Abstract
Key message
An allele of Pm2 for wheat powdery mildew resistance was identified in a putative Agropyron cristatum -derived line and used in wheat breeding programs.
Abstract
Powdery mildew (caused by Blumeria graminis f. sp. tritici, Bgt) is one of the most devastating wheat diseases worldwide. It is important to exploit varied sources of resistance from common wheat and its relatives in resistance breeding. KM2939, a Chinese breeding line, exhibits high resistance to powdery mildew at both the seedling and adult stages. It carries a single dominant powdery mildew resistance (Pm) allele of Pm2, designated Pm2b, the previous allelic designation Pm2 will be re-designated as Pm2a. Pm2b was mapped to chromosome arm 5DS and flanked by sequence characterized amplified region (SCAR) markers SCAR112 and SCAR203 with genetic distances of 0.5 and 1.3 cM, respectively. Sequence tagged site (STS) marker Mag6176 and simple sequence repeat (SSR) marker Cfd81 co-segregated with SCAR203. Pm2b differs in specificity from donors of Pm2a, Pm46 and PmLX66 on chromosome arm 5DS. Allelism tests indicated that Pm2b, Pm2a and PmLX66 are allelic. Therefore, Pm2b appears to be a new allele at the Pm2 locus. The closely linked markers were used to accelerate transfer of Pm2b to wheat cultivars in current production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Powdery mildew, caused by the fungal ascomycete Blumeria graminis f. sp. tritici (Bgt), is a devastating disease of wheat, affecting grain yield and quality (Morgounov et al. 2012). With popularization of semi-dwarf, high-yielding cultivars, increased utilization of nitrogen fertilizer and improved irrigation, the disease has increased in importance in wet and warm areas (Bennett 1984; Zhuang and Li 1993; Zhuang 2003). Certain agricultural practices and chemical controls have been followed to reduce the disease, but these approaches have also increased production costs, polluted the environment and speeded to encourage the occurrence of new pathogen variants (Hardwick et al. 1994; Ryabchenko et al. 2003; Parks et al. 2008). The most effective, economical, environmentally sound and consistently used method for control is host resistance (Huang et al. 2000; Huang and Röder 2004).
More than 60 Pm genes/alleles located at 45 loci have been identified, including the formally designated genes Pm1–Pm50, and a few temporarily designated genes (e.g., McIntosh et al. 2012; Mohler et al. 2013; Xiao et al. 2013). However, when a qualitative resistance gene is over popularized in production, new isolates capable of overcoming the resistance usually appear some years later, and may induce epidemic ‘boom-bust’ cycles such as occurred for Pm8 and Yr9 in China following increases in the respective virulence genes and loss of resistance to powdery mildew and stripe rust, respectively (Graybosch 2001; Ryabchenko et al. 2003; Li and Zeng 2002; Wan et al. 2007). In fact, many Pm genes have lost effectiveness due to the race-specific nature of resistance and excessive deployment of single resistance genes (Xiao et al. 2013). Therefore, a constant search and transfer of new and effective sources of powdery mildew resistance are necessary to counter the continuous evolution of virulence in Bgt.
In the past, exploitation of resistance genes/alleles mainly focused on cultivated wheat. Over time, the genetic base for disease resistance has been narrowed by replacing highly variable landraces with high yielding, pure-line cultivars in many parts of the world, and the more homogeneous genetic backgrounds of cultivated species have led to unprecedented challenges in resistance breeding (Johnson 1992; Gupta et al. 2010; Muhammad et al. 2011; Karsai et al. 2012). Progenies of distant hybridization between common wheat and its wild species could be important sources of wheat disease resistance. A few Pm genes from non-progenitor (that is, other than A, B and D genome diploids) species have been identified, such as Pm7, Pm8, Pm17 and Pm20 from Secale cereale (Heun et al. 1990; Friebe et al. 1994, 1996; Hsam and Zeller 1997) and Pm21 from Haynaldia villosa (Chen et al. 1995). These genes were introgressed into the wheat genome in large alien translocation or addition lines, and can be detected by genomic in situ hybridization (GISH). Some other genes were also identified in the progenies from distant hybridizations, but no cytologically detectable signals were observed by genomic in situ hybridization techniques. Such genes include Pm40 and Pm43 (He et al. 2009; Luo et al. 2009) and stripe rust resistance genes Yr50 and YrL693 (Liu et al. 2013; Huang et al. 2014) putatively derived from Thinopyrum intermedium. In all cases, the resistant lines containing these genes were selected several generations after the original inter-specific crosses with selection for disease resistance, but without monitoring for alien chromatin by cytological or marker methods.
With the development of molecular markers, including restriction fragment length polymorphism (RFLP), random amplified polymorphic DNA (RAPD), simple sequence repeat (SSR), sequence tagged site (STS) and amplified fragment length polymorphism (AFLP) markers, the above resistance genes were mapped to wheat chromosomes. The wheat-derived markers tightly linked to these resistance genes can be used for marker-assisted selection (MAS) to improve the efficiency of selection in wheat breeding (Gupta et al. 2010). MAS has been successfully performed in wheat breeding programs to transfer Th. intermedium-derived barley yellow dwarf virus resistance gene Bdv2 into commercial cultivars (Zhang et al. 2004), but in this case there is evidence demonstrating that a small alien segment is present. Alien-derived leaf rust resistance genes Lr9 (from Ae. umbellulata), Lr24 (from Th. ponticum) and Lr47 (from Ae. speltoides) have been transferred to other common wheat cultivars by use of markers (Nocente et al. 2007) that were either within the non-combining alien segments, and therefore behaving as ‘perfect’ markers, or were linked wheat-derived markers.
Agropyron cristatum (2n = 4x = 28, PPPP), a wild relative of wheat, possesses many desirable traits for improving wheat, including resistance to major diseases, such as powdery mildew, rusts and barley yellow dwarf virus, tolerance to drought and salinity, and various high-yielding characteristics, e.g., high tiller number, and larger floret and high kernel numbers (Dong et al. 1992; Li et al. 1997; Martín et al. 1999; Wu et al. 2006, 2010). Transfer of these alien traits to wheat is important for wheat improvement, including powdery mildew resistance (Luan et al. 2010; Chen et al. 2013).
KM2939, a putative A. cristatum-derived breeding line, shows high resistance to powdery mildew at both the seedling and adult stages. The objectives of this study were to study the inheritance of powdery mildew resistance in KM2939, determine the chromosomal location of the resistance gene, investigate the utility of closely linked markers of the resistance gene in different genetic backgrounds, and rapidly transfer the resistance gene to susceptible cultivars in current production.
Materials and methods
Plant materials
The materials used in this study included wheat lines KM2939, X3986-2, Huixianhong, Mingxian 169, Shimai 15, Fukuho, Laizhou 953 and CMH83.605, and A. cristatum accession Z559. KM2939 was derived from the cross 4201/CMH83.605//FC. Line 4201 was selected from a cross of Laizhou 953 and a glossy wheat-A. cristatum line selected from selfed hybrids of A. cristatum accession Z559 and Japanese introduction Fukuho. Line FC was derived from selfed hybrids of A. cristatum accession Z559 and Fukuho, and CMH83.605 was a wheat line introduced from Mexico. X3986-2 is an indigenous germplasm that has a known Pm gene PmX3986-2 discovered by our laboratory (Ma et al. 2014). KM2939 showed high resistance to powdery mildew, while Shimai 15, Huixianhong and Mingxian 169 were all tested by different Bgt isolates and showed highly susceptible with infection type (IT) 4.
To analyze the inheritance of powdery mildew resistance, KM2939 was crossed with Huixianhong, Mingxian 169 and Shimai 15 to construct segregating populations, and F1, F2, F3, BC1F1 and BC2F1 materials were tested for resistance to the Bgt isolate E09 that is avirulent on KM2939 and virulent on Huixianhong, Mingxian 169 and Shimai 15. The BC2F1 population from cross with KM2939 and Shimai 15 were used as mapping population.
Disease assessment at the seedling stage
KM2939 was tested for reaction to 27 single-pustule-derived powdery mildew isolates by separate inoculations in a temperature-controlled greenhouse at the Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing (Supplementary Table S1). Meanwhile, 40 wheat lines carrying known Pm genes or gene combinations were used to compare their reaction patterns with KM2939 (Supplementary Table S1) (Ma et al. 2014). These Bgt isolates were collected from different wheat ecological regions of China, and each isolate contains a different combination of virulences to resistance genes (Zhou et al. 2002). The method of disease infection and evaluation was described in An et al. (2013).
PCR amplification and marker analysis
Genomic DNA was extracted from the leaf tissues of young seedlings following a phenol/chloroform method (Sharp et al. 1988). Equal amounts of DNA from 10 resistant and 10 susceptible BC2F1 plants of cross KM2939/Shimai 15 were bulked to produce resistant and susceptible pools for bulked segregant analysis (BSA) (Michelmore et al. 1991). Simple sequence repeat (SSR) primers randomly distributed on all 21 chromosomes were used to amplify DNA from the two parents, and resistant and susceptible bulks in a polymorphic marker survey.
Twenty-five expressed sequence tags (ESTs) mapping to the Pm2-containing bin of chromosome arm 5DS were converted to STS markers and tested against both the parents and bulks; these included BE498794, BG604817, BE352603, BE404490, BE498665, BE636795, BE499257, BF291319, BG313707, BE591974, BE500291, BE498878, BE446058, BE591275, BQ167501, BE496976, BE443538, BE435260, BF291319, BE498665, BE499257, BE636795, BE443751, BE352603 and BG604817 (Ma et al. 2011; Gao et al. 2012; http://wheat.pw.usda.gov/cgi-bin/gbrowse/WheatPhysicalESTMaps/#search).
The resulting polymorphic markers were genotyped on the BC2F1 plants of KM2939/3*Shimai 15. PCR amplification and separation and visualization of the PCR products followed Xu et al. (2012). The PCR profile was as follows: 1 cycle at 94 °C for 3 min, followed by 35 cycles at 94 °C for 30 s, 50–60 °C (depending on specific primers) for 40 s, 72 °C for 40 s, and a final extension at 72 °C for 5 min. Appropriate amounts of PCR products mixed with loading buffer (98 % formamide, 0.25 % bromophenol, 0.25 % xylene cyanol and 10 mM EDTA) were separated on 8 % non-denaturing polyacrylamide gels (Acrylamide: Bisacrylamide = 25:1 or 39:1) with 1× TBE buffer (90 mM tris–borate, 2 mM EDTA, pH 8.3), and visualized by silver straining (Xu et al. 2012).
Linkage mapping
Chi squared (χ 2) tests for goodness-of-fit were made to evaluate deviations of observed phenotypic data from theoretically expected segregation ratios. Linkage maps with genetic distances between markers and the resistance gene were constructed by the software MAPMAKER 3.0 with an LOD threshold of 3.0 (Lincoln et al. 1992). Map distances were derived from recombination values using the Kosambi function (Kosambi 1944).
Application of the resistance gene in wheat breeding programs
KM2939 was crossed with susceptible recurrent parents Shimai 15, Shixin 828, Kenong 199 and Gaocheng 8901, followed by two backcrosses to the recurrent parents. The closely linked markers were evaluated for applicability in diagnosing the resistance gene in each of the recipients. Progeny plants with the marker alleles from the resistant donor were retained for MAS. Finally, the resistances of selected progeny plants were verified using Bgt isolate E09.
A near isogenic line (NIL) was developed using susceptible cultivar Shimai 15 as the recurrent parent. To assess the genetic similarity of selected lines to the backcross parent, they were surveyed with 284 SSR markers distributed across all 21 chromosomes. Markers with polymorphism between KM2939 and Shimai 15 were selected from the 284 SSR markers, and used to screen the segregating materials. Estimation of the recipient genomic composition (RGC) followed the method of Xue et al. (2010), and was calculated by the formula: RGC = (2BB + AB) × 100 %/2 (BB + AB), where BB represents homozygous marker loci of the recurrent parent Shimai 15, and AB represents heterozygous loci.
Results
Inheritance of powdery mildew resistance in KM2939
When inoculated with Bgt isolate E09, KM2939 showed a hypersensitive reaction with IT 0, whereas Shimai 15, Huixianhong and Mingxian 169 were highly susceptible to IT 4. F1 seedlings of KM2939/Shimai 15, KM2939/Huixianhong, and KM2939/Mingxian 169 were all resistant to IT 0, and the F2 populations segregated 3,275 resistant to 1,079 susceptible plants (\(\chi^{2}_{3:1}\) = 0.11, df = 1, P = 0.74), 255 to 76 (\(\chi^{2}_{3:1}\) = 0.73, df = 1, P = 0.39) and 190 to 60 (\(\chi^{2}_{3:1}\) = 0.80, df = 1, P = 0.37), respectively, all fitting single dominant gene segregation ratios (Table 1). When F2 populations of KM2939/Huixianhong and KM2939/Mingxian 169 were transplanted to the field, 293 and 214 plants survived to produce F3 seeds, respectively. Twenty-four plants of each F2:3 family were evaluated for powdery mildew response. The ratios of homozygous resistant (RR): segregating (Rr):homozygous susceptible (rr) families from KM2939/Huixianhong and KM2939/Mingxian 169 were consistent with the expected 1:2:1 (Table 1). BC1F1 populations of KM2939/2*Shimai 15 and KM2939/2*Mingxian 169, and a BC2F1 population of KM2939/3*Shimai 15 all segregated with expected ratios of 1:1 (Table 1). We concluded that powdery mildew resistance in KM2939 was conferred by a single dominant gene, which was designated Pm2b.
Molecular mapping of Pm2b
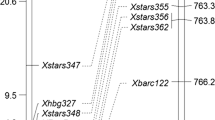
Initially, 284 SSR markers randomly located on all 21 wheat chromosomes were tested for polymorphism between the parents and bulks, and only the marker Cfd40 located on both chromosome arms 5DS and 5AS showed a consistent polymorphism between both the parents and resistant and susceptible bulks (Supplementary Fig. S1). To determine and saturate the genomic region near Pm2b based on the location of Cfd40, 42 and 36 SSR markers on 5DS and 5AS, respectively, were tested on the parents and bulks. Only SSR markers Cfd81, Cfd78 and Gpw302 on 5DS showed polymorphisms between the parents and bulks (Supplementary Fig. S1), whereas none of the markers located on 5AS displayed such polymorphisms. Primer pairs CFD40, CFD81, CFD78 and GPW302 were further used to genotype the BC2F1 population of KM2939/3*Shimai 15 to determine the genetic distance between the four marker loci and Pm2b (Fig. 1). Marker loci Xcfd40, Xcfd81, Xcfd78 and Xgpw302 were closely linked to Pm2b with genetic distances 9.1, 1.3, 4.5 and 10.2 cM, respectively (Fig. 2a). AFLP-derived SCAR markers SCAR112 and SCAR203 linked to the known Pm gene MlBrock (Li et al. 2009) also mapped close to Pm2b with genetic distances of 0.4 and 1.3 cM, respectively (Fig. 2a). Twenty-five ESTs, previously mapped to bin 5DS1-0-0.63 containing Pm2a, were also tested for polymorphism between the parents and bulks, and polymorphic marker Mag6176 was derived by conversion from EST BE498794. This is the same as the Pm2a-linked marker (Xmag6176) described by Ma et al. (2011). It was located 1.3 cM proximal to Pm2b (Fig. 2a). The loci order of linked markers was Xscar112–Pm2b–Xscar203/Xmag6176/Xcfd81–Xcfd78–Xcfd40–Xgpw302. Based on the previous studies, marker loci Xcfd81, Xscar112 and Xscar203 were all assigned to the deletion bin 5DS-1-0-0.63 (Ma et al. 1994; Gao et al. 2012; Huang et al. 2012). Therefore, Pm2b flanked by these markers should be located in the same chromosome bin (Fig. 2b). As EST BE498794 was previously mapped in bin 5DS-1-0-0.63 (http://wheat.pw.usda.gov/cgi-bin/gbrowse/WheatPhysicalESTMaps/#search), its converted EST-SSR marker locus Xmag6176 linked with Pm2b must be located in the same chromosome position.
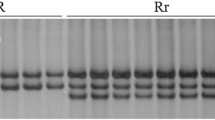
Examples of amplification patterns of Pm2b-linked polymorphic markers Cfd81 (a), and Mag6176 (b) from selected KM2939/3*Shimai 15 F1 plants in 8 % silver-stained non-denaturing polyacrylamide gels. Lane M, pUC18/MspI; P1, P2 KM2939 and Shimai 15; Lanes: R heterozygous resistant plants, S homozygous susceptible plants. Arrows indicate polymorphic bands
Comparison of Pm2b and known Pm genes on chromosome arm 5DS
Twenty-seven Bgt isolates were used to compare the seedling stage powdery mildew reactions conferred by Pm2b, Pm2a, and temporarily designated Pm genes Pm46, PmLX66 and PmX3986-2 (Supplementary Table S1). KM2939 was resistant to 25 of 27 isolates (92.6 %), except for E21 and E32. This was a wider range of effectiveness than conferred by the donor of Pm2a, a widely used gene in Chinese wheat breeding programs (19 of 27 isolates (70.4 %) avirulent). Pm46, a temporarily designated Pm gene in German cultivar Tabasco, conferred resistance to 23 of 27 (85.2 %) isolates, PmLX66, a temporarily designated gene in Liangxing 66, one of the most widely grown cultivars in China, conferred resistance to 20 of 27 (74.1 %) isolates, and PmX3986-2, a temporarily designated gene in an indigenous germplasm, conferred resistance to 16 of 27 (Ma et al. 2014). All the three genes conferred narrower resistance spectra than Pm2b to the isolates tested. Data for 14 differential Bgt isolates are shown in Table 2; the total data are provided in Supplementary Table S1. The resistance spectrum of Pm2b was clearly different from those for Pm2a, Pm46, PmLX66 and PmX3986-2. It was concluded that Pm2b is different from other Pm genes located on chromosome arm 5DS.
To further clarify the allelic relationships of Pm2b and other Pm genes on chromosome arm 5DS, a total of 6,112 F2 plants from KM2939//Ulka/8*Cc (Pm2a) were inoculated with culture E09 that is avirulent to both the parents. No susceptible F2 plant was found, demonstrating that Pm2b and Pm2a are likely at the same locus. Also no susceptible plant occurred among 1,112 and 292 F2 plants from crosses KM2939/Liangxing 66 and Liangxing 66/KM2939 tested with the same isolate, indicating that Pm2b and PmLX66 were also allelic or closely linked in repulsion.
Applicability of the closely linked markers in MAS and development of an NIL
Of the markers linked to Pm2b, Cfd81, SCAR112, SCAR203 and Gpw302 showed polymorphisms between KM2939 and recipient cultivars Shimai 15, Kenong 199, Shixin 828 and Gaocheng 8901, whereas markers Cfd40, Cfd78 and Mag6176 were polymorphic only between KM2939 and one or two of the recipients.
For MAS, susceptible cultivar Gaocheng 8901 was selected as a high quality wheat recipient. Using the closely linked markers, we selected a high gluten quality, powdery mildew resistant, wheat line after backcrossing and selfing for several generations. This line is now taken part in our own yield comparation test. For transferring Pm2b to more susceptible cultivars in current production, BC2F1 populations of KM2939/Shimai 15, and BC1F1 plants from crosses of KM2939 with Kenong 199 and Shixin 828 are being subjected to MAS using Pm2b-linked markers. F1 hybrids of KM2939 and other cultivars in current production, such as Han 7086, Han 6172, Ji 5265, Luyuan502, Zhou 16, Zhou 8425B and Zhoumai 22, have also been obtained, and will be backcrossed with the cultivars and subjected to MAS in the near future.
To develop an NIL with Pm2b, Shimai 15, a susceptible cultivar widely grown on the North China Plain, was selected as a recurrent parent. A BC2F1 population of KM2939/3*Shimai 15 was obtained, and 100 BC2F1 plants possessing resistance to Bgt isolate E09 and having the appropriate Cfd81 allele were selected prior to background selection. To evaluate the genetic similarity of the selected lines to the backcross parent Shimai 15, 97 polymorphic SSR markers randomly distributed on all 21 wheat chromosomes were used for background selection. A BC2F1 plant, coded as KS148, with 91 homozygous marker loci identical to Shimai 15 and 6 heterozygous marker loci was selected. RGC of KS148 was thus 96.9 %. Three other BC2F1 plants had RGC values of 95.9, 95.5 and 95.4 %, respectively.
Discussion
Exploitation of powdery mildew resistance genes from progenies of distant hybridization
Exploitation of resistance genes from progenies of distant hybridization is an important and effective way of increasing diversity of resistance to powdery mildew in wheat. Unfortunately, many translocation lines have questionable value in wheat improvement, because they often have large and complex chromosome segments that do not fully compensate for lost wheat chromatin or carry additional deleterious features (linkage drag) (Young and Tanksley 1989). If the alien chromosome segment is small, it is more likely to have value in wheat improvement. Occasionally, valuable traits are transferred to recipient genotypes without detectable cytological changes (Kuraparthy et al. 2007a, b). In the Kuraparthy et al. (2007a, b) reports, authenticity of alien origin was supported by the presence of linked markers derived from the alien source and availability of alien addition lines used as the donor sources and experimental controls. Disease resistance genes identified in such lines in wheat also include powdery mildew resistance genes Pm40 and Pm43 (He et al. 2009; Luo et al. 2009) putatively derived from Th. intermedium, and stripe rust resistance genes Yr50 and YrL693 also putatively derived from Th. intermedium (Liu et al. 2013; Huang et al. 2014). Irrespective of origin, these genes are important sources of resistance.
Agropyron cristatum is a perennial Triticeae species and a valuable source of resistance to many wheat diseases, including powdery mildew (Li et al. 1995). Since it was successfully crossed with common wheat, attempts have been made to introgress useful traits to wheat (Li et al. 1997; Wu et al. 2006; Luan et al. 2010). The Chinese breeding line KM2939 is a derivative of A. cristatum. All known common wheat parents in the KM2939 pedigree were tested against Bgt isolate E09, and they were all highly susceptible to powdery mildew with the IT 4. However, Pm2b is mostly likely a wheat gene rather than an A. cristatum derived gene. Firstly, over several years of observations in the field, KM2939 was genetically and agronomically uniform. Secondly, KM2939 has 42 chromosomes and thus far GISH analysis and molecular marker special for the A. cristatum genome have failed to detect A. cristatum chromatin (data not shown). Thirdly, the powdery mildew resistance gene in KM2939 behaved as a single Mendelian unit and was mapped by wheat-derived markers, with the two closest flanking markers only 0.6 and 1.3 cM from Pm2b. Importantly, Pm2b appears to be allelic with the known wheat genes. Therefore, Pm2b is more likely a wheat gene derived from unknown source.
Relationships between Pm2b and known Pm genes on chromosome 5D
There are three Pm genes reported on chromosome arm 5DL; viz. Pm34 flanked by the SSR markers Barc177 and Barc144 (Miranda et al. 2006), Pm35 linked to Cfd26 at a distance of 11.9 cM (Miranda et al. 2007) and Pm-M53 flanked by Wmc289 and Gwm292 (Li et al. 2010). These three genes are at genetic distances of about 92, 41 and 55 cM, respectively, from Pm2b based on reported maps (Somers et al. 2004). Pm2b confers a significantly different response spectrum from Pm34 and Pm35 when tested with an array of 27 Bgt isolates (Supplementary Table S1). Obviously, Pm2b differs from these genes. Pm2a, identified in the wheat landrace Ulka and located on chromosome arm 5DS, is linked to SSR marker Cfd81 with a genetic distance of 2.0 cM (Qiu et al. 2006), and distance of 3.3 cM from Pm2b (Fig. 2a). Temporarily designated Pm genes reported on chromosome arm 5DS include MlBrock which co-segregated with Cfd81 in cultivar Brock (Li et al. 2009), PmD57-5D flanked by Mag6176 and Gwm205 in line D57 (Ma et al. 2011), Pm46 flanked by Mp510 and Gwm205 in German cultivar Tabasco (Gao et al. 2012), PmLX66 flanked by Cfd81 and SCAR203 in Chinese wheat cultivar Liangxing 66 (Huang et al. 2012) and PmX3986-2 flanked by Cfd81 and SCAR112 in an indigenous germplasm (Ma et al. 2014). Among these, PmD57-5D and Mlbrock are likely to be Pm2a (Li et al. 2009; Ma et al. 2011). Pm46, PmLX66 and PmX3986-2 are at genetic distances of 1.8, 4.1 and 0.7 cM from Pm2b based on reported maps (Huang et al. 2012; Gao et al. 2012). To clarify the relationships between Pm2b and Pm2a, Pm46, PmLX66 and PmX3986-2, 27 Bgt isolates were tested on a panel of wheat genotypes including KM2939, Ulka/8*Cc (with Pm2a), Tabasco (with Pm46), Liangxing 66 (with PmLX66) and X3986-2 (with PmX3986-2). The results indicated that KM2939 confers a different reaction pattern compared to lines with Pm2a, Pm46, PmLX66 and PmX3986-2 (Table 1). Therefore, Pm2b is a different specificity from Pm2a, Pm46, PmLX66 and PmX3986-2. In a test of allelism of Pm2b and Pm2a using 6,112 F2 plants from KM2939/Ulka/8*Cc inoculated with isolate E09, no susceptible plant was found. Similar results were obtained for Pm2b and PmLX66 using 1,112 and 292 F2 plants from reciprocal crosses KM2939/Liangxing 66 and Liangxing 66/KM2939, respectively. Therefore, Pm2b is apparently located at the same locus as Pm2a and PmLX66. Pm46 was previously shown to be closely linked, but not allelic, to Pm2a (Gao et al. 2012). That is, Pm2b is also closely linked, but not allelic, to Pm46. Thus, based on response spectrum and allelism tests, Pm2b appears to be a novel allele of Pm2a, and was therefore designated Pm2b; the previous allelic designation Pm2 will be re-designated as Pm2a.
Transferring Pm2b into wheat cultivars
Whether a resistance gene has potential in wheat improvement depends, to some extent, on the agronomic traits of the donor. Fortunately, KM2939 possesses some valuable inheritances of high-yield characteristics, e.g., much higher kernel numbers compared with main cultivars in China (P < 0.01), and as for other agronomic traits, such as spike length, spike number, spikelet number, thousand kernel weight and sterile spikelet number, KM2939 appears to perform competitively with current leading cultivars (data not shown). Therefore, KM2939 is an attractive high-yielding line that will attract the attention of breeders without the need for intensive pre-breeding prior to incorporation of the resistance gene into breeding populations.
Seven molecular markers were closely linked to Pm2b in the BC2F1 population KM2939/3*Shimai 15. However, not all the closely linked markers identified in genetic studies diagnose a resistance gene in all genetic backgrounds. To use these markers, it is necessary to trace linked alleles in specific crosses. Likewise, three RAPD markers linked to Pm25 cannot diagnose Pm25 in some backgrounds carrying it due to excessive genetic distances between the markers and Pm25 (Shi et al. 1998). Hartl et al. (1995) reported that it was difficult to identify all cultivars possessing Pm1 and Pm2a using closely linked RFLP markers. A similar result was obtained for Pm2a using an STS marker converted from the corresponding RFLP marker linked to Pm2a (Mohler and Jahoor 1996). Therefore, it is necessary to confirm the availability of the closely linked markers in different genetic backgrounds for MAS. After verification, four of the seven markers can be used as more effective markers for selection of Pm2b in different genetic backgrounds. Work on transferring Pm2b into different susceptible cultivars has been carried out for several years by MAS, and we have obtained an advanced line currently in our own yield comparation test, an NIL with RGC of 96.9 %, and several selected populations that show resistance to powdery mildew at the both seedling and adult stages.
Author contribution statement
P Ma Experimental implementation, data analysis and manuscript preparation. H. Xu Production of the mapping population and genetic map. Y. Xu Production of the genetic map. Y. Qie MAS analysis. Q. Luo Genomic in situ hybridization and molecular marker detection. X. Zhang, X. Li Germplasm creation and field investigation. Y. Zhou Powdery mildew tests. D. An, L. Li Study concept and design.
Abbreviations
- AFLP:
-
Amplified fragment length polymorphism
- Bgt :
-
Blumeria graminis f. sp. tritici
- BSA:
-
Bulked segregant analysis
- GISH:
-
Genomic in situ hybridization
- MAS:
-
Marker-assisted selection
- NIL:
-
Near-isogenic line
- Pm :
-
Powdery mildew resistance gene
- RAPD:
-
Random amplified polymorphic DNA
- RFLP:
-
Restriction fragment length polymorphism
- RGC:
-
Recipient genome composition
- SCAR:
-
Sequence characterized amplified region
- SSR:
-
Simple sequence repeat
- STS:
-
Sequence tagged site
References
An DG, Zheng Q, Zhou YL, Ma PT, Lv ZL, Li LH, Li B, Luo QL, Xu HX, Xu YF (2013) Molecular cytogenetic characterization of a new wheat-rye 4R chromosome translocation line resistant to powdery mildew. Chromosome Res 21:419–432
Bennett FGA (1984) Resistance to powdery mildew in wheat: a review of its use in agriculture and breeding programmes. Plant Pathol 3:279–300
Chen PD, Qi LL, Zhou B, Zhang SZ, Liu DJ (1995) Development and molecular cytogenetic analysis of wheat-Haynaldia villosa 6VS/6AL translocation lines specifying resistance to powdery mildew. Theor Appl Genet 91:1125–1128
Chen PD, You CF, Hu Y, Chen SW, Zhou B, Cao AZ, Wang XE (2013) Radiation-induced translocations with reduced Haynaldia villosa chromatin at the Pm21 locus for powdery mildew resistance in wheat. Mol Breed 31:477–484
Dong YS, Zhou RH, Xu SJ, Li LH, Cauderon Y, Wang RRC (1992) Desirable characteristics in perennial Triticeae collected in China for wheat improvement. Hereditas 116:175–178
Friebe B, Heun M, Tuleen N, Zeller FJ, Gill BS (1994) Cytogenetically monitored transfer of powdery mildew resistance from rye into wheat. Crop Sci 34:621–625
Friebe B, Jiang J, Raupp WJ, McIntosh RA, Gill BS (1996) Characterization of wheat-alien translocations conferring resistance to diseases and pests: current status. Euphytica 91:59–87
Gao HD, Zhu FF, Jiang YJ, Wu JZ, Yan W, Zhang QF, Jacobi A, Cai SB (2012) Genetic analysis and molecular mapping of a new powdery mildew resistant gene Pm46 in common wheat. Theor Appl Genet 125:967–973
Graybosch RA (2001) Uneasy unions: quality effects of rye chromatin transfers to wheat. J Cereal Sci 33:3–16
Gupta PK, Langridge P, Mir RR (2010) Marker-assisted wheat breeding: present status and future possibilities. Mol Breed 261:145–161
Hardwick NV, Jenkins JEE, Collins B, Groves SJ (1994) Powdery mildew (Erysiphe graminis) on winter wheat: control with fungicides and the effects on yield. Crop Prot 13:93–98
Hartl L, Weiss H, Stephan U, Zeller FJ, Jahoor A (1995) Molecular identification of powdery mildew resistance genes in common wheat (Triticum aestivum L.). Theor Appl Genet 90:601–606
He RL, Chang ZJ, Yang ZJ, Yuan ZY, Zhan HX, Zhang XJ, Liu JX (2009) Inheritance and mapping of powdery mildew resistance gene Pm43 introgressed from Thinopyrum intermedium into wheat. Theor Appl Genet 118:1173–1180
Heun M, Friebe B, Bushuk W (1990) Chromosomal location of the powdery mildew resistance gene of Amigo wheat. Phytopathology 80:1129–1133
Hsam SLK, Zeller FJ (1997) Evidence of allelism between genes Pm8 and Pm17 and chromosomal location of powdery mildew and leaf rust resistance genes in the common wheat cultivar ‘Amigo’. Plant Breed 116:119–122
Huang XQ, Röder MS (2004) Molecular mapping of powdery mildew resistance genes in wheat: a review. Euphytica 137:203–223
Huang XQ, Hsam SLK, Zeller FJ, Wenzel G, Mohler V (2000) Molecular mapping of the wheat powdery mildew resistance gene Pm24 and marker validation for molecular breeding. Theor Appl Genet 101:407–414
Huang J, Zhao ZH, Song FJ, Wang XM, Xu HX, Huang Y, An DG, Li HJ (2012) Molecular detection of a gene effective against powdery mildew in the wheat cultivar Liangxing 66. Mol Breed 30:1737–1745
Huang Q, Li X, Chen WQ, Xiang ZP, Zhong SF, Chang ZJ, Zhang M, Zhang HY, Tan FQ, Ren ZL, Luo PG (2014) Genetic mapping of a putative Thinopyrum intermedium-derived stripe rust resistance gene on wheat chromosome 1B. Theor Appl Genet 127:843–853
Johnson R (1992) Past, present and future opportunities in breeding for disease resistance, with examples from wheat. Euphytica 63:3–22
Karsai I, Vida G, Petrovics S, Petcu E, Kobiljski B, Ivanovska S, Bedö Z, Veisz O (2012) Assessment of the spatial genotypic and phenotypic diversity present in the various winter wheat breeding programs in Southeast Europe. Euphytica 186:139–151
Kosambi DD (1944) The estimation of map distance from recombination values. Ann Eugen 12:172–175
Kuraparthy V, Chhuneja P, Dhaliwal HS, Kaur S, Bowden RL, Gill BS (2007a) Characterization and mapping of cryptic alien introgression from Aegilops geniculata with new leaf rust and stripe rust resistance genes Lr57 and Yr40 in wheat. Theor Appl Genet 114:1379–1389
Kuraparthy V, Sood S, Chhuneja P, Dhaliwal HS, Kaur S, Bowden RL, Gill BS (2007b) A cryptic wheat-Aegilops triuncialis translocation with leaf rust resistance gene Lr58. Crop Sci 47:1995–2003
Li Z, Zeng S (2002) Wheat rust in China (In Chinese). China Agricultural Press, Beijing
Li LH, Dong YS, Zhou RH, Li XQ, Li P, Yang XM (1995) Cytogenetics and self-fertility of intergeneric hybrids between Triticum aestivum L. and Agropyron cristatum (L.) Gaertn. Chin J Genet 22:105–112
Li LH, Li XQ, Li P, Dong YC, Zhao GS (1997) Establishment of wheat-Agropyron cristatum alien addition lines. I. Cytology of F3, F2BC1, BC4 and BC3F1 progenies. Acta Genet Sin 24:154–159
Li GQ, Fang TL, Zhu J, Gao LL, Li S, Xie CJ, Yang ZM, Sun QX, Liu ZY (2009) Molecular identification of a powdery mildew resistance gene from common wheat cultivar Brock (In Chinese). Acta Agron Sin 35:1613–1619
Li T, Zhang ZY, Hu YK, Duan XY, Xin ZY (2010) Identification and molecular mapping of a resistance gene to powdery mildew from the synthetic wheat line M53. J Appl Genet 52:137–143
Lincoln S, Daly M, Lander E (1992) Constructing genetic maps with Mapmaker/EXP30 Whitehead Institute Techn Rep, 3rd edn. Whitehead Institute, Cambridge
Liu J, Chang Z, Zhang X, Yang Z, Li X, Jia J, Zhan H, Guo H, Wang J (2013) Putative Thinopyrum intermedium-derived stripe rust resistance gene Yr50 maps on wheat chromosome arm 4BL. Theor Appl Genet 126:265–274
Luan Y, Wang XG, Liu WH, Li CY, Zhang JP, Gao AN, Wang YD, Yang XM, Li LH (2010) Production and identification of wheat-Agropyron cristatum 6P translocation lines. Planta 232:501–510
Luo PG, Luo HY, Chang ZJ, Zhang HY, Zhang M, Ren ZL (2009) Characterization and chromosomal location of Pm40 in common wheat: a new gene for resistance to powdery mildew derived from Elytrigia intermedium. Theor Appl Genet 118:1059–1064
Ma ZQ, Sorrells ME, Tanksley SD (1994) RFLP markers linked to powdery mildew resistance genes Pm1, Pm2, Pm3, and Pm4 in wheat. Genome 37:871–875
Ma HQ, Kong ZX, Fu BS, Li N, Zhang LX, Jia HY, Ma ZQ (2011) Identification and mapping of a new powdery mildew resistance gene on chromosome 6D of common wheat. Theor Appl Genet 123:1099–1106
Ma PT, Xu HX, Luo QL, Qie YM, Zhou YL, Xu YF, Han HM, Li LH, An DG (2014) Inheritance and genetic mapping of a gene for seedling resistance to powdery mildew in wheat line X3986-2. Euphytica 200:149–157
Martín A, Cabrera A, Esteban E, Hernández P, Ramírez MC, Rubiales D (1999) A fertile amphiploid between diploid wheat (Triticum tauschii) and crested wheatgrass (Agropyron cristatum). Genome 42:519–524
McIntosh RA, Dubcovsky J, Rogers WJ, Morris C, Appels R, Xia XC (2012) Catalogue of gene symbols for wheat: 2012 supplement. http://www.wheat.pw.usda.gov
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Miranda LM, Murphy JP, Marshall D, Leath S (2006) Pm34: a new powdery mildew resistance gene transferred from Aegilops tauschii Coss. to common wheat (Triticum aestivum L.). Theor Appl Genet 113:1497–1504
Miranda LM, Murphy JP, Marshall D, Cowger C, Leath S (2007) Chromosomal location of Pm35, a novel Aegilops tauschii derived powdery mildew resistance gene introgressed into common wheat (Triticum aestivum L.). Theor Appl Genet 114:1451–1456
Mohler V, Jahoor A (1996) Allele-specific amplification of polymorphic sites for the detection of powdery mildew resistance loci in cereals. Theor Appl Genet 93:1078–1082
Mohler V, Bauer C, Schweizer G, Kempf H, Hartl L (2013) Pm50: a new powdery mildew resistance gene in common wheat derived from cultivated emmer. J Appl Genet 54:259–263
Morgounov A, Tufan HA, Sharma R, Akin B, Bagci A, Braun HJ, Kaya Y, Keser M, Payne TS, Sonder K, McIntosh R (2012) Global incidence of wheat rusts and powdery mildew during 1969–2010 and durability of resistance of winter wheat variety Bezostaya 1. Eur J Plant Pathol 132:323–340
Muhammad IK, Mir AK, Ma HX, Gul SSK, Abdul JK, Tila M (2011) Selection of parents for crossing based on genotyping and phenotyping for stripe rust (Puccinia striiformis) resistance and agronomic traits in bread wheat breeding. Cytol Genet 45:379–394
Nocente F, Gazza L, Pasquini M (2007) Evaluation of leaf rust resistance genes Lr1, Lr9, Lr24, Lr47 and their introgression into common wheat cultivars by marker-assisted selection. Euphytica 155:329–336
Parks R, Carbone I, Murphy JP, Marshall D, Cowger C (2008) Virulence structure of the eastern US wheat powdery mildew population. Plant Dis 92:1047–1082
Qiu YC, Sun XL, Zhou RH, Kong XY, Zhang SS, Jia JZ (2006) Identification of microsatellite markers linked to powdery mildew resistance gene Pm2 in wheat. Cereal Res Commun 34:1267–1273
Ryabchenko AS, Serezhkina GV, Mishina GN, Andreev LN (2003) Morphological variability of wheat powdery mildew in the context of its parasitic adaptation to wheat-Aegilops lines with different resistance. Biol Bull Acad Sci 30:255–261
Sharp PJ, Kreis M, Shewry PR, Gale MD (1988) Location of β-amylase sequences in wheat and its relatives. Theor Appl Genet 75:286–290
Shi AN, Leath S, Murphy JP (1998) A major gene for powdery mildew resistance transferred to common wheat from wild einkorn wheat. Phytopathology 88:144–147
Si QM, Zhang XX, Duan XY, Sheng BQ, Zhou YL (1992) On gene analysis and classification of powdery mildew (Erysiphe graminis f. sp. tritici) resistant wheat varieties. Acta Phytopathol Sin 22:349–355
Somers DJ, Isaac P, Edwards K (2004) A high-density wheat microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114
Wan A, Chen X, He Z (2007) Wheat stripe rust in China. Aust J Agric Res 58:605–619
Wu J, Yang XM, Wang H, Li HJ, Li LH, Li X, Liu WH (2006) The introgression of chromosome 6P specifying for increased numbers of florets and kernels from Agropyron cristatum into wheat. Theor Appl Genet 114:13–20
Wu M, Zhang JP, Wang JC, Yang XM, Gao AN, Zhang XK, Liu WH, Li LH (2010) Cloning and characterization of repetitive sequences and development of SCAR markers specific for the P genome of Agropyron cristatum. Euphytica 172:363–372
Xiao MG, Song FJ, Jiao JF, Wang XM, Xu HX, Li HJ (2013) Identification of the gene Pm47 on chromosome 7BS conferring resistance to powdery mildew in the Chinese wheat landrace Hongyanglazi. Theor Appl Genet 126:1397–1403
Xu HX, Yin DD, Li LH, Wang QX, Li XQ, Yang XM, Liu WH, An DG (2012) Development and application of EST based markers specific for chromosome arms of rye (Secale cereale L.). Cytogenet Genome Res 136:220–228
Xue SL, Li GQ, Jia HY, Li F, Gao Y, Xu F, Tang MZ, Wang Y, Wu XY, Zhang ZZ, Zhang LX, Kong ZX, Ma ZQ (2010) Marker-assisted development and evaluation of near-isogenic lines for scab resistance QTLs of wheat. Mol Breed 25:397–405
Young ND, Tanksley SD (1989) RFLP analysis of the size chromosomal segments retained around Tm-2 locus of tomato during backcross breeding. Theor Appl Genet 77:353–359
Zhang ZY, Xu JS, Xu QJ, Larkin P, Xin ZY (2004) Development of novel PCR markers linked to the BYDV resistance gene Bdv2 useful in wheat for marker-assisted selection. Theor Appl Genet 109:433–439
Zhou YL, Duan XY, Chen G, Sheng BQ, Zhang Y (2002) Analyses of resistance genes of 40 wheat cultivars or lines to wheat powdery mildew. Acta Phytopathol Sin 32:301–305
Zhuang QS (2003) Wheat improvement and pedigree analysis in China. China Agriculture Press, Beijing
Zhuang QS, Li ZQ (1993) Present status of wheat breeding and related genetic study in China. Wheat Inf Serv 76:1–15
Acknowledgments
We are grateful to Dr. R.A. McIntosh, University of Sydney, Australia for critically reviewing drafts of this paper. This research was financially supported by the National High-Tech Research and Development Program of China No. 2011AA1001, the National Natural Science Foundation of China No. 31171550, the National Scientific and Technological Supporting Program of China No. 2013BAD01B02 and the Chinese Academy of Sciences No. XDA08030107.
Conflict of interest
The authors (Pengtao Ma, Hongxing Xu, Yunfeng Xu, Lihui Li, Yanmin Qie, Qiaoling Luo, Xiaotian Zhang, Xiuquan Li, Yilin Zhou and Diaoguo An) declare that our experiments comply with the current laws of China and we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Beat Keller.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, P., Xu, H., Xu, Y. et al. Molecular mapping of a new powdery mildew resistance gene Pm2b in Chinese breeding line KM2939. Theor Appl Genet 128, 613–622 (2015). https://doi.org/10.1007/s00122-015-2457-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-015-2457-5