Abstract
Main conclusion
Fine mapping of wheat powdery mildew-resistance gene Pm4e to a 0.19 cM interval with sequence-based markers provides the foundation for map-based cloning and marker-assisted selection with breeder-friendly markers.
Powdery mildew caused by Blumeria graminis f. sp. tritici is a wheat foliar disease that poses a serious threat to global wheat production. Pm4 is a resistance gene locus that has played a key role in controlling this disease in wheat production and a few resistance alleles of this locus have been identified. We have previously mapped the Pm4e allele to a 6.7 cM interval on chromosome 2AL. In this study, Pm4e was delimited to a 0.19 cM interval flanked by Xwgrc763 and Xwgrc865, through employment of a larger segregating population, derived from the cross of resistant parent D29 with susceptible parent Yangmai 158 (Y158), and enrichment of the genetic interval with markers developed on Chinese Spring (C.S.) survey sequence. In this interval, Pm4e co-segregated with a few markers, some of which were either D29-dominant or Y158-dominant, implying great sequence variation in the interval between D29 and Y158. Most of these co-segregation markers could not differentiate the Pm4 alleles from each other. Survey of 55 wheat cultivars with four co-dominant markers showed that the Pm4e-co-segregating loci always co-exist. Annotation of the Pm4e interval-corresponding C.S. sequence revealed more than a dozen resistance gene analogs clustered in a 2.4 Mb region, although C.S. is susceptible to the Pm4e-avirulent isolate Bgt2. This study has established the foundation for map-based cloning of Pm4e. Moreover, some of the co-dominant markers developed in this study could help in marker-assisted transfer of Pm4e into elite cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Powdery mildew caused by Blumeria graminis f. sp. tritici (Bgt) is a devastating wheat foliar disease, predominant in regions with cool and maritime climate, and causes severe yield losses once epidemics occur (Everts and Leath 1992). To control this disease through breeding, a number of resistance genes have been identified. With the recent characterization of Pm55, Pm57, Pm58, Pm59, Pm60, and Pm61, 88 formally designated powdery mildew-resistance genes or alleles mapping to 57 different loci (Pm1–Pm61, Pm18 = Pm1c, Pm22 = Pm1e, Pm23 = Pm4c, Pm31 = Pm21) have been documented so far (Zhang et al. 2016; Liu et al. 2017; McIntosh et al. 2017; Wiersma et al. 2017; Zou et al. 2017; Tan et al. 2018; Sun et al. 2018), and some of them have played important roles in stabilizing wheat yield.
More and more studies demonstrated that the resistance gene locus could evolve into multiple alleles or carry tightly arranged resistance genes, for example, Pm1 (Liang et al. 2016; Yao et al. 2007), Pm2 (Ma et al. 2015; Sánchez et al. 2016), Pm3 (Yahiaoui et al. 2004; Bhullar et al. 2010; Zhao et al. 2016), Pm4 (Briggle 1966; Hao et al. 2008; Schmolke et al. 2012; Li et al. 2017), Pm5 (Huang et al. 2003), and Pm24 (Huang et al. 2000; Xue et al. 2012), for wheat powdery mildew resistance. Five alleles at the Pm4 locus have been reported. Pm4a was introduced from T. dicoccum accession Khapli and T. durum accession Yuma (Briggle 1966; Ma et al. 2004). Pm4b was a T. carthlicum gene (The et al. 1979). Both Pm4c and Pm4e were identified in common wheat cultivars (Hao et al. 2008; Li et al. 2017). A resistance gene originated from T. monococcum was assumed to be Pm4d (Schmolke et al. 2012). Moreover, a few other powdery mildew-resistance genes mapped on chromosome 2AL could be either allelic or closely linked to the Pm4, such as pmX (Fu et al. 2013), PmLK906 (Niu et al. 2008) and PmPS5A (Zhu et al. 2005).
Pm4 is one of the most widely used powdery mildew-resistance locus and can be found alone or in combination with other powdery mildew-resistance genes in many resistance cultivars in China (Huang et al. 1997). Pm4a has been overcome due to the emergence of new virulent strains and its wide utilization in breeding programs globally (Duan et al. 2002) and in China (Li et al. 2017); however, the allelic expansion of the Pm4 locus has increased the resistance duration of this locus. Investigation of the allelic variations in detail is, therefore, important, not only for increase of the allelic diversity, but also for understanding the host–pathogen interaction and resistance gene pyramiding (Srichumpa et al. 2005; Koller et al. 2018).
This study was aimed at fine mapping of Pm4e, with an ultimate goal of resolving the complexity of Pm4 locus and providing breeders with friendly markers that can be efficiently used in marker-assisted breeding.
Materials and methods
Plant materials
D29 containing Pm4e is a common wheat landrace introduced from Sichuan province of China, without known pedigree. CI14123 (Khapli/8*CC), Armada and 81-7241 are three common wheat lines containing Pm4a, Pm4b, and Pm4c, respectively. Yangmai158 (Y158) is an elite Chinese common wheat cultivar, developed by the Yangzhou Lixiahe Regional Institute of Agricultural Sciences, China. Chinese Spring is a common wheat landrace. Sumai No.3, a common wheat cultivar, was used as the susceptible control in resistance evaluation. The segregation population was derived from selfing of BC6F1 plants with heterozygous Pm4e. In addition, 55 Chinese wheat cultivars or lines from different ecological areas were used in survey with Pm4e-linked markers.
Resistance evaluation
According to the method described by Xu et al. (2008), all the seedlings grown in rectangular trays were inoculated at one leaf stage by dusting the conidiospores of Pm4e-avirulent isolate Bgt2 freshly increased on susceptible cultivar Sumai No. 3. Sumai No. 3 and Y158 were used as susceptible controls. After inoculation, the trays were placed in a growth chamber set with over 80% humidity, 14 h light, and a 22 °C/18 °C temperature day/night cycle. Disease symptoms were scored 7 day post-inoculation when the control plants of Sumai No.3 and Y158 were heavily diseased, according to 0–5 scale as mentioned by Yao et al. (2007). The 0, 1, 2, 3, 4, and 5 in this scale represent no visible symptoms, visible necrosis without sporulation, sparse sporulation, moderate sporulation, abundant sporulation, and abundant sporulation with mycelia covering more than 80% of the leaf area, respectively. A score of 0–2 was considered as resistant and 3–5 as susceptible.
DNA extraction and PCR amplification
DNA was extracted from young seedling tissue using the procedure of Ma et al. (1994). For bulked segregant analysis (BSA) (Michelmore et al. 1991), resistant and susceptible bulks were made by combination of equal amount of DNA from ten homozygous resistant and ten homozygous susceptible F2 plants, respectively, following progeny testing.
Polymerase chain reaction of markers was performed in PE9600 thermal cycler (Perkin Elmer), in a volume of 10 μl containing 10–20 ng of template, 2 pmol of each of the primers, 2 nmol of each of the deoxynucleoside triphosphates, 15 nmol of MgCl2, 0.1 U Taq DNA polymerase, and 1 × PCR buffer. The PCR profile was set as one cycle of 94 °C for 3 min, followed by 36 cycles of 94 °C for 30 s, 50–60 °C (depending on the specific primers) for 40 s, and 72 °C for 50 s, and a final extension at 72 °C for 5 min. The PCR products were separated on 8% non-denaturing polyacrylamide gels with a 19:1, 29:1, or 39:1 acrylamide/bisacrylamide ratio, and then silver-stained as described by Santos et al. (1993). PCR products from amplification with the Pm4a co-segregating marker STS-BCD1231 (Ma et al. 2004) were separated on a 1% agarose gel, and viewed under UV light after staining with ethidium bromide.
Recombinant screening, marker development, and map construction
Recombinant screening was performed with the published Pm4e-flanking markers with Xhbg327 at one side and Xgdm93 at the other side (Li et al. 2017). Marker Xsts-bscd1231, which co-segregated with Pm4e, was also used in genotyping. The obtained recombinants were transferred to greenhouse to obtain the corresponding F2:3 progenies. To narrow down the Pm4e interval, the genomic information from the corresponding Chinese Spring (C.S.) chromosome 2AL sequence available via the IWGSC wheat sequence repository at URGI-INRA-Versailles, France (http://wheat-urgi.versailles.inra.fr/) was used to develop additional PCR-based markers for mapping and genotyping by taking advantage of simple sequence repeats (SSR), cleaved amplified polymorphism (CAP), single nucleotide polymorphism (SNP), and repeats junctions. In addition, the corresponding T. urartu 2AL scaffolds and T. durum 2AL contigs were also used to develop markers. All primer sequences were designed using MacVector (Accelryes, UK). If the PCR products of a marker were monomorphic sizewise between the parents, they were digested with restriction enzymes EcoRI, HhaI, RsaI, MspI, HindIII, MboI, and AluI to reveal potential sequence composition variations. Each digestion was performed in a 5 μl reaction mix with 0.05 μg DNA, according to supplier’s manual.
Physical mapping and gene annotation
The sequences associated with the two flanking markers most closely linked to Pm4e were used as queries in search against the C.S. chromosome 2A sequence available via the IWGSC to determine the boundaries of the interval. The gene annotation information of the interval was then retrieved from IWGSC RefSeq v1.0 annotation (https://urgi.versailles.inra.fr/download/iwgsc/IWGSC_RefSeq_Annotations/v1.0/).
Linkage analysis
Linkage analysis was conducted with Mapmaker 3.0 (Lincoln et al. 1993). An LOD score of 3.0 was used as the threshold for linkage. Genetic distances were estimated using Kosambi function (Kosambi 1944). Chi-squared (χ2) tests for goodness-of-fit were used to evaluate deviations of the observed data from the expected segregation ratios.
Results
Phenotyping and genotyping of the segregation population
Li et al. (2017) found, using an F2 population derived from the cross of D29 with Y158, that Pm4e is a dominant-resistance gene and was 4.9 cM from Xgdm93 at one side and 1.8 cM from Xhbg327 at the other side (Li et al. 2017). To fine map this gene, we surveyed a population of 772 plants derived from selfing of BC6F1 plants with Pm4e, which were obtained in development of the Pm4e near isogenic lines using Y158 as the recurrent parent. Inoculation with Bgt2 of the population seedlings yielded 575 resistant plants and 197 susceptible plants, which fit well the expected 3:1 segregation ratio (\(\chi^{2}_{3:1} = \, 0.0017\), P = 0.90–0.95) and was in consistent with the conclusion by Li et al. (2017). Genotyping these plants with Xsts_bcd1231, Xgdm93, and Xhbg327 showed that Xsts_bcd1231 co-segregated with the phenotype and was encompassed in the 3.51 cM Xgdm93–Xhbg327 interval (Fig. 1a). Fifty-three plants with recombination occurring between Xgdm93 and Xhbg327 were identified and phenotypically and genotypically verified using the progenies derived from selfing.
High-density mapping
To enrich the Pm4e interval with more markers, a total of 176 markers, majority of which are SSR-based, were designed based on the C.S. genomic DNA sequence corresponding to the interval and homologous scaffolds or contigs from T. urartu and T. durum. However, only 14 markers detected length or presence/absence polymorphism between D29 and Y158 as well as between the resistant and susceptible pools (Table 1). Of these polymorphic markers, except for WGRC349 derived from the T. urartu scaffold50306, all others were developed based on the Chinese Spring 2A survey sequence. Digestion of the monomorphic PCR products with restriction enzymes revealed that WGRC421 and WGRC427 detected polymorphism between the two parents when digested with restriction enzymes HhaI and EcoRI, respectively. All these polymorphic markers were mapped to the Pm4e interval using the data from recombinant genotyping, resulting in a reduction of the average adjoining marker interval to 0.38 cM (Fig. 1b). We were, therefore, able to delimit Pm4e to a 0.19 cM genetic interval flanked by Xwgrc763 and Xwgrc865 (Fig. 1b).
Besides Xsts-bcd1231, Xwgrc872, Xwgrc869, Xwgrc883, Xwgrc929, and Xwgrc1096 showed co-segregation with Pm4e (Fig. 1b). These markers mapped to an approximately 360-kb block of C.S. 2AL. In the Xwgrc763 and Xwgrc865 interval, except for Xwgrc763, Xwgrc872, and Xwgrc869, the other four marker loci were either D29-dominant (Xwgrc929) or Y158-dominant (Xwgrc883, Xwgrc865, Xwgrc1096) (Fig. 2), implying great sequence variation in the Pm4e block.
Polymorphic pattern of markers WGRC763 (a), WGRC872 (b), WGRC869 (c), BCD1231 (d), WGRC883 (e) and WGRC865 (f) in CI14123 (Pm4a, 1), Armada (Pm4b, 2), 81-7241 (Pm4c, 3), D29 (Pm4e, 4) and Y158 (5). a, c On 8% 39:1 acrylamide/bisacrylamide gels; b, e, f on 8% 29:1 acrylamide/bisacrylamide gels; d on a 1% agarose gel. Arrows indicate the polymorphic bands mapping to chromosome 2A. M1 and M2, DNA markers pUC19/MspI and D2000, respectively. Numbers to the left indicate molecular size in bp
Comparative mapping of Pm4e and Pm4b
Wu et al. (2018) recently published a marker map of Pm4b. We surveyed all the Pm4b-linked markers in this map but the SRAP marker Me8/Em7−220 and found that only one marker ICS13 detected polymorphism between D29 and Y158. Xics13 was mapped 0.71 cM distal to the Pm4e interval (Fig. 1b) and 1.3 cM proximal to Pm4b (Wu et al. 2018). Applying the markers’ mapping to the Pm4e interval to lines contains Pm4a, Pm4b, and Pm4c, we showed that Xwgrc883 differentiated the Pm4e line from the Pm4b and Pm4c lines (Fig. 2). WGRC883 could be a co-dominant marker for Pm4b and Pm4c (Fig. 2e).
Gene annotation of the Pm4e interval-corresponding C.S. sequence
The flanking marker loci Xwgrc908 (the closer locus Xwgrc763 was not used due to its involvement in a potential inversion, see Fig. 4) and Xwgrc865 defined a 6.1 Mb sequence region of C.S. chromosome 2A (IWGSC, v1.0). According to the gene annotations of IWGSC RefSeq v1.0, this region contains 122 high confidence genes (Electronic Supplementary Table 1), some of which are homologs of known disease resistance or defense-associated proteins, such as NBS–LRR-resistance proteins, receptor-like protein kinases, harpin-induced-like protein, and thaumatin-like protein. The NBS–LRR-resistance gene analogs (RGAs) are mainly clustered into two regions, defined by TraesCS2A01G560600.1-TraesCS2A01G560900.1 and TraesCS2A01G564200.1-TraesCS2A01G564900.1. To verify the linkage of these RGAs with Pm4e, markers were designed and used in survey of D29 and Y158. Five markers produced the expected PCR products in at least one of the lines, but WGRC3242, designed based on the NBS–LRR analog TraesCS2A01G560700.1, was the only one detected polymorphism (Fig. 3a). As expected, Xwgrc3242 co-segregated with Pm4e. Although Xwgrc3242 was monomorphic between D29 and C.S. (Fig. 3a), C.S. was susceptible to the isolate Bgt2, implying that C.S. does not possess the Pm4e allele. However, it is possible that C.S. carries a gene allelic to Pm4e.
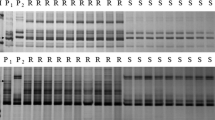
Polymorphic pattern of TraesCS2A01G560700.1-derived marker WGRC3242, resolved in the 8% 29:1 acrylamide/bisacrylamide gel (a) and comparison of resistance response of D29 and C.S. to Bgt2 at seedling stage (b). Y158 was the susceptible control. In a, D (D29), Y (Y158), R (resistant pool), S (susceptible pool), C (C.S.), M, DNA marker pUC19/MspI. Arrow indicates the mapped polymorphic band. Numbers to the left indicate molecular size in bp
Pm4e haplotype in cultivars
Fifty-five lines from different ecological regions of China were surveyed with co-dominant markers WGRC763, WGRC872, WGRC869, and WGRC982. The results showed that Xwgrc872 and Xwgrc869, which co-segregated with Pm4e, always co-existed in eight of the lines (Table 2). Different from this, recombination of Xwgrc982 with Xwgrc872 and Xwgrc869 was observed in 11 of the lines that produced a product same as in D29. However, no recombination was observed between Xwgrc872/Xwgrc869 and Xwgrc763 (Fig. 1b). Xwgrc763 was the other flanking marker locus and was closer to the Pm4e than Xwgrc982. Since WGRC763, WGRC872, and WGRC869 could not differentiate among the four Pm4 alleles, it appeared that a Pm4 haplotype was detected in these lines. Examining these lines with the commonly used Pm4a-co-segregating dominant marker STS-BCD1231 yielded supportive results (Table 2, Fig. 1b).
Discussion
Multiple genes for powdery mildew resistance on the long arm of wheat chromosome 2A have been reported. The existence of five formally designated Pm4 genes and at least five temporarily designated genes closely linked to Pm4 locus makes it one of the complex regions as previously known for Pm1, Pm2, Pm3, and Pm5 loci (Li et al. 2017). Unlike the well-understood true allelic series of Pm3 locus, no candidate gene has been cloned for the Pm4 locus so far. Moreover, none of the genes or alleles at or near the Pm4 locus has been fine mapped except Pm4b (Wu et al. 2018). The fine mapping of Pm4e and the development of new markers linked to the Pm4 locus are hoped to lay the foundation for the map-based cloning of Pm4 and its utilization in breeding programs. It is still necessary to determine whether or not the Pm4 locus represents a true allelic series or paralogous copies of one gene. It is noted that multiple copies of RGAs exist in the Pm4e-interval-corresponding C.S. sequence.
Among the five so-called Pm4 alleles, Pm4d was reasoned to be a gene linked, instead of allelic, to Pm4a and Pm4e (Li et al. 2017). Using resistance spectrum test (Li et al. 2017) and marker WGRC883, we were able to distinguish Pm4e from the other Pm4 alleles. In consistence with their allelic relationship, all markers co-segregated with Pm4e, but the Y158-dominant Xwgrc883 identified a common haplotype in the Pm4-carrying lines (Fig. 2). Among these markers, BCD1231 and the BCD1231-derived STS markers have been shown to co-segregate with Pm4 alleles in a few studies (Ma et al. 1994, 2004; Niu et al. 2010; Fu et al. 2013; Li et al. 2017). In a fine mapping study, Pm4a was indeed mapped to the same position as Pm4e (data not shown). The Pm4e interval also overlaps with Pm4b interval reported by Hao et al. (2008). However, this is not in line with the mapping position of Pm4b reported by Wu et al. (2018) (Fig. 4). Alignment of the markers on the Pm4e map to the C.S. 2A sequence revealed a near-perfect collinearity of the Pm4e interval apart from an inversion involving Xwgrc763 and Xwgrc929/Xwgrc872/Xwgrc1096 (Fig. 4). Comparing to the C.S. sequence, the Pm4b interval appears to be involved in a large inversion event that causes the relative position change of Pm4b (Fig. 4). However, this still requires verification due to the inconsistency of Pm4b map in Hao et al. (2008) and Wu et al. (2018). A comparative high-resolution mapping and allelism test of all these genes might be able to clarify some of the issues.
Alignment of the markers on the Pm4e and Pm4b maps (Wu et al. 2018) to the C.S. 2A genomic DNA sequence. The marker order in the Pm4e map was not drawn in proportional to the genetic distance and the physical positions in bp of the C.S. map was not drawn in proportional to the physical distance
Although Pm4e has been mapped to an interval as small as 0.19 cM, the corresponding physical distance is approximately 6.1 Mb in C.S. This is equal to a physical/genetic distance ratio of 32.3 Mb/cM, far greater than the 1.7 Mb/cM for flowering repressor gene VRN2 (Yan et al. 2004) and the 1 Mb/cM in the case of Mla (Wei et al. 1999), thus makes Pm4e cloning more difficult. In the marker block co-segregating with Pm4e, four markers were either D29-dominant or Y158-dominant, suggesting that great sequence variations are present between D29 and Y158, which could inhibit recombination occurrence, as in the case of the Pm1 locus (Liang et al. 2016). Nevertheless, the presence of co-dominant markers (Xwgrc872 and Xwgrc869) in the block implies that it is still possible to reach higher mapping resolution through using larger mapping population or altering the susceptible mapping parents.
Currently, most of the cloned race-specific disease-resistance genes in cereal crops encode CC–NBS–LRR-type proteins, for example, Pm3b (Yahiaoui et al. 2004), Pm2 (Sánchez et al. 2016), Pm60 (Zou et al. 2017), Pm21 (He et al. 2018), Lr1 (Cloutier et al. 2007), and Sr33 (Periyannan et al. 2013) in wheat. In the 6.1 Mb C.S. sequence corresponding to Pm4e, more than a dozen RGAs are distributed over a 2.4 Mb region, but they do not provide resistance to the Pm4e-avirulent isolate Bgt2. It is worth examining if Pm4e is evolved from one of these RGAs, which is now under way in our lab.
The diagnostic markers previously developed for the Pm4 locus were either dominant or mapped away from the gene. Thus, for selection of the Pm4 genes, more makers are often needed, and sometimes, an additional time-consuming step of progeny testing is required to confirm the heterozygosity. In this study, we identified co-dominant marker loci Xwgrc763 and Xwgrc982 that were tightly linked to Pm4e, and more importantly, co-dominant marker loci Xwgrc872 and Xwgrc869 that co-segregated with Pm4e. They could be markers of choice in marker-assisted selection (MAS) of Pm4e and pyramiding the Pm4 genes with other genes to obtain more stable and durable resistance against powdery mildew.
Author contribution statement
Khan NU conducted genotyping, phenotyping, data analysis, and prepared the draft; N Li contributed to experiment design and data analysis and preparation of the draft; T Shen, P Wang, WB Tang, and Z Zhang participated in genotyping and phenotyping; S Ma contributed to sequence analysis; HY Jia and ZX Kong contributed to implementation of the project, Z Ma conceived the project, designed the experiments, and reviewed the article.
Change history
05 September 2018
Unfortunately, the style of the units was incorrectly published (“cm” instead of “cM”) throughout the original article.
References
Bhullar NK, Zhang ZQ, Wicker T, Keller B (2010) Wheat gene banks as a source of new alleles of the powdery mildew resistance gene Pm3: a large scale allele mining project. BMC Plant Bio 10:88
Briggle LW (1966) Transfer of resistance to Erysiphe graminis f. sp. tritici from Khapli emmer and Yuma durum to hexaploid wheat. Crop Sci 6:459–461
Cloutier S, McCallum BD, Loutre C, Banks TW, Wicker T, Feuillet C, Keller B, Jordan MC (2007) Leaf rust resistance gene Lr1, isolated from bread wheat (Triticum aestivum L.) is a member of the large psr567 gene family. Plant Mol Biol 65:93–106
Duan S, Xu Y, Wu X (2002) Research progress of pathogen virulence, resistance genes and resistance breeding of wheat powdery mildew. J Triticeae Crops 22:83–86 (in Chinese)
Everts KL, Leath S (1992) Effect of early season powdery mildew on development, survival, and yield contribution of tillers of winter wheat. Phytopathology 82:1273–1278
Fu BS, Yang C, Li N, Ma HQ, Kong ZX, Zhang LX, Jia HY, Ma ZQ (2013) pmX: a recessive powdery mildew resistance gene at the Pm4 locus identified in wheat landrace Xiaohongpi. Theor Appl Genet 126:913–921
Hao Y, Liu A, WangY Feng D, Gao J, Li X, Liu S, Wang H (2008) Pm23: a new allele of Pm4 located on chromosome 2AL in wheat. Theor Appl Genet 117:1205–1212
He H, Zhu S, Zhao R, Jiang Z, Ji Y, Ji J, Qui D, Li H, Bie T (2018) Pm21, encoding a typical CC-NBS-LRR protein, confers broad-spectrum resistance to wheat powdery mildew disease. Mol Plant 11:879–882
Huang XQ, Hsam SLK, Zeller FJ (1997) Identification of powdery mildew resistance genes in common wheat (Triticum aestivum L. em Thell.). IX. Cultivars, land races and breeding lines grown in China. Plant Breed 116:233–238
Huang XQ, Hsam SLK, Zeller FJ, Wenzel G, Mohler V (2000) Molecular mapping of the wheat powdery mildew resistance gene Pm24 and marker validation for molecular breeding. Theor Appl Genet 101:407–414
Huang XQ, Wang LX, Xu MX, Röder MS (2003) Microsatellite mapping of the powdery mildew resistance gene Pm5e in common wheat (Triticum aestivum L.). Theor Appl Genet 106:858–865
Koller T, Brunner S, Herren G, Hurni S, Keller B (2018) Pyramiding of transgenic Pm3 alleles in wheat results in improved powdery mildew resistance in the field. Theor Appl Genet 131:861–871
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Li N, Jia HY, Kong ZX, Tang WB, Ding YX, Liang JC, Ma HQ, Ma ZQ (2017) Identification and marker-assisted transfer of a new powdery mildew resistance gene at the Pm4 locus in common wheat. Mol Breed 37:79
Liang JC, Fu BS, Tang WB, Khan NU, Li N, Ma ZQ (2016) Fine mapping of two powdery mildew resistance genes located at the Pm1 cluster. Plant Genome. https://doi.org/10.3835/plantgenome2015.09.0084
Lincoln SE, Daly MJ, Lander ES (1993) Constructing linkage maps with MAPMAKER/Exp version 3. 0: a tutorial reference manual, vol 3. Whitehead Institute for Medical Res, Cambridge
Liu W, Koo DH, Xia Q, Li C, Bai F, Song Y, Friebe B, Gill BS (2017) Homoeologous recombination-based transfer and molecular cytogenetic mapping of powdery mildew-resistant gene Pm57 from Aegilops searsii into wheat. Theor Appl Genet 30:841–848
Ma ZQ, Sorrells ME, Tanksley SD (1994) RFLP markers linked to powdery mildew resistance genes Pm1, Pm2, Pm3 and Pm4 in wheat. Genome 37:871–875
Ma ZQ, Wei JB, Chen SH (2004) PCR-based markers for the powdery mildew resistance gene Pm4a in wheat. Theor Appl Genet 109:140–145
Ma PT, Xu HX, Xu YF, Li LH, Qie YM, Luo QL, Zhang XT, Li XQ, Zhou YL, An DG (2015) Molecular mapping of a new powdery mildew resistance gene Pm2b in Chinese breeding line KM2939. Theor Appl Genet 128:613–622
McIntosh RA, Dubcovsky J, Rogers WJ, Morris C, Xia XC (2017) Catalogue of gene symbols for wheat: 2017 Supplement. https://shigen.nig.ac.jp/wheat/komugi/genes/macgene/supplement2017.pdf. Accessed 4 Aug 2018
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Niu JS, Wang BQ, Wang YH, Cao AZ, Qi ZJ, Shen TM (2008) Chromosome location and microsatellite markers linked to a powdery mildew resistance gene in wheat line “Lankao 90(6)”. Plant Breed 127:346–349
Niu JS, Jia HY, Yin J, Wang BQ, Ma ZQ, Shen TM (2010) Development of an STS marker linked to powdery mildew resistance genes PmLK906 and Pm4a by gene chip hybridization. Agric Sci China 9:331–336
Periyannan S, Moore J, Ayliffe M, Bansal U, Wang XJ, Huang L, Deal K, Luo MC, Kong XY, Bariana H, Mago R, McIntosh R, Dodds P, Dvorak J, Lagudah E (2013) The gene Sr33, an ortholog of barley Mla genes, encodes resistance to wheat stem rust race Ug99. Science 341:786–788
Sánchez MJ, Steuernage B, Ghosh S, Herren G, Hurni S, Adamski N, Vrána J, Kubaláková M, Krattinger SG, Wicker T, Doležel J, Keller B, Wulff BBH (2016) Rapid gene isolation in barley and wheat by mutant chromosome sequencing. Genome Biol 17:221
Santos FR, Pena SD, Epplen JT (1993) Genetic and population study of a Y-linked tetranucleotide repeat DNA polymorphism with a simple non-isotopic technique. Hum Genet 90:655–656
Schmolke M, Mohler V, Hartl L, Zeller FJ, Hsam SLK (2012) A new powdery mildew resistance allele at the Pm4 wheat locus transferred from einkorn (Triticum monococcum). Mol Breed 29:449–456
Srichumpa P, Brunner S, Keller B, Yahiaoui N (2005) Allelic series of four powdery mildew resistance genes at the Pm3 locus in hexaploid bread wheat. Plant Physiol 139:885–895
Sun HG, Hu JH, Song W, Qiu D, Cui L, Wu PP, Zhang HJ, Liu HW, Yang L, Qu YF, Li YH, Li T, Cheng W, Zhou Y, Liu ZY, Li JT, Li HJ (2018) Pm61: a recessive gene for resistance to powdery mildew in wheat landrace Xuxusanyuehuang identified by comparative genomics analysis. Theor Appl Genet. https://doi.org/10.1007/s00122-018-3135-1
Tan CC, Li GQ, Cowger C, Carver BF, Xu XY (2018) Characterization of Pm59, a novel powdery mildew resistance gene in Afghanistan wheat landrace PI 181356. Theor Appl Genet 131:1145–1152
The TT, McIntosh RA, Bennett EGA (1979) Cytogenetical studies in wheat. IX. Monosomic analyses, telocentric mapping and linkage relationships of genes Sr21, Pm4 and Mle. Aust J Biol Sci 32:115–125
Wei FS, Gobelman-Werner K, Morroll SM, Kurth J, Mao L, Wing R, Leister D, Schulze-Lefert P, Wise RP (1999) The Mla (powdery mildew) resistance cluster is associated with three NBS-LRR gene families and suppressed recombination within a 240-kb DNA interval on chromosome 5S (1HS) of barley. Genetics 153:1929–1948
Wiersma AT, Pulman JA, Brown LK, Cowger C, Olson EL (2017) Identification of Pm58 from Aegilops tauchii. Theor Appl Genet 130:1123–1133
Wu PP, Xie JZ, Hu JH, Qiu D, Liu ZY, Li JT, Li MM, Zhang HJ, Li Yang, Liu HW, Zhou Y, Zhang ZJ, Li HJ (2018) Development of molecular markers linked to powdery mildew resistance gene Pm4b by combining SNP discovery from transcript sequencing data with bulked segregant analysis (BSR-Seq) in wheat. Front Plant Sci. https://doi.org/10.3389/fpls.2018.00095
Xu HX, Yao GQ, Xiong L, Yang LL, Jiang YM, Fu BS, Zhao WF, Zhang ZZ, Zhang CQ, Ma ZQ (2008) Identification and mapping of pm2026: a recessive powdery mildew resistance gene in an einkorn (Triticum monococcum L.) accession. Theor Appl Genet 117:471–477
Xue F, Wang CY, Li C, Duan XY, Zhou YL, Zhao NJ, Wang YJ, Ji WQ (2012) Molecular mapping of a powdery mildew resistance gene in common wheat landrace Baihulu and its allelism with Pm24. Theor Appl Genet 125:1425–1432
Yahiaoui N, Srichumpa P, Dudler R, Keller B (2004) Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J 37:528–538
Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J (2004) The wheat Vrn2 gene is a flowering repressor down-regulated by vernalization. Science 303:1640–1644
Yao GQ, Zhang JJ, Yang LL, Xu HX, Jiang YM, Xiong L, Zhang CQ, Zhang ZZ, Ma ZQ, Sorrells ME (2007) Genetic mapping of two powdery mildew resistance genes in einkorn (Triticum monococcum L.) accessions. Theor Appl Genet 114:351–358
Zhang R, Sun B, Chen J, Cao A, Xing L, Feng Y, Lan C, Chen P (2016) Pm55, a developmental-stage and tissue-specific powdery mildew resistance gene introgressed from Dasypyrum villosum into common wheat. Theor Appl Genet 129:1975–1984
Zhao CZ, Li YH, Dong HT, Geng MM, Liu WH, Li F, Ni ZF, Wang XJ, Xie CJ, Sun QX (2016) Molecular cloning, functional verification, and evolution of TmPm3, the powdery mildew resistance gene of Triticum monococcum L. Genet Mol Res 15(2):gmr.15028056
Zhu Z, Zhou R, Kong X, Dong Y, Jia J (2005) Microsatellite markers linked to two powdery mildew resistance genes introgressed from Triticum carthlicum accession PS5 into common wheat. Genome 48:585–590
Zou SH, Wang H, Li YW, Kong ZS, Tang DZ (2017) The NB-LRR gene Pm60 confers powdery mildew resistance in wheat. N Phytol 218:298–309
Acknowledgements
This project was partially supported by National Key Research and Development Program (2016YFD0101802), NSFC Funds 30025030, 30771344, 30771165 and 31501302, Fundamental Research Fund for the Central Universities (KJQN201633), Jiangsu Collaborative Innovation Initiative for Modern Crop Production, ‘111’ project B08025, Innovation Team Program for Jiangsu Universities (2014). The first author also acknowledges the financial support from Chinese Scholarship Council for Ph. D program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
The original version of this article was revised: The incorrect units has been corrected.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ullah, K.N., Li, N., Shen, T. et al. Fine mapping of powdery mildew resistance gene Pm4e in bread wheat (Triticum aestivum L.). Planta 248, 1319–1328 (2018). https://doi.org/10.1007/s00425-018-2990-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-018-2990-y