Abstract
Key message
Powdery mildew resistance gene Pm55 was physically mapped to chromosome arm 5VS FL 0.60–0.80 of Dasypyrum villosum . Pm55 is present in T5VS·5AL and T5VS·5DL translocations, which should be valuable resources for wheat improvement.
Abstract
Powdery mildew caused by Blumeria graminis f. sp. tritici is a major wheat disease worldwide. Exploiting novel genes effective against powdery mildew from wild relatives of wheat is a promising strategy for controlling this disease. To identify novel resistance genes for powdery mildew from Dasypyrum villosum, a wild wheat relative, we evaluated a set of Chinese Spring-D. villosum disomic addition and whole-arm translocation lines for reactions to powdery mildew. Based on the evaluation data, we concluded that the D. villosum chromosome 5V controls post-seedling resistance to powdery mildew. Subsequently, three introgression lines were developed and confirmed by molecular and cytogenetic analysis following ionizing radiation of the pollen of a Chinese Spring-D. villosum 5V disomic addition line. A homozygous T5VS·5AL translocation line (NAU421) with good plant vigor and full fertility was further characterized using sequential genomic in situ hybridization, C-banding, and EST-STS marker analysis. A dominant gene permanently named Pm55 was located in chromosome bin 5VS 0.60–0.80 based on the responses to powdery mildew of all wheat-D. villosum 5V introgression lines evaluated at both seeding and adult stages. This study demonstrated that Pm55 conferred growth-stage and tissue-specific dependent resistance; therefore, it provides a novel resistance type for powdery mildew. The T5VS·5AL translocation line with additional softness loci Dina/Dinb of D. villosum provides a possibility of extending the range of grain textures to a super-soft category. Accordingly, this stock is a new source of resistance to powdery mildew and may be useful in both resistance mechanism studies and soft wheat improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Powdery mildew, caused by Blumeria graminis (DC.) E.O. Speer f. sp. tritici Em. Marchal is one of the most economically important diseases in many wheat (Triticum aestivum L.) growing regions with cool or maritime climates. The denser canopies of modern semi-dwarf cultivars produced under high fertility conditions favor powdery mildew development with consequent losses in yield and grain quality. Resistant cultivars are considered to be a cost-effective and environment friendly way of controlling this disease. Although more than 70 genes/alleles (Pm genes) conferring powdery mildew resistance have been identified in wheat (McIntosh et al. 2013), most of them are ineffective due to the presence of virulence in the pathogen. Hence, identification of new resistance sources in adapted germplasm is an important and long-term objective in achieving durable and broad-spectrum resistance.
Powdery mildew resistance in wheat has heavily relied on the species of the tertiary gene pool to discover new resistance sources. Among the powdery mildew resistance genes that are currently available, Pm7, Pm8, Pm17, and Pm20 were introduced from cereal rye (Secale cereale L.) (McIntosh et al. 2013), Pm40 (Luo et al. 2009), Pm43 (He et al. 2009), and PmL962 (Shen et al. 2015) were introgressed from Thinopyrum intermedium (Host) Barkworth & D. R. Dewey and Pm51 came from Th. ponticum (Podp.) Barkworth & D.R. Dewey (Zhang et al. 2014a), suggesting alien gene transfer is an important means of increasing the genetic diversity of powdery mildew resistance in common wheat. In the tertiary gene pool, Dasypyrum villosum (L.) Candargy is a valuable species for wheat improvement because of its resistance to cereal cyst nematodes (Zhang et al. 2012a), wheat streak mosaic virus (Zhang et al. 2005), stem rust (Qi et al. 2011), cereal eyespot (Yildirim et al. 1998), and powdery mildew (Chen et al. 1995). The all-stage powdery mildew resistance gene Pm21, originating from chromosome arm 6VS of D. villosum, has remained as an effective source of resistance for more than 20 years and has been used widely in breeding powdery mildew resistant cultivars in China (Chen et al. 1995; Li et al. 2007). Except for chromosome 6V, it is not clear if other chromosomes of D. villosum have powdery mildew resistance genes.
Several wheat-D. villosum chromosome addition and substitution lines have been developed in the past 30 years (Gradzielewska 2006; De Pace et al. 2011). However, when the targeted alien genes from the D. villosum V genome are used in wheat improvement, they need to be in the form of a cytologically stable, compensating wheat-alien whole-arm (Robertsonian) translocation (Qi et al. 2007). Liu et al. (2011) developed a set of compensating T. aestivum–D. villosum Robertsonian translocation lines, with the exception of the 2VS and 5VL arms. In addition, eight whole-arm translocations, including T1VS·1BL, T1VL·1DL, T2VS·2DL, T2VL·2DS, T4VS·4DL, T5VS·5DL, T6VS·6AL, and T6VL·6AS (Chen et al. 1995; Zhang et al. 2005, 2010, 2013, 2014b), which originated from the D. villosum accession GP005 (introduced from the Cambridge Botanical Garden, UK), have been developed in our laboratory. The T5VS·5DL translocation line carrying grain softness genes Dina/Dinb from D. villosum has been transferred into current varieties by backcrossing (Zhang et al. 2015). Agronomic characteristics showed that T5VS·5DL translocation lines had higher powdery mildew resistance at the early flowering and grain filling stages than its recurrent parents, implying that a resistance gene may locate on the alien chromatin.
To confirm this resistance, T. aestivum–D. villosum 5V introgression lines and a set of wheat-D. villosum addition and whole-arm translocation lines were evaluated for response to powdery mildew in both seedling and adult stages. The resistance characteristics and physical location of a novel powdery mildew resistance gene on the short arm of chromosome 5V were investigated in this study.
Materials and methods
Plant materials
The lines used in this study included T. aestivum cv. Chinese Spring (CS), Yangmai 15, Zhengmai 9405, D. villosum accession GP005, T. durum cv. 1286 (AABB), T. durum cv. 1286-D. villosum (GP005) amphiploid (AABBVV), and other T. aesitivum–D. villosum genetic stocks listed in Table 1. D. villosum accession GP005 is the donor of the V chromatins in the present study and its chromosomes were numbered #4 by De Pace et al. (2011). All of these materials were maintained at the Cytogenetics Institute at Nanjing Agricultural University (CINAU). Three nulli-tetrasomic lines (N5AT5D, N5BT5D, and N5DT5A), kindly provided by the Wheat Genetics and Genomics Resource Center, Kansas State University, were also used in the study.
Development of T. aestivum–D. villosum 5V introgression lines
To develop T. aesitivum–D. villosum 5V introgression lines, flowering spikes of CS-D. villosum disomic addition 5V line, DA5V, were cut off retaining flag leaves and irradiated with 1200 rad of γ-rays from a 60Co source. The matured pollen freshly harvested from irradiated spikes within 2 days after irradiation was pollinated to emasculated florets of CS. M1 and M2 plants were backcrossed to CS. In BC2F2, three CS-5V introgression lines, including a Robertsonian translocation T5VS·5AL (NAU421), were identified using genomic in situ hybridization (GISH), sequential chromosome C-banding, and molecular markers.
Cytogenetic analysis
The protocol of chromosome preparation of root tip cells (RTCs) and C-banding followed Gill et al. (1991) and GISH analysis of RTCs and pollen mother cells (PMCs) were according to Chen et al. (1995). The genomic DNA of D. villosum was labeled with fluorescein-12-dUTP, and used as a probe to detect the chromosome fragment of 5V. After hybridization with the probes, chromosomes were counterstained with propidium idodide (PI) and mounted in Vectashield (Roche Co., Burlingame, CA, USA). Signals were examined with an Olympus BX60 epifluorescence microscope (Olympus Co., Tokyo, Japan). GISH images of RTCs and PMCs were captured with a SPOT Cooled Color Digital Camera (Diagnostic Instruments, Sterling Heights, MI, USA).
Molecular marker analysis
Six previously reported 5V-specific EST-PCR markers (CINAU41, CINAU42, CINAU43, 5EST-237, 5EST-238, and Xwmc233) were used to screen the alien chromatin in wheat genetic background (Cao et al. 2009; Zhang et al. 2012b). Among them, CINAU42 is specific for chromosome arm 5VL and the others are specific for 5VS. Genomic DNA was isolated from young leaves according to instructions accompanying the DNAsecure Plant Kit (Tiangen Biotech Co., Ltd., Beijing, China). PCR amplifications were conducted in a 25 μL reaction mixture containing 1× Taq DNA polymerase buffer, 0.8 mmol/L MgCl2, 0.8 mmol/L dNTPs, 200 μmol/L primers, 2 units DNA polymerase, and 50 ng genomic DNA as template. The samples were denaturated at 95 °C for 4 min and subjected to 35 cycles of 30 s of denaturation at 94 °C, 55–60 °C (depending on the specific primers) for 45 s, and 1.2 min elongation at 72 °C, with a final extension at 72 °C for 10 min. The PCR products were analyzed on 10 % nondenaturing polyacrylamide gels with a 39:1 ratio of acrylamide: bisacrylamide. Gels were silver stained and photographed.
Field trials
The wheat-D. villosum genetic stocks and their genetic background parents (Table 1) were planted in the field nurseries in Nanjing, China, consecutively for 3 years. Each line was planted in a four-row (1 m long) plot, with 25 cm spacing between rows. The planting rate was 10 plants per row. To investigate the inheritance of powdery mildew resistance introgressed from D. villosum chromosome arm 5VS, T5VS·5AL (NAU421) was backcrossed to the powdery mildew susceptible cultivar (cv.) Yangmai 15. The BC1F1 (NAU421/2*Yangmai 15) and F2 (NAU421/Yangmai 15) populations were planted in the field nurseries in Nanjing in 2014 growing season and F2:3 populations were evaluated in 2015 growing season. Common wheat ‘Sumai 3’ was used to increase Bgt inoculum and as the susceptible control. All plants were covered with plastic sheeting for protection during the winter seasons. A local field collection of Bgt (mixed races) was used to infect all materials at the jointing stage. In addition, adult-plant reactions to powdery mildew on NAU415 (T5VS·5DL) and NAU421 (T5VS·5AL) were simultaneously assessed at the Institute of Plant Protection, Chinese Academy of Agricultural Sciences in Beijing, China. Also, NAU222 (T5VS·5DL) and NAU421 (T5VS·5AL), along with their respective recurrent parents Yangmai 15 and CS, were tested at Zhengzhou in Henan province, China, using local Bgt field inoculum during 2015. Reactions evaluated at the heading and grain filling stages were scored on a 0–4 infection type (IT) scale, and were then separated into two classes, resistant (R, IT 0–2) and susceptible (S, IT 3–4).
Greenhouse test
The seedlings of NAU421 (T5VS·5AL) and a set of lines carrying known Pm genes or gene combinations were tested for their reactions to 24 single-pustule-derived Bgt virulent isolates (E01, E02, E05, E06, E07, E09, E11, E13, E15, E16, E17, E18, E20, E21, E23-1, E23-2, E26, E30-1, E30-2, E32, E49, E50, E60, and E69) using separate artificial inoculation in a temperature-controlled greenhouse at the Institute of Plant Protection, the Chinese Academy of Agricultural Sciences, Beijing, China (Zhou et al. 2002). In addition, the powdery mildew responses of lines NAU415 (T5VS·5DL), NAU421 (T5VS·5AL), CS, Yangmai 15, and NAU222 (T5VS·5DL) were simultaneously evaluated at the one- to seven-leaf stages in Nanjing. Six seeds per line were planted in a single pot, with one Sumai 3 plant being used as the susceptible control in every pot. A Nanjing field collection of Bgt conidiospores and single spore-derived isolates, E20, E26, and E31 were used for separate tests under controlled greenhouse conditions. Inoculations were carried out by shaking conidiospores from susceptible seedlings of Sumai 3 held above the test plants. The response of each plant was recorded on a 0–4 IT scale system at 15 days after inoculation.
Results
Origin of the powdery mildew resistance
Seedling reactions of a set of T. aestivum–D. villosum chromosomes 1V to 7V disomic addition lines and eight whole-arm translocation lines (Table 1) to the local Bgt isolates in Nanjing were evaluated in the greenhouse. Results showed that the disomic addition line DA6V and T6VS·6AL translocation line 92R137 had a high level of resistance (IT 0), which was similar to the donor D. villosum GP005 while other lines were susceptible (IT 3–4) at the seeding stage. When the lines were tested in the field at adult stages, in addition to DA6V and 92R137, disomic addition line DA5V and T5VS·5DL translocation line NAU415 also exhibited a high level of resistance (IT 0; −1). These results suggested that in addition to Pm21 on chromosome arm 6VS (Chen et al. 1995), chromosome 5V may carry adult-plant resistance to powdery mildew.
Development of T. aesitivum–D. villosum 5V introgression lines
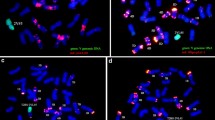
To confirm if D. villosum chromosome 5V carries a resistance gene to powdery mildew, pollen of DA5V was irradiated with γ-rays. Three CS-D. villosum 5V introgression lines were developed in the progenies following irradiated pollen treatment. Two of the lines, NAU122 and NAU421, were Robertsonian translocation lines with 2n = 42, pairing as 21 bivalents at meiotic metaphase I. The 5VL-specific marker CINAU42 was present in NAU122, but all five 5VS-specific markers were absent, indicating that NAU122 was a T5VL·W translocation line (Fig. 1a, b). In contrast, all five 5VS-specific markers were present and CINAU42 was absent in NAU421, indicating that it was a T5VS·W translocation line (Fig. 1c, d). Absence of 5AS-specific bands of CINAU-41, 5EST-237, and 5EST-238 in NAU421 (Fig. 2) indicated that chromosome arm 5AS had been replaced by 5VS. Sequential chromosome C-banding (Fig. 1f) and GISH (Fig. 1g) showed that the opposite arm of the translocated chromosome in NAU421 was chromosome 5AL (Gill et al. 1991). Thus, the translocated chromosome in NAU421 was T5VS·5AL. NAU111 was a telosomic addition line (2n = 42 + 2t) (Fig. 1e). Molecular marker analysis showed that all five 5VS-specific markers were present whereas the 5VL-specific marker CINAU42 was absent, indicating that NAU111 was a disomic chromosome 5VS telosomic addition line. The three T. aesitivum–D. villosum 5V introgression lines developed were used to evaluate the powdery mildew response in detail.
Mitotic and meiotic GISH analyses of wheat-D. villosum chromosome 5V introgression lines. Total genomic DNA of D. villosum labeled with digoxigenin-11-dUTP was used as probe. D. villosum chromatin fluoresced with a yellowish-green color and wheat chromatin fluoresced red. a, b Mitotic and meiotic GISH patterns of NAU122 containing a pair of wheat-D. villosum chromosome arm 5VL Robertsonian translocation chromosomes; c, d GISH patterns of NAU421 with a pair of wheat-D. villosum 5VS Robertsonian translocation chromosomes; e mitotic GISH patterns of NAU111, a ditelosomic 5VS addition line; f, g sequential C-banding and GISH patterns of the translocated chromosome in NAU421
Electrophoresis patterns of EST-PCR marker 5EST-237. Chromosome associations of individual bands are shown on the right. The 5VS-specific fragment is present in NAU415, NAU222, NAU421, and NAU111. The 5DS-specific fragment is absent in NAU415 and NAU222; the 5AS-specific fragment is absent in NAU421
Powdery mildew responses in different stages and conditions
Powdery mildew tests at early flowering and grain filling stages in Beijing field conditions showed that the leaves, stems, and spikes of NAU415 (T5VS·5DL; Zhang et al. 2010) and NAU421 (T5VS·5AL) were all highly resistant (IT, 0–0). When tested under field conditions in Zhengzhou, NAU421 (T5VS·5AL) and NAU222 (T5VS·5DL; Zhang et al. 2015) also exhibited a highly resistant response (IT, 0–1) on all tissues while their respective recurrent parents CS and Yangmai 15 were susceptible (IT, 4) (Fig. 3). Under field conditions in Nanjing, DA5V, NAU415 (T5VS·5DL), NAU421 (T5VS·5AL), NAU222 (T5VS·5DL), and NAU111 (Dt5VS) showed a highly resistant response (IT, 0–1) on leaves and stems but had a susceptible phenotype (IT, 2–3) on spikes when tested in 2013, 2014, and 2015. The non-5VS control lines NAU122 (T5VL·W), CS, and Yangmai 15 had highly susceptible IT on all tissues and this resistance, therefore, was confirmed to be derived from the chromosome arm 5VS.
Powdery mildew reactions of T5VS·5DL translocation line NAU222 (N) and its parent Yangmai 15 (Y). Upper Phenotypes of NAU222 and Yangmai 15 at about 10 days after inoculation at different leaf development stages; Lower powdery mildew reactions of NAU222 (N) and Yangmai 15 (Y) at the early flowering stages (left) and grain filling (right) at under Zhengzhou field conditions
The seedlings of NAU415 and a set of wheat genotypes carrying known Pm genes or gene combinations were tested for reaction to 24 virulent Bgt isolates at the one- to two-leaf stages. The results of the mildew response from NAU415 showed susceptibility to all 24 virulent isolates with an IT score of 3–4. In contrast, the T6VS·6AL line 92R137 carrying Pm21 was highly resistant to these isolates with IT scores of 0–0. To investigate the growth stage at which the resistance was expressed, lines NAU415 (T5VS·5DL), NAU421 (T5VS·5AL), CS, NAU222 (T5VS·5DL), and Yangmai 15 were tested at the one- to seven-leaf stages with mixed races and three Bgt isolates E20, E26, and E31, respectively. All lines were equally susceptible at the one- and two-leaf stages, but some resistance was expressed at the three-leaf stage, and then increased to a fully effective level at the five-leaf and later stages (Fig. 3). Thus, this powdery mildew resistance gene(s) was expressed in a specific plant growth stage on targeted tissue in common wheat.
Genetic analysis of powdery mildew resistance
The segregation patterns of the BC1F1 population from NAU421/2*Yangmai 15 and F2 and F2:3 populations from NAU421/Yangmai 15 are shown in Table 2. When inoculated with the Nanjing field collection of Bgt conidiospores at the adult stages, the F1 plants showed infection types similar to the resistant parent NAU421 (T5VS·5AL), indicating that the resistance was dominant. The BC1F1 population segregated in a ratio of 1 resistant:1 susceptible (χ 2 = 0.91, P > 0.05) and the F2’s segregated in 3 resistant:1 susceptible (χ 2 = 3.26, P > 0.05) ratio. In addition, the pooled data of 75 F2:3 lines from NAU421/Yangmai 15 segregated as expected for single gene ratio:16 homozygous resistant: 39 segregating: 20 homozygous susceptible (χ2 = 0.54, P > 0.05). The combined results from GISH and molecular marker analysis confirmed the presence of a T5VS·5AL translocation chromosome. Similar outcomes were achieved for line NAU415, but in that case a T5VS·5DL translocation chromosome was involved. The results verified that a dominant powdery mildew resistance response gene, permanently designated as Pm55, was located on chromosome arm 5VS.
Physical location of Pm55
To confirm the physical location of Pm55, the lines containing different chromosome 5V segments were evaluated for reactions to powdery mildew in five- to seven-leaf stage plants (Fig. 4). The 5V disomic addition line DA5V, T5VS·5DL translocation line NAU415, T5VS·5AL translocation line NAU421, and 5VS ditelosomic addition line NAU111 all showed high level of resistance (IT, 0–0), whereas the T5VL·W translocation line NAU122 was highly susceptible (IT, 4). The two Zhengmai 9405 intercalary translocation lines NAU5VS-4 and NAU5VS-5 (Zhang et al. 2012b) showed differential reactions. Line NAU5VS-4, which carried a FL 0.60–1.00 5VS segment, was resistant, whereas line NAU5VS-5 with a FL 0.80–1.00 5VS segment was susceptible along with the recurrent parent Zhengmai 9405. The different responses to powdery mildew indicated that the resistance gene is located in the 5VS FL 0.60–0.80 region.
Discussion
Alien gene transfer has played an important role in increasing the genetic diversity for wheat improvement. Several disease resistance genes have been introduced to hexaploid wheat from D. villosum (Qi et al. 2011; Zhang et al. 2005; Chen et al. 1995) by Robertsonian translocations, including the all-stage powdery mildew resistance gene Pm21 located on chromosome 6V. Using a CS-D. villosum chromosome 5V disomic addition line irradiated with γ-ray, we successfully developed CS-D. villosum chromosome 5V introgression lines NAU415 (T5VS·5DL), NAU421 (T5VS·5AL), NAU111 (Dt5VS), NAU122 (T5VL·W), NAU5VS-4 (T5VS-W·W), and NAU5VS-5 (T5VS-6AS·6AL). A comparative analysis of these introgression lines confirmed that a novel dominant gene for powdery mildew resistance, designated Pm55, was successfully introduced into common wheat. This gene was physically mapped in the chromosome region 5VS FL0.60–0.80.
Pm55, conferred growth-stage and tissue-specific dependent resistance. Thus, translocation lines with this gene were susceptible at seedling stage against the mixed Bgt races from Nanjing field and 24 single isolates, but showed high resistance after the 5-leaf stage. However, visual assessment showed that plenty of pathogen spores were present in glume and awn of NAU222 (T5VS·5DL) at late filling stage under Nanjing field condition, but no spores were observed in leaves and stems. In contrast, pathogen spores were not observed in glume and awn of NAU222 (T5VS·5DL) and NAU421 (T5VS·5AL) under Zhengzhou and Beijing field conditions, respectively. This may be due to the different isolates in the three places. Pm55 as a developmental-stage and tissue-specific powdery mildew resistance gene may have a different resistance mechanism from both all-growth stage and adult-plant resistance genes. A similar reaction has been reported for stripe rust resistance. Two cultivars, Otane and Advantage, with adult-plant resistance to stripe rust on the flag leaf, were susceptible in the spikes (Cromey 1989). We hypothesize that the spike and leaf of wheat may be involved in two host immunity systems. Wheat germplasm with spike infections due to presence of Pm55 might reduce the utility of this gene in wheat cultivar development. But, late growth stage epidemics of powdery mildew can result in significant yield loss and grain quality deterioration, and Pm55 could be pyramided with other slow-mildewing resistance gene(s) to develop durable resistance cultivars. Studies of field performance in adult plants in different genetic backgrounds are needed to ascertain the level of resistance conferred by Pm55.
To continue advances in wheat production while reducing the need for chemical control, new sources of disease resistance genes must be exploited. Pm55 will be of benefit to breeding programs working with powdery mildew disease. Molecular markers are powerful tools in breeding programs as they enable transfer of resistance genes without a need to perform disease tests. EST-PCR markers that target coding regions can be developed by designing primers based on EST sequences. Coding regions are more highly conserved between species, making the EST-derived markers more likely to be transferrable across taxonomic boundaries (Wang et al. 2010). This transferability is especially useful for marker development in species where whole genome sequences are not yet available, such as D. villosum. Molecular marker 5EST-237 was developed from an EST sequence of wheat homoeologous group 5 (Zhang et al. 2012b). To determine if the 730-bp band for this marker was specific for chromosome 5VS, we identified chromosome arm 5VS in segregating populations by GISH and observed a complete association between chromosome 5VS and 5EST-237. In the case of NAU421 with T5VS·5AL, the 730-bp band substituted for 5AS-specific band (750 bp) and was associated with chromosome arm 5VS in NAU421/Yangmai 15 F2 populations. Similar results were obtained in regard to the T5VS·5DL translocation line where the 730-bp 5VS band replaced the 800-bp 5DS-specific band (Zhang et al. 2015). Therefore, the 730-bp band of 5EST-237 can be used to track chromosome 5VS in a wheat genetic background. In other words, the co-dominant marker 5EST-237 can be used for marker-assisted selection of Pm55 when either NAU421 (T5VS·5AL) or NAU421 (T5VS·5DL) is used as a donor parent.
Grain endosperm texture, i.e. hardness or softness, is an important trait that determines the end-use quality of wheat flour. It is mainly controlled by the completely linked Pin genes at the distal end of chromosome 5DS (Morris and Beecher 2012). Although orthologous puroindoline genes on chromosomes 5A and 5B are not present in cultivated wheat, expressed orthologs are present in the diploid donors of the A and B genomes (Li et al. 2008) as well as in chromosome arm 5VS of D. villosum where they were designated as Dina/Dinb (Zhang et al. 2010). T5VS·5AL translocation line NAU421 simultaneously has softness loci Pina-D1a/Pinb-D1a on 5DS and Dina-V1a/Dinb-V1a on 5VS, which provides a possibility of extending the range of grain textures to a super-soft category. Therefore, the negative side of these translocations if they can be successfully exploited is that their use will be restricted to soft wheat breeding programs. However, it should be possible to mutate one or other of the softness genes to achieve hard textured derivatives if future powdery mildew resistant derivatives are proven to have acceptable agronomic performance. Both Zhengmai 9405 intercalary translocation lines NAU5VS-4 and NAU5VS-5 carry Dina-V1a/Dinb-V1a and have soft grain texture (Zhang et al. 2012b), but produce differential powdery mildew reactions. Line NAU5VS-4 was resistant, whereas line NAU5VS-5 was susceptible, suggesting the softness locus is distal to Pm55.
In conclusion, a novel powdery mildew resistance gene Pm55 was identified and physically mapped to chromosome bin 5VS FL 0.60–0.80 of D. villosum. Pm55 becomes effective after the 5-leaf stage and is present in two Robertsonian T5VS·5AL and T5VS·5DL translocation lines. In these lines Pm55 is tightly linked to the softness locus Dina/Dinb, and currently has potential only for soft wheat improvement. A co-dominant molecular marker 5EST-237 for Pm55 was developed for marker-assisted selection in breeding programs.
Author contribution statement
Conceived and designed the experiments: Ruiqi Zhang; Performed the cytogenetic experiments: Bingxiao Sun, Juan Chen and Yigao Feng; Evaluated the powdery mildew resistance: Aizhong Cao and Liping Xing; Wrote the paper: Ruiqi Zhang, Caixia Lan and Peidu Chen.
References
Cao YP, Cao AZ, Wang XE, Chen PD (2009) Screening and application of EST-based PCR markers specific to individual chromosomes of Haynaldia villosa. Acta Agron Sin 35:1–10
Chen PD, Qi LL, Zhou B, Zhang SZ, Liu DJ (1995) Development and molecular cytogenetic analysis of wheat-Haynaldia villosa 6VS/6AL translocation lines specifying resistance to powdery mildew. Theor Appl Genet 91:1125–1128
Cromey MG (1989) Infection and control of stripe rust in wheat spikes. N Z J Crop Hortic Sci 17:159–164
De Pace C, Vaccino P, Cionini G, Pasquini M, Bizzarri M, Qualset CO (2011) Dasypyrum. In: Kole C (ed) Wild crop relatives: genomic and breeding resources, cereals, vol 1, chapter 4. Springer, Heidelberg, pp 185–292
Gill BS, Friebe B, Endo TR (1991) Standard karyotype and nomenclature system for description of chromosome bands and structural aberrations in wheat (Triticum aestivum). Genome 34:830–839
Gradzielewska A (2006) The genus Dasypyrum—part 2. Dasypyrum villosum—a wild species used in wheat improvement. Euphytica 152:441–454
He RL, Chang ZJ, Yang ZJ, Yuan ZY, Liu JX, Zhan HX, Zhang XJ (2009) Inheritance and mapping of a powdery mildew resistance Pm43 introgressed from Thinopyrum intermedium into wheat. Theor Appl Genet 118:1173–1180
Li GP, Chen PD, Zhang SZ, Wang XE, He ZH, Zhang Y, Zhao H, Huang HY, Zhou XC (2007) Effects of the 6VS.6AL translocation on agronomic traits and dough properties of wheat. Euphytica 155:305–313
Li WL, Huang L, Gill BS (2008) Recurrent deletions of puroindoline genes at the grain hardness locus in four independent lineages of polyploid wheat. Plant Physiol 146:200–212
Liu C, Qi LL, Liu WX, Zhao WC, Wilson J, Friebe B, Gill BS (2011) Development of a set of compensating Triticum aestivum–Dasypyrum villosum Robertsonian translocation lines. Genome 54:836–844
Luo PG, Luo HY, Chang ZJ, Zhang HY, Zhang M, Ren ZL (2009) Characterization and chromosomal location of Pm40 in common wheat: a new gene for resistance to powdery mildew derived from Elytrigia intermedium. Theor Appl Genet 118:1059–1064
McIntosh RA, Dubcovsky J, Rogers J, Morris C, Appels R, Xia XC (2013) Catalogue of gene symbols for wheat. In: KOMUGI-integrated wheat science database at http://www.shigen.nig.ac.jp/wheat/komugi/genes/download.jsp
Morris CF, Beecher BS (2012) The distal portion of the short arm of wheat (Triticum aestivum L.) chromosome 5D controls endosperm vitreosity and grain hardness. Theor Appl Genet 125:247–254
Qi LL, Friebe B, Zhang P, Gill BS (2007) Homoeologous recombination, chromosome engineering and crop improvement. Chromosome Res 15:3–19
Qi LL, Pumphrey MO, Friebe B, Zhang P, Qian C, Bowden RL, Rouse MN, Jin Y, Gill BS (2011) A novel Robertsonian translocation event leads to transfer of a stem rust resistance gene (Sr52) effective against race Ug99 from Dasypyrum villosum into bread wheat. Theor Appl Genet 123:159–167
Shen XK, Ma LX, Zhong SF, Liu N, Zhang M, Chen WQ, Zhou YL, Li HJ, Chang ZJ, Li X, Bai GH, Zhang HY, Tan FQ, Ren ZL, Luo PG (2015) Identification and genetic mapping of the putative Thinopyrum intermedium-derived dominant powdery mildew resistance gene PmL962 on wheat chromosome arm 2BS. Theor Appl Genet 128:517–528
Wang MJ, Zhang Y, Lin ZS, Ye XG, Yuan YP, Ma W, Xin ZY (2010) Development of EST–PCR markers for Thinopyrum intermedium chromosome 2Ai#2 and their application in characterization of novel wheat-grass recombinants. Theor Appl Genet 121:1369–1380
Yildirim A, Jones SS, Murray TD (1998) Mapping a gene conferring resistance to Pseudocercosporella herpotrichoides on chromosome 4V of Dasypyrum villosum in a wheat background. Genome 41:1–6
Zhang QP, Li Q, Wang XE, Wang HY, Lang SP, Wang Y, Wang SL, Chen PD, Liu DJ (2005) Development and characterization of a Triticum aestivum–Haynaldia villosa translocation line T4VS/4DL conferring resistance to wheat spindle streak mosaic virus. Euphytica 145:317–320
Zhang RQ, Cao YP, Wang XE, Feng YG, Chen PD (2010) Development and characterization of a Triticum aestivum–D. villosum T5VS.5DL translocation line with soft grain texture. J Cereal Sci 51:220–225
Zhang JJ, Yuan HX, Zhang RQ, Xing XP, Dai JL, Niu JS, Li HL, Chen PD (2012a) Analysis of resistance to Heterodera filipjevi in Triticum aestivum–Dasypyrum villosum germplasm. Acta Agron Sin 38:1–8
Zhang RQ, Wang XE, Chen PD (2012b) Molecular and cytogenetic characterization of a small alien-segment translocation line carrying the softness genes of Haynaldia villosa. Genome 55:639–646
Zhang W, Zhang RQ, Feng YG, Bie TD, Chen PD (2013) Distribution of highly repeated DNA sequences in Haynaldia villosa and its application in the identification of alien chromatin. Chin Sci Bull 58:890–897
Zhang H, Li GR, Zhang XJ, Li X, Guo HJ, Gong WP, Jia JQ, Qiao LY, Ren YK, Yang ZJ, Chang ZJ (2014a) Chromosomal location and comparative genomics analysis of powdery mildew resistance gene Pm51 in a putative wheat-Thinopyrum ponticum introgression line. PLoS One 9(11):e113455. doi:10.1371/journal.pone.0113455
Zhang RQ, Zhang MY, Wang XE, Chen PD (2014b) Introduction of chromosome segment carrying the seed storage protein genes from chromosome 1V of Dasypyrum villosum showed positive effect on bread-making quality of common wheat. Theor Appl Genet 127:523–533
Zhang RQ, Hou F, Feng YG, Zhang W, Zhang MY, Chen PD (2015) Characterization of a Triticum aestivum–Dasypyrum villosum T2VS·2DL translocation line expressing a longer spike and more kernels traits. Theor Appl Genet 128:2415–2425
Zhou YL, Duan XY, Chen G, Sheng BQ, Zhang Y (2002) Analyses of resistance genes of 40 wheat cultivars or lines to wheat powdery mildew. Acta Phytopathol Sin 32:301–305
Acknowledgments
This study was supported by National Natural Science Foundation of China (No. 30871519). We thank Prof. Bob McIntosh, Plant Breeding Institute, University of Sydney, Cobbitty, NSW, Australia, for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have no conflicts of interest and agree with publication.
Additional information
Communicated by S. S. Xu.
Rights and permissions
About this article
Cite this article
Zhang, R., Sun, B., Chen, J. et al. Pm55, a developmental-stage and tissue-specific powdery mildew resistance gene introgressed from Dasypyrum villosum into common wheat. Theor Appl Genet 129, 1975–1984 (2016). https://doi.org/10.1007/s00122-016-2753-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-016-2753-8