Abstract
Triticum monococcum accession TA2026 showed resistance to wheat powdery mildew. To identify the resistance gene and transfer it to common wheat, genetic analysis and molecular mapping were conducted using an F2 population and derived F3 families from the cross of TA2026 × M389. The results indicated that TA2026 possessed a recessive powdery mildew resistance gene. This gene was mapped to the terminal portion of chromosome 5AmL and flanked by SSR marker loci Xcfd39 and Xgwm126. Eight RFLP markers previously mapped to the terminal chromosome 5AmL were converted into STS markers. Three loci, detected by MAG1491, MAG1493 and MAG1494, the STS markers derived from RFLP probes CDO1312, PSR164 and PSR1201, respectively, were linked to this resistance gene with Xmag1493 only 0.9 cM apart from it. In addition, the STS marker MAG2170 developed from the tentative consensus wheat cDNA encoding the Mlo-like protein identified a locus co-segregating with Xmag1493. This is the first recessive powdery mildew resistance gene identified on chromosome 5Am, and is temporarily designated pm2026. We have successfully transferred it to a tetraploid background, and this resistance stock will now be used as the bridge parent for its transfer to common wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Common wheat (Triticum aestivum L.), one of the major staple food crops of humankind, is constantly challenged by many diseases such as rust, powdery mildew, and Fusarium head blight. Powdery mildew is a devastating foliar disease of wheat caused by Blumeria graminis f. sp tritici (Bgt). The identification and utilization of powdery mildew resistance genes has played a great role in curbing this disease and in maintaining stable wheat production. Currently, the most studied powdery mildew resistance in wheat is the qualitative resistance controlled by major genes, which generally has a race-specific nature. However, because of the co-evolution of pathogen virulence and host resistance, the wide and extensive use of race-specific resistance genes can lead to the rapid emergence of new virulent pathogen strains (McDonald and Linde 2002). Virulent Bgt strains have been found for all commonly used resistance genes (Niewoehner and Leath 1998; Clarkson 2000; Duan et al. 2002). Thus, scientists are working on identification and characterization of new resistance genes.
Sixty-two powdery mildew resistance genes distributed on all chromosomes but 3A, 2D, 3D, and 4D in the wheat genome have been reported (http://wheat.pw.usda.gov/GG2/pubs.shtml; Qiu et al. 2005; Zhu et al. 2006; Sun et al. 2006; Yao et al. 2007; Miranda et al. 2007), and 35 powdery mildew resistance loci have been determined (Miranda et al. 2007). Only nine of them are recessive, including Pm5(a–e) on the long arm of chromosome 7B (Hsam et al. 2001; Huang et al. 2003), Pm9 and mlRD30 on chromosome 7A (Schneider et al. 1991; Singrün et al. 2004), Pm26 on chromosome 2BS (Rong et al. 2000), and PmY212 on chromosome 5DL (Sun et al. 2006). Except for Pm5a and Pm26, which originated from cultivated and wild emmer wheat, respectively, the other Pm5 alleles, Pm9 and mlRD30 were identified in common wheat.
The employment of molecular markers has greatly expedited the identification, cloning, and utilization of useful genes. Molecular markers linked to more than 32 powdery mildew resistance genes have been reported. Through map-based cloning, a few genes in wheat, including Pm3b (Yahiaoui et al. 2004), Q (Faris et al. 2003), Lr10 (Feuillet et al. 2003), Vrn1 (Yan et al. 2003), and Gpc-B1 QTL (Uauy et al. 2006), have been isolated. By marker-assisted selection, different powdery mildew resistance genes were successfully pyramided into elite cultivars (Liu et al. 2000). Singrün et al. (2003) and Srichumpa et al. (2005) investigated allelic relationships of Pm1 and Pm3 loci, respectively, using markers closely linked to them. In the last 7 years, with the help of molecular markers, 18 powdery mildew resistance genes in wheat were reported, including 14 from the relatives of wheat, for example, PmU (Qiu et al. 2005), Mlm2033 and Mlm80 (Yao et al. 2007) from diploid wheat, Pm30 (Liu et al. 2002), Pm31 (Xie et al. 2003), Pm33 (Zhu et al. 2005), and MlZec1 (Mohler et al. 2005) from tetraploid wheat, Pm34 (Miranda et al. 2006), Pm35 (Miranda et al. 2007), PmY201 and PmY212 (Sun et al. 2006) from Aegilops squarrosa, and PmY39 from Ae. umbellulata (Zhu et al. 2006). In addition, Srnić et al. (2005) identified two powdery mildew resistance genes on chromosome 7A transferred from T. monococcum subsp. monococcum and T. timopheevii subsp. armeniacum. These results demonstrated that wheat relatives are a reservoir of valuable genes to be discovered.
In the present study, we identified and mapped a new recessive powdery mildew resistance gene in an einkorn accession by using molecular markers. The resistance was maintained when transferred into a tetraploid background.
Materials and methods
Plant materials
Triticum monococcum accessions TA2026 and TA2033, M389, T. turgidum var. dicoccoides accession T323 and the common wheat cultivar Sumai No. 3 were used in this study. TA2026 and TA2033 were provided by Dr. B. S. Gill, Wheat Genetic Resource Center at Kansas State University. Among the tetraploid wheat germplasm maintained in the authors’ laboratory, T323 flowers at about the same time as TA2026.
Resistance evaluation
Seedlings were grown in rectangular trays placed in a growth chamber each with 72 4 × 4 cm wells and were inoculated at the one-leaf stage with pathogen isolate Bgt19 according to Yao et al. (2007). Sumai No. 3, used as the susceptible control, was planted randomly in the trays. TA2026 and M389 were also evaluated using the local field Bgt composite collected from Nanjing, Jiangsu and 18 Bgt isolates other than Bgt19, which were all the single-spore progenies of the composite. Evaluation data were collected 10 days after inoculation. Resistance performance of each plant was scored on a 0–5 scale, representing no visible symptom, necrosis without sporulation, sparse sporulation, moderate sporulation, abundant sporulation, and abundant sporulation with more than 80% of the leaf area covered with mycelia, respectively.
Marker analysis
DNA was extracted from young seedling tissues following the procedure described by Ma et al. (1994). For bulked segregant analysis, DNA bulks were prepared by combining equal amounts of DNA from six resistant or six susceptible plants derived from the F2 population. Progeny tests showed that all five bulked susceptible F2 plants segregated in F3 progenies, whereas the bulked resistant F2 plants bred true for the resistance.
SSR markers from the gwm series (Röder et al. 1998), cfd series (Guyomarc’h et al. 2002), cfa series (Sourdille et al. 2003), barc and wmc series (http://www.wheat.pw.usda.gov) that were mapped to the A genome of wheat were selected for polymorphism survey. PCR was performed either in a PE9600 thermal cycler (Perkin Elmer, Norwalk, CT, USA) or PTC-225 thermal cycler (MJ research) in a volume of 10 μl containing 10–20 ng of template DNA, 2 pmol of each of the primers, 2 nmol of each of the dNTPs, 15 nmol of MgCl2, 0.1 U of Taq DNA polymerase, and 1× PCR buffer. The PCR profile included: one cycle of 94°C 3 min, followed by 35 cycles of 94°C 30 s, 50–60°C (depending on the specific primers) 30 s and 72°C 50 s, and a final extension at 72°C for 5 min. PCR products were separated in 8% non-denaturing polyacrylamide gels with a 19:1, 25:1 or 39:1 ratio of acrylamide and bisacrylamide, and then silver-stained as described by Santos et al. (1993).
Eight RFLP markers mapped to the terminal portion of chromosome 5AmL of T. monococcum (Dubcovsky et al. 1996), including CDO1312, PSR164, PSR1201, WG114, BCD1302, ABG498, ABG366, and ABG394, were converted into STS markers by using the corresponding sequences retrieved from the NCBI nucleotide database. As the terminal chromosome 5AL is syntenic to rice chromosome 3 (Sorrells et al. 2003), these RFLP probe sequences were also used in querying against rice genomic sequences deposited in http://www.tigr.org/tdb/e2k1/osa1/pseudomolecules/ to identify homologous rice PAC or BAC clones. Five ESTs including BE444616, BQ169076, BE591666, BE490291, and BE443205 that have been mapped to the terminal deletion bin (5AL17-0.78-1.00) of chromosome 5AL in the deletion bin map (http://www.graingenes.org) were also used in the STS marker development.
STS primers were designed using MACVECTOR V8.0 (Accelrys, UK). PCRs were performed according to the procedure described earlier. Primer sequence information of a few STS markers is listed in Table 1. Cleaved amplification polymorphism was explored when the STS primers did not reveal size variation between PCR products of the parents, by digesting the products with restriction enzymes AfaI, AluI, MboI, MspI, HaeIII, HindIII, SalI, or TaqI (TaKaRa Bio. Co. Ltd., Dalian, China). Each digestion was performed according to the supplier’s manual in a 10 μl reaction mix with 5 μl PCR product. Digested products were separated on 8% non-denaturing polyacrylamide gels and silver-stained.
Linkage analysis
MAPMAKER Macintosh V2.0 (Lander et al. 1987) was used to establish the linkage map with the map distance calculated using the Kosambi mapping function (Kosambi 1944). A LOD score of 3.0 was used as the threshold for declaration of linkage.
Chromosome counting
Seeds were germinated on wet filter papers placed in Petri dishes at room temperature. Roots ~1 cm long were excised and pretreated in ice-water for 24 h, then fixed in 3:1 (v/v) absolute ethanol-acetic acid in the refrigerator. Root tip squashing and chromosome counting followed the procedures described in Ma et al. (1991).
Results
Inheritance of the powdery mildew resistance in TA2026
Seven days after the inoculation, the control and susceptible parents showed distinct disease symptoms but the resistant parents were still clear from the disease. TA2026 was highly resistant to the field Bgt composite and only sparse sporulation was observed on its leaves 10 days after the inoculation. When challenged with the 19 isolates, it showed resistance to all but Bgt14. TA2026 had a resistance score of 0–2 and M389 had a score of 4–5.
To investigate the inheritance of the powdery mildew resistance in TA2026, segregating populations were challenged with Bgt19, which was routinely used in powdery mildew resistance evaluation in the authors’ laboratory. All F1 plants from the cross involving M389 and TA2026 were as susceptible as M389. Among 228 F2 plants derived from this cross, 64 had a score of 0–2 (3, 22 and 39 plants with a score of 0, 1 and 2, respectively), 164 had a score of 3–5 (31, 63 and 70 plants with a score of 3, 4 and 5, respectively). To verify this result and to determine the genotypes of the F2 plants, progeny testing was performed at F3 with more than 30 seedlings tested for each F3 line. All the F2 plants with a score of 0–1, 35 of the 39 plants with a score of 2, and 5 of the 31 plants with a score of 3 produced progenies showing no segregation in resistance and all with a score less than 2. All F2 plants with a score of 4–5, 4 of the 39 plants with a score of 2, and 26 of the 31 plants with a score of 3 produced progenies with a score of 4–5. Of them, 118 segregated for resistance in a recessive manner. Thus, based on the screening results from both generations, among the 228 F2 plants, 65 were homozygous resistant to Bgt19 and 45 were homozygous susceptible, fitting the 1:2:1 segregation ratio (χ 2 = 3.79, P = 0.15). These results indicated that a single recessive resistance gene controls resistance in TA2026 to Bgt19.
Yao et al. (2007) reported that TA2033 possesses a dominant powdery mildew resistance gene on the long arm of chromosome 7A. The cross of TA2033 with TA2026 produced resistant F1 plants. Of 264 F2 plants derived from this cross, only 40 had a susceptible phenotype with a score of 3–5, fitting the expected 13:3 segregation ratio for the coexistence of a dominant gene and a recessive gene (χ 2 = 2.01, P = 0.16). This result further confirmed the presence of a recessive gene in TA2026. This gene was temporarily designated pm2026.
Mapping pm2026
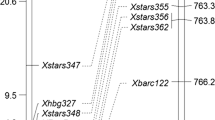
We surveyed 172 SSR markers previously mapped to the A genome of wheat and found that Xcfd39 differentiated the parents and bulks (Fig. 1a). F2 plants derived from M389 × TA2026 were genotyped with this marker and close association of Xcfd39 with pm2026 was observed (Fig. 2). Because Somers et al. (2004) mapped Xcfd39 to the long arm of chromosome 5A near the telomere, we surveyed 11 additional SSR markers previously mapped on chromosome 5AmL. Five more polymorphic loci were detected by markers CFA2141, CFA2155, CFA2185, GWM6, and GWM126 and were associated with pm2026 (Fig. 2). The order of these marker loci agreed well with that in the published 5AL map (Somers et al. 2004; Sourdille et al. 2004). The pm2026 gene was flanked by Xcfd39 and Xgwm126, with the latter towards the telomere.
Polymorphic patterns detected with CFD39 (a), MAG1491 (b), MAG1493 (c), MAG1494 (d) and MAG2170/AluI (e) in 8% non-denatured polyacrylamide gels with a 19:1 (a), 25:1 (b, c) or 39:1 (d, e) ratio of acrylamide and bis-acrylamide. The arrows indicate the polymorphic bands. M: pUC19/MspI, the numbers to its left are the band size (bp). Lanes 1, 2, 3 and 4 represent TA2026, M389, R and S pools, respectively
Linkage of pm2026 with STS markers converted from RFLP markers on chromosome 5AmL
To identify more markers linked with pm2026, STS markers converted from eight RFLP markers located at the terminal portion of chromosome 5AmL were surveyed. Three loci, detected by MAG1491, MAG1493 and MAG1494, the STS markers derived from RFLP probes CDO1312, PSR164 and PSR1201, respectively, were polymorphic between the parents (Fig. 1b–d) and linked with pm2026 (Fig. 2). Like Xcfd39, Xmag1493 was also proximal to pm2026; however, it displayed closer linkage to the resistance gene.
Linkage of pm2026 with STS markers developed from ESTs
STS markers developed based on five ESTs mapped to the 5AL terminal deletion bin did not detect polymorphism between the parents. Since RFLP probes CDO1312 and PSR164 have homologs on the overlapping BAC clones OJ1015F07 and OJA1015F07 of rice chromosome 3, respectively, the gene prediction results of these two BACs were checked. On OJA1015F07 there is an Mlo homolog that is distal to OJ1015F07 and separated from the PSR164 homolog by one gene. Thus, we amplified this Mlo homolog in TA2026 and M389 using the primer set MAG2170 designed based on the tentative consensus wheat cDNA sequence TC267529 encoding the Mlo-like protein. A single 1,560 bp band was obtained in both accessions with the identity confirmed by sequencing (data not shown). After digesting the PCR products with AluI, which explores the single nucleotide polymorphism at a recognition site of this enzyme, co-dominant polymorphic bands were detected between the parents (Fig. 1e) and they showed co-segregation with Xmag1493.
Transfer of pm2026
To transfer pm2026 to common wheat, TA2026 was crossed with the susceptible emmer accession T323. Seventeen BC1F1 plants susceptible to Bgt19 were obtained by backcrossing the F1 plants with T323 as the recurrent parent. These plants were genotyped at the co-dominant Xmag1491 locus. Because pm2026 is a recessive gene, BC1F1 plants heterozygous at Xmag1491 segregated as expected for resistance in their progenies, while those homozygous at Xmag1491 bred true for susceptibility. We then examined the progenies with resistant segregants derived from one of the BC1F1 plants by observing squash preparations of the root meristematic cells and genotyping the Xmag1493 locus closest to pm2026. The results showed that these plants had 28 somatic chromosomes (data not shown) and all the resistant segregants had the TA2026 allele of Xmag1493 (Fig. 3). Therefore, we concluded that the recessive gene pm2026 had been successfully transferred to this tetraploid genetic background and was expressed normally.
Discussion
In this study, we identified and mapped a recessive powdery mildew resistance gene in the T. monococcum accession TA2026, which was resistant to the local field Bgt composite and 18 of the 19 Bgt isolates tested. Even though Bgt14 was virulent against TA2026, it accounted for only 3% of the 33 single-spore progenies from the Bgt composite (LL Yang and ZQ Ma, unpublished data). Given that Bgt19 was virulent to Pm1, Pm3, Pm5, Pm7, and Pm8, the identification of pm2026 that confers resistance to this isolate would be a useful supplement to the powdery mildew resistance gene pool currently employed in China’s wheat breeding programs.
Currently, seven powdery mildew resistance genes have been reported from einkorn wheat (Hsam et al. 1998; Shi et al. 1998; Qiu et al. 2005; Srnić et al. 2005; Yao et al. 2007), but none of these is recessive gene. We mapped pm2026 to chromosome 5AmL, 0.9 cM from the Mlo homolog. Pm23, identified in common wheat (McIntosh et al. 1998), is so far the only powdery mildew resistance gene reported on the chromosome 5A, but its position on this chromosome is not known. Thus, the allelic relationship of pm2026 with Pm23 is to be determined. Sun et al. (2006) reported the recessive powdery mildew resistance gene PmY212 on 5DL. According to mapping positions of the pm2026 and PmY212 linked markers in the NW genetic marker map (Xue et al. 2008), these two genes could not be a pair of homoeoalleles. Other recessive powdery mildew resistance genes documented so far distribute in the seventh homoeologous chromosome group of wheat with the exception of Pm26 on chromosome 2B. Thus, pm2026 is a new powdery mildew resistance gene.
It is interesting to note that the recessive pm2026 was tightly linked to Xmag2170, the marker developed from the Mlo homolog whose recessive alleles in barley govern broad-spectrum resistance against powdery mildew (Büschges et al. 1997). This result implies that it is not the Mlo allelic variation in TA2026 that conditions the resistance. A majority of the dominant resistance genes cloned so far encode either NBS-LRR or receptor kinase-like proteins. However, recessive genes conferring disease resistances are far more diversified. Mlo encodes a trans-membrane protein with the function limiting host cell entry of the powdery mildew fungi (Büschges et al. 1997). PMR4, EDR1, EDR2, PMR5, and PMR6 in Arabidopsis whose recessive mutants conditioning resistance to powdery mildew pathogen Erysiphe cichoracearum encode a callose synthase (Nishimura et al. 2003), a putative MAP3K (Frye et al. 2001), an unknown protein (Tang et al. 2005), a plant-specific unknown protein (Vogel et al. 2004), and a pectate lyase-like protein (Vogel et al. 2002), respectively. The former three genes negatively regulate SA-inducible defense responses, while the latter two affect pectin composition SA-independently. Though Mlo is conserved in different plant species (Elliott et al. 2002), no Mlo mutants conferring powdery mildew resistance in wheat have been reported to date. Thus, exploration and investigation of recessive resistance genes in wheat would not only expand the resistance gene sources, but also be helpful in understanding the resistance mechanisms. The good collinearity between the pm2026 region and its syntenic rice chromosome as well as the flanking markers tightly linked to pm2026 could facilitate fine mapping and map-based cloning of this gene.
Phenotypic selection for recessive genes is more difficult and often requires more generations. With molecular markers closely linked to them, plants with the desirable alleles or genes can be identified without selfing. Therefore, identification and employment of the linked markers would expedite the breeding process. Moreover, closely linked markers could help diminish deleterious linkage drag probably present in gene transfers from wild relatives. With the help of the STS marker MAG1491, we initiated the transfer of pm2026 into T. turgidum accession T323 through backcrossing. Cytological examination and resistance evaluation of the BC1F2 progenies derived from T323*2/TA2026 indicated that pm2026 had been successfully transferred and that it maintains the resistance to Bgt19. To use it in breeding programs, pm2026 in the tetraploid line is now being backcrossed to an elite common wheat cultivar susceptible to powdery mildew. The new line with pm2026 will also be useful for investigating resistance to individual pathogen isolates.
References
Büschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, van Daelen R, van der Lee T, Diergaarde P, Groenendijk J, Töpsch S, Vos P, Salamini F, Schulze-Lefert P (1997) The barley Mlo gene: a novel control element of plant pathogen resistance. Cell 88:695–705
Clarkson JDS (2000) Virulence survey report for wheat powdery mildew in Europe, 1996–1998. http://www.crpmb.org/2000/1204clarkson
Duan S, Xu Y, Wu X (2002) Research progress of pathogen virulence, resistance genes and resistance breeding of wheat powdery mildew (in Chinese). J Triticeae Crops 22:83–86
Dubcovsky J, Luo M-C, Zhong G-Y, Bransteitter R, Desai A, Kilian A, Kleinhofs A, Dvorák J (1996) Genetic map of diploid wheat, Triticum mococcum L., and its comparison with maps of Hordeum vulgare L. Genetics 143:983–999
Elliott C, Zhou F, Spielmeyer W, Panstruga R, Schulze-Lefert P (2002) Functional conservation of wheat and rice Mlo orthologs in defense modulation to the powdery mildew fungus. Mol Plant Microbe Interact 15:1069–1077
Faris JD, Fellers JP, Brooks SA, Gill BS (2003) A bacterial artificial chromosome contig spanning the major domestication locus Q in wheat and identification of a candidate gene. Genetics 164:311–321
Feuillet C, Travella S, Stein N, Albar L, Nublat A, Keller B (2003) Map-based isolation of the leaf rust disease resistance gene Lr10 from the hexaploid wheat (Triticum aestivum L.) genome. Proc Natl Acad Sci USA 100:15253–15258
Frye CA, Tang D, Innes RW (2001) Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc Natl Acad Sci USA 98:373–378
Guyomarc’h H, Sourdille P, Edwards KJ, Bernard M (2002) Studies of the transferability of microsatellites derived from Triticum tauschii to hexaploid wheat and to diploid related species using amplification, hybridization and sequence comparisons. Theor Appl Genet 105:736–744
Hsam SLK, Huang XQ, Ernst F, Hartl L, Zeller FJ (1998) Chromosomal location of genes for resistance to powdery mildew in common wheat (Triticum aestivum L. em Thell.). 5. Alleles at the Pm1 locus. Theor Appl Genet 96:1129–1134
Hsam SLK, Huang XQ, Zeller FJ (2001) Chromosomal location of genes for resistance to powdery mildew in common wheat (Triticum aestivum L. em Thell.). 6. Alleles at the Pm5 locus. Theor Appl Genet 102:127–133
Huang XQ, Wang LX, Xu MX, Röder MS (2003) Microsatellite mapping of the powdery mildew resistance gene Pm5e in common wheat (Triticum aestivum L.). Theor Appl Genet 106:858–865
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Lander ES, Green P, Abrahamson J, Barlow A, Daley MJ, Lincoln SE, Newburg L (1987) Mapmaker: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Liu J, Liu D, Tao W, Li W, Wang S, Chen P, Cheng S, Gao D (2000) Molecular marker-facilitated pyramiding of different genes for powdery mildew resistance in wheat. Plant Breed 119:21–24
Liu Z, Sun Q, Ni Z, Nevo E, Yang TM (2002) Molecular characterization of a novel powdery mildew resistance gene Pm30 in wheat originating from wild emmer. Euphytica 123:21–29
Ma ZQ, Zhao YH, Liu DJ (1991) Incorporation of restoring gene of Aegilops umbellulata into wheat. Genome 34:727–732
Ma ZQ, Sorrells ME, Tanksley SD (1994) RELP markers linked to powdery mildew resistance genes Pm1, Pm2, Pm3 and Pm4 in wheat. Genome 37:871–875
McDonald BA, Linde C (2002) Pathogen population genetics, evolution potential and durable resistance. Annu Rev Phytopathol 40:349–379
McIntosh RA, Hart GE, Devos KM, Gale MD, Rogers WJ (1998) Catalogue of gene symbols for wheat. In Proc. 9th International Wheat Genetics Symposium, vol 5, Saskatoon, 2–7 Aug 1998. Univ. Extension Press, Saskatoon
Miranda LM, Murphy JP, Marshall DS, Leath S (2006) Pm34: a new powdery mildew resistance gene transferred from Aegilops tauschii Coss. to common wheat (Triticum aestivum L.). Theor Appl Genet 113:1497–1504
Miranda LM, Murphy JP, Marshall D, Cowger C, Leath S (2007) Chromosomal location of Pm35, a novel Aegilops tauschii derived powdery mildew resistance gene introgressed into common wheat (Triticum aestivum L.). Theor Appl Genet 114:1451–1456
Mohler V, Zeller FJ, Wenzel G, Hsam SLK (2005) Chromosomal location of genes for resistance to powdery mildew in common wheat (Triticum aestivum L. em Thell.). 9. Gene MlZec1 from the Triticum dicoccoides-derived wheat line Zecoi-1. Euphytica 142:161–167
Niewoehner AS, Leath S (1998) Virulence of Blumeria graminis f. sp. tritici on winter wheat in the eastern United States. Plant Dis 82:64–68
Nishimura MT, Stein M, Hou B-H, Vogel JP, Edwards H, Somerville SC (2003) Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301:969–972
Qiu YC, Zhou RH, Kong XY, Zhang SS, Jia JZ (2005) Microsatellite mapping of a Triticum urartu Tum. derived powdery mildew resistance gene transferred to common wheat (Triticum aestivum L.). Theor Appl Genet 111:1524–1531
Röder MS, Korzun V, Wendehake K, Plaschke J, Tixier M-H, Leroy P, Ganal MW (1998) A microsatellite map of wheat. Genetics 149:2007–2023
Rong JK, Millet E, Manisterski J, Feldman M (2000) A new powdery mildew resistance gene: introgression from wild emmer into common wheat and RFLP-based mapping. Euphytica 115:121–126
Santos FR, Pena SDJ, Epplen JT (1993) Genetic and population study of a Y-linked tetranucleotide repeat DNA polymorphism with a simple non-isotopic technique. Hum Genet 90:655–656
Schneider DM, Heun M, Fischbeck G (1991) Inheritance of the powdery mildew resistance gene Pm9 in relation to Pm1 and Pm2 of wheat. Plant Breed 107:161–164
Shi AN, Leath S, Murphy JP (1998) A major gene for powdery mildew resistance transferred to common wheat from wild einkorn wheat. Phytopathology 88:144–147
Singrün C, Hsam SLK, Hartl L, Zeller FJ, Mohler V (2003) Powdery mildew resistance gene Pm22 in cultivar Virest is a member of the complex Pm1 locus in common wheat (Triticum aestivum L. em Thell.). Theor Appl Genet 106:1420–1424
Singrün C, Hsam SLK, Zeller FJ, Wenzel G, Mohler V (2004) Localization of a novel recessive powdery mildew resistance gene from common wheat line RD30 in the terminal region of chromosome 7AL. Theor Appl Genet 109:210–214
Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114
Sorrells ME, Rota ML, Bermudez-Kandianis CE, Greene RA, Kantety R, Munkvold JD, Miftahudin , Mahmoud A, Ma X, Gustafson PJ, Qi L, Echalier B, Gill BS, Matthews DE, Lazo GR, Chao S, Anderson OD, Edwards H, Linkiewicz AM, Dubcovsky J, Akhunov ED, Dvorak J, Zhang D, Nguyen HT, Peng J, Lapitan NLV, Gonzalez-Hernandez JL, Anderson JA, Hossain K, Kalavacharla V, Kianian SF, Choi D-W, Close TJ, Dilbirligi M, Gill KS, Steber C, Walker-Simmons MK, McGuire PE, Qualset CO (2003) Comparative DNA sequence analysis of wheat and rice genomes. Genome Res 13:1818–1827
Sourdille P, Cadalen T, Guyomarc’h H, Snape JW, Perretant MR, Charmet G, Boeuf C, Bernard S, Bernard M (2003) An update of the Courtot × Chinese Spring intervarietal molecular marker linkage map for the QTL detection of agronomic traits in wheat. Theor Appl Genet 106:530–538
Sourdille P, Singh S, Cadalen T, Brown-Guedira GL, Gay G, Qi L, Gill BS, Dufour P, Murigneux A, Bernard M (2004) Microsatellite-based deletion bin system for the establishment of genetic-physical map relationships in wheat (Triticum aestivum L.). Funct Integr Genomics 4:12–25
Srichumpa P, Brunner S, Keller B, Yahiaoui N (2005) Allelic series of four powdery mildew resistance genes at Pm3 locus in hexaploid bread wheat. Plant Physiol 139:885–895
Srnić G, Murphy JP, Lyerly JH, Leath S, Marshall DS (2005) Inheritance and chromosomal assignment of powdery mildew resistance genes in two winter wheat germplasm lines. Crop Sci 45:1578–1586
Sun XL, Liu D, Zhang HQ, Huo NX, Zhou RH, Jia JZ (2006) Identification and mapping of two new genes conferring resistance to powdery mildew from Aegilops tauschii (Coss.) schmal. J Integr Plant Biol 48:1204–1209
Tang D, Ade J, Frye CA, Innes RW (2005) Regulation of plant defense responses in Arabidopsis by EDR2, a PH and START domain-containing protein. Plant J 44:245–257
Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J (2006) A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 314:1298–1301
Vogel JP, Raab TK, Schiff C, Somerville SC (2002) PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell 14:2095–2106
Vogel JP, Raab TK, Somerville CR, Somerville SC (2004) Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. Plant J 40:968–978
Xie C, Sun Q, Ni Z, Yang TM, Nevo E, Fahima T (2003) Chromosomal location of a Triticum dicoccoides-derived powdery mildew resistance gene in common wheat by using microsatellite markers. Theor Appl Genet 106:341–345
Xue SL, Zhang ZZ, Lin F, Kong ZX, Cao Y, Li CJ, Yi HY, Mei MF, Zhu HL, Wu JZ, Xu HB, Zhao DM, Tian DG, Zhang CQ, Ma ZQ (2008) A high-density intervarietal map of the wheat genome enriched with markers derived from expressed sequence tags. Theor Appl Genet. doi:10.1007/s00122-008-0764-9
Yahiaoui N, Srichumpa P, Dudler R, Keller B (2004) Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J 37:528–538
Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J (2003) Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci USA 100:6263–6268
Yao G, Zhang J, Yang L, Xu H, Jiang Y, Xiong L, Zhang C, Zhang Z, Ma Z, Sorrells ME (2007) Genetic mapping of two powdery mildew resistance genes in einkorn (Triticum monococcum L.) accessions. Theor Appl Genet 114:351–358
Zhu Z, Zhou R, Kong X, Dong Y, Jia J (2005) Microsatellite markers linked to 2 powdery mildew resistance genes introgressed from Triticum carthlicum accession PS5 into common wheat. Genome 48:585–590
Zhu Z, Zhou R, Kong X, Dong Y, Jia J (2006) Microsatellite marker identification of a Triticum aestivum-Aegilops umbellulata substitution line with powdery mildew resistance. Euphytica 150:149–153
Acknowledgments
This project was partially supported by NSFC Funds (30025030, 30771344, 30771165), MOE program for outstanding young faculty, and NAU Youth Fund for Scientific Innovation (Y200602). We thank all the lab staffs and graduate students who have contributed to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hongxing Xu and Guoqi Yao contributed equally.
Communicated by A. Kilian.
Rights and permissions
About this article
Cite this article
Xu, H., Yao, G., Xiong, L. et al. Identification and mapping of pm2026: a recessive powdery mildew resistance gene in an einkorn (Triticum monococcum L.) accession. Theor Appl Genet 117, 471–477 (2008). https://doi.org/10.1007/s00122-008-0791-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-008-0791-6