent and-independent pathways in Arabidopsis. Biochem Biophys Res Commun 515:699–705
Liu D, Zhao Q, Cui X, Chen R, Li X, Qiu B, Ge F (2019b) A transcriptome analysis uncovers Panax notoginseng resistance to Fusarium solani induced by methyl jasmonate. Genes Genomics 41:1383–1396
Lymperopoulos P, Msanne J, Rabara R (2018) Phytochrome and phytohormones: working in tandem for plant growth and development. Front Plant Sci 9:1–14
Ma C, Wang ZQ, Zhang LT, Sun MM, Lin TB (2014) Photosynthetic responses of wheat (Triticum aestivum L.) to combined effects of drought and exogenous methyl jasmonate. Photosynthetica 52:377–385
Maksymiec W (2007) Signaling responses in plants to heavy metal stress. Acta Physiol Plant 29:177–187
Mandaokar A, Browse J (2009) MYB108 acts together with MYB24 to regulate jasmonate-mediated stamen maturation in Arabidopsis. Plant Physiol 149:851–862
Mehta S, James D, Reddy MK (2019a) Omics technologies for abiotic stress tolerance in plants: current status and prospects. In: Recent approaches in omics for plant resilience to climate change. Springer, Cham, pp 1–34
Mehta S, Singh B, Dhakate P, Rahman M, Islam MA (2019b) Rice, marker-assisted breeding, and disease resistance. In: Disease resistance in crop plants. Springer, Cham, pp 83–111
Mehta S, Lal SK, Sahu KP, Venkatapuram AK, Kumar M, Sheri V, Varakumar P, Vishwakarma C, Yadav R, Jameel MR, Ali M, Achary VMM, Reddy MK (2020) CRISPR/Cas9-Edited Rice: A New Frontier for Sustainable Agriculture. In: New Frontiers in Stress Management for Durable Agriculture. Springer, Singapore, pp 427–458
Mehta S, Singh B, Patra A, Tripathi A, Easwaran M, Choudhary JR, Choudhary M, Aggarwal SK (2020) Maize microbiome: current insights for the sustainable agriculture. In: Microbiomes and Plant Health. Academic Press, Massachusetts, pp 267–297
Mehta S, Chakraborty A, Roy A, Singh IK, Singh A (2021) Fight Hard or Die Trying: Current Status of Lipid Signaling during Plant–Pathogen Interaction. Plants 10:1098
Mehta S, Gogna M, Singh B, Patra A, Singh IK, Singh A (2021) Silicon: a plant nutritional “non-entity” for mitigating abiotic stresses. In: Plant stress biology. Springer, Singapore, pp 17–49
Mei C, Qi M, Sheng G, Yang Y (2006) Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol Plant Microbe Interact 19:1127–1137
Miranshahi B, Sayyari M (2018) Methyl jasmonate mitigates drought stress injuries and affects essential oil of Summer savory. J Agric Sci Technol 18:1635–1645
Mockaitis K, Estelle M (2008) Auxin receptors and plant development: a new signaling paradigm. Annu Rev Cell Dev Biol 24:55–80
Mohamed HI, Latif HH (2017) Improvement of drought tolerance of soybean plants by using methyl jasmonate. Physiol Mol Biol Plants 23:545–556
Mueller-Uri F, Parthier B, Nover L (1988) Jasmonate-induced alteration of gene expression in barley leaf segments analyzed by in-vivo and in-vitro protein synthesis. Planta 176:241–247
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Noriega G, Cruz DS, Batlle A, Tomaro M, Balestrasse K (2012) Heme oxygenase is involved in the protection exerted by Jasmonic acid against Cadmium stress in Soybean roots. J Plant Growth Regul 31:79–89
Oliveira MB, Junior ML, Grossi-de-Sá MF, Petrofeza S (2015) Exogenous application of methyl jasmonate induces a defense response and resistance against Sclerotinia sclerotiorum in dry bean plants. J Plant Physiol 182:13–22
Pandey N, Iqbal Z, Pandey B, Sawant SV (2017) Phytohormones and drought stress: plant responses to transcriptional regulation. In: Mechanism of plant hormone signaling under stress. Wiley, New Jersey, pp 477–504
Pauwels L, Barbero GF, Geerinck J, Tilleman S, Grunewald W, Pérez AC, García-Casado G (2010) NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464:788–791
Pedranzani H, Vigliocco A, Miersch O, Abdala G (2007) Cold and water stresses produce changes in endogenous jasmonates in two populations of Pinus pinaster Ait. Plant Growth Regul 52:111–116
Pedranzani H, Racagni G, Alemano S, Miersch O, Ramírez I, Peña-Cortés H, Taleisnik E, Machado-Domenech E, Abdala G (2013) Salt tolerant tomato plants show increased levels of jasmonic acid. Plant Growth Regul 41:149–158
Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol 28:489–521
Poonam S, Kaur H, Geetika S (2013) Effect of jasmonic acid on photosynthetic pigments and stress markers in Cajanus cajan (L.) Millsp. seedlings under copper stress. Am J Plant Sci 4:817–823
Qi T, Huang H, Song S, Xie D (2015) Regulation of jasmonate-mediated stamen development and seed production by a bHLH-MYB complex in Arabidopsis. Plant Cell 27:1620–1633
Qin F, Kodaira KS, Maruyama K, Mizoi J, Tran LSP, Fujita Y, Yamaguchi-Shinozaki K (2011) SPINDLY, a negative regulator of gibberellic acid signaling, is involved in the plant abiotic stress response. Plant Physiol 157:1900–1913
Rahman M, Sultana S, Nath D, Kalita S, Chakravarty D, Mehta S, Wani SH, Islam MA (2019) Molecular breeding approaches for disease resistance in sugarcane. In: Disease Resistance in Crop Plants. Springer, Cham, pp 131–155
Rajput LS, Aggarwal SK, Mehta S, Kumar S, Nataraj V, Shivakumar M, Maheshwari HS, Yadav S, Goswami D (2021) Role of WRKY Transcription Factor Superfamily in Plant Disease Management. In: Plant Stress Biology. Springer, Singapore, pp 335–361
Ritonga FN, Chen S (2020) Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 9:560
Rosahl S, Feussner I (2004) Oxylipins. In: Plant lipids: biology, utilisation and manipulation. Blackwell, Oxford, pp 329–454
Ruan JJ, Zhou YX, Zhou ML, Yan J, Khurshid M, Weng WF, Cheng JP, Zhang K (2019) Jasmonic acid signaling pathway in plants. Int J Mol Sci 20:2479
Sadeghipour O (2017) Amelioration of salinity tolerance in cowpea plants by seed treatment with methyl jasmonate. Legume Res 40:1100–1106
Sahil, Keshan R, Patra A, Mehta S, Abdelmotelb KF, Lavale SA, Chaudhary M, Aggarwal SK, Chattopadhyay A (2021) Expression and Regulation of Stress-Responsive Genes in Plants Under Harsh Environmental Conditions. In: Harsh Environment and Plant Resilience: Molecular and Functional Aspects. Springer Nature, Switzerland AG, pp 25–44
Salimi F, Shekari F, Hamzei J (2016) Methyl jasmonate improves salinity resistance in German chamomile (Matricaria chamomilla L.) by increasing activity of antioxidant enzymes. Acta Physiol Plant 38:1–14
Saltveit ME (2000) Wound induced changes in phenolic metabolism and tissue browning are altered by heat shock. Postharvest Biol Technol 21:61–69
Savchenko T, Kolla VA, Wang CQ, Nasafi Z, Hicks DR, Phadungchob B, Chehab WE, Brandizzi F, Froehlich J, Dehesh K (2014) Functional convergence of oxylipin and abscisic acid pathways controls stomatal closure in response to drought. Plant Physiol 164:1151–1160
Sayyari M, Babalar M, Kalantari S, Martínez-Romero D, Guillén F, Serrano M, Valero D (2011) Vapour treatments with methyl salicylate or methyl jasmonate alleviated chilling injury and enhanced antioxidant potential during postharvest storage of pomegranates. Food Chem 124:964–970
Sharma P, Sharma MMM, Patra A, Vashisth M, Mehta S, Singh B, Tiwari M, Pandey V (2020) The role of key transcription factors for cold tolerance in plants. In: Transcription factors for abiotic stress tolerance in plants. Academic, London, pp 123–152
Sharma P, Sharma MMM, Malik A, Vashisth M, Singh D, Kumar R, Singh B, Patra A, Mehta S, Pandey V (2021) Rhizosphere, Rhizosphere Biology, and Rhizospheric Engineering. In: Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management. Springer, Cham, pp 577–624
Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds TR, He SY (2010) Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468:400–405
Shimizu T, Miyamoto K, Miyamoto K, Minami E, Nishizawa Y, Iino M (2013) OsJAR1 contributes mainly to biosynthesis of the stress induced jasmonoyl-isoleucine involved in defense responses in rice. Biosci Biotechnol Biochem 77:1556–1564
Siddiqi KS, Husen A (2019) Plant response to jasmonates: current developments and their role in changing environment. Bull Natl Res Cent 43:153
Singh P, Dave A, Vaistij FE, Worrall D, Holroyd GH, Wells JG, Kaminski F, Graham IA, Roberts MR (2017) Jasmonic acid-dependent regulation of seed dormancy following maternal herbivory in Arabidopsis. New Phytol 214:1702–1711
Singh B, Mehta S, Aggarwal SK, Tiwari M, Bhuyan SI, Bhatia S, Islam MA (2019) Barley, disease resistance, and molecular breeding approaches. In: Disease resistance in crop plants. Springer, Cham, pp 261–299
Sirhindi G, Mir MA, Sharma P, Gill SS, Kaur H, Mushtaq R (2015) Modulatory role of jasmonic acid on photosynthetic pigments, antioxidants and stress markers of Glycine max L. under nickel stress. Physiol Mol Biol Plants 21:559–565
Song S, Qi T, Huang H, Ren Q, Wu D, Chang C, Xie D (2011) The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect Jasmonate-regulated stamen development in Arabidopsis. Plant Cell 23:1000–1013
Staswick PE, Yuen GY, Lehman CC (1998) Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J 15:747–754
Staswick PE, Tiryaki I, Rowe ML (2002) Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 14:1405–1415
Sun TP (2010) Gibberellin signal transduction in stem elongation & leaf growth. In: Plant hormones. Springer, Dordrecht, pp 303–328
Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G (2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448:661–665
Tian D, Peiffer M, De Moraes CM, Felton GW (2014) Roles of ethylene and jasmonic acid in systemic induced defense in tomato (Solanum lycopersicum) against Helicoverpa zea. Planta 239:577–589
Trischuk RG, Schilling BS, Low NH, Gray GR, Gusta LV (2014) Cold acclimation, de-acclimation and re-acclimation of spring canola, winter canola and winter wheat: the role of carbohydrates, cold-induced stress proteins and vernalization. Environ Exp Bot 106:156–163
Viswanath KK, Varakumar P, Pamuru RR, Basha SJ, Mehta S, Rao AD (2020) Plant lipoxygenases and their role in plant physiology. J Plant Biol 63:83–95
Vriezen WH, Achard P, Harberd NP, Van Der Straeten D (2004) Ethylene mediated enhancement of apical hook formation in etiolated Arabidopsis thaliana seedlings is gibberellin dependent. Plant J 37:505–516
War AR, Paulraj MG, Ignacimuthu S, Sharma HC (2015) Induced resistance to Helicoverpa armigera through exogenous application of jasmonic acid and salicylic acid in groundnut, Arachis hypogaea. Pest Manag Sci 71:72–82
Wasternack C (2014) Action of jasmonates in plant stress responses and development applied aspects. Biotechnol Adv 32:31–39
Wasternack C, Hause B (2002) Jasmonates and octadecanoids: signals in plant stress responses and development. Prog Nucleic Acid Res Mol Biol 72:165–221
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111:1021–1058
Wasternack C, Hause B (2019) The missing link in jasmonic acid biosynthesis. Nat Plants 5:776–777
Wasternack C, Song S (2017) Jasmonates: biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J Exp Bot 68:1303–1321
Weber H, Chetelat A, Caldelari D, Farmer EE (1999) Divinyl ether fatty acid synthesis in late blight-diseased potato leaves. Plant Cell 11:485–493
Wu H, Wu X, Li Z, Duan L, Zhang M (2012) Physiological evaluation of drought stress tolerance and recovery in cauliflower (Brassica oleracea L.) seedlings treated with methyl jasmonate and coronatine. J Plant Growth Regul 31:113–123
Wu X, Ding C, Baerson SR, Lian F, Lin X, Zhang L, Wu C, Hwang SY, Zeng R, Song Y (2019) The roles of jasmonate signalling in nitrogen uptake and allocation in rice (Oryza sativa L.). Plant Cell Environ 42:659–672
Wuebbles DJ, Fahey DW, Hibbard KA, DeAngelo B, Doherty S, Hayhoe K, Horton R, Kossin JP, Taylor AM, Weaver CP (2017) Climate science special report: a sustained assessment activity of the US global change research program. In: US global change research program. Washington, DC, p 669
Xiang C, Oliver DJ (1998) Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10:1539–1550
Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280:1091–1094
Yadav B, Jogawat A, Lal SK, Lakra N, Mehta S, Shabek N, Narayan OP (2021) Plant mineral transport systems and the potential for crop improvement. Planta 253:45
Yan Z, Chen J, Li X (2013) Methyl jasmonate as modulator of cd toxicity in Capsicum frutescens var fasciculatum seedlings. Ecotoxicol Environ Saf 98:203–209
Yan Z, Zhang W, Chen J, Li X (2015) Methyl jasmonate alleviates cadmium toxicity in Solanum nigrum, by regulating metal uptake and antioxidative capacity. Biol Plant 59:373–381
Zandalinas SI, Mittler R, Balfagón D, Arbona V, Gómez-Cadenas A (2018) Plant adaptations to the combination of drought and high temperatures. Physiol Plant 162:2–12
Zhai Q, Zhang X, Wu F, Feng H, Deng L, Xu L, Li C (2015) Transcriptional mechanism of jasmonate receptor COI1-mediated delay of flowering time in Arabidopsis. Plant Cell 27:2814–2828
Zhang X, Sheng J, Li F, Meng D, Shen L (2012) Methyl jasmonate alters arginine catabolism and improves postharvest chilling tolerance in cherry tomato fruit. Postharvest Biol Technol 64:160–167
Zhang X, Zhu Z, An F, Hao D, Li P, Song J, Guo H (2014) Jasmonate-activated MYC2 represses ETHYLENE INSENSITIVE3 activity to antagonize ethylene-promoted apical hook formation in Arabidopsis. Plant Cell 26:1105–1117
Zhang H, Zhang Q, Zhai H, Li Y, Wang X, Liu Q, He S (2017) Transcript profile analysis reveals important roles of jasmonic acid signalling pathway in the response of sweet potato to salt stress. Sci Rep 7:1–12
Zhao ML, Wang JN, Shan W, Fan JG, Kuang JF, Wu KQ, Li XP, Chen WX, He FY, Chen JY, Lu WJ (2013) Induction of jasmonate signalling regulators MaMYC2s and their physical interactions with MaICE1 in methyl jasmonate-induced chilling tolerance in banana fruit. Plant Cell Environ 36:30–51
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sahil et al. (2021). Jasmonic Acid for Sustainable Plant Growth and Production Under Adverse Environmental Conditions. In: Husen, A. (eds) Plant Performance Under Environmental Stress . Springer, Cham. https://doi.org/10.1007/978-3-030-78521-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-78521-5_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-78520-8
Online ISBN: 978-3-030-78521-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)