Abstract
Although reduced blood lactate concentrations ([lac−]B) have been observed during whole-body exercise following inspiratory muscle training (IMT), it remains unknown whether the inspiratory muscles are the source of at least part of this reduction. To investigate this, we tested the hypothesis that IMT would attenuate the increase in [lac−]B caused by mimicking, at rest, the breathing pattern observed during high-intensity exercise. Twenty-two physically active males were matched for 85% maximal exercise minute ventilation \( \left( {\dot V_{{\text{E}}} \max } \right) \) and divided equally into an IMT or a control group. Prior to and following a 6 week intervention, participants performed 10 min of volitional hyperpnoea at the breathing pattern commensurate with 85% \( \dot V_{{\text{E}}} \max . \) The IMT group performed 6 weeks of pressure-threshold IMT; the control group performed no IMT. Maximal inspiratory mouth pressure increased (mean ± SD) 31 ± 22% following IMT and was unchanged in the control group. Prior to the intervention in the control group, [lac−]B increased from 0.76 ± 0.24 mmol L−1 at rest to 1.50 ± 0.60 mmol L−1 (P < 0.05) following 10 min volitional hyperpnoea. In the IMT group, [lac−]B increased from 0.85 ± 0.40 mmol L−1 at rest to 2.02 ± 0.85 mmol L−1 following 10 min volitional hyperpnoea (P < 0.05). After 6 weeks, increases in [lac−]B during volitional hyperpnoea were unchanged in the control group. Conversely, following IMT the increase in [lac−]B during volitional hyperpnoea was reduced by 17 ± 37% and 25 ± 34% following 8 and 10 min, respectively (P < 0.05). In conclusion, increases in [lac−]B during volitional hyperpnoea at 85% \( \dot V_{{\text{E}}} \max \) were attenuated following IMT. These findings suggest that the inspiratory muscles were the source of at least part of this reduction, and provide a possible explanation for some of the IMT-mediated reductions in [lac−]B, often observed during whole-body exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Specific respiratory muscle training (RMT) can be performed using voluntary isocapnic hyperpnoea (VIH), flow-resistive loading, or pressure-threshold loading; with the exception of VIH, these are commonly referred to as inspiratory muscle training (IMT). Ventilatory endurance is enhanced with all three techniques, whereas IMT also increases diaphragm thickness (Downey et al. 2007; Enright et al. 2006) and the maximal strength, shortening velocity and power of the inspiratory muscles (for a full review see McConnell and Romer 2004). Furthermore, well controlled studies have shown improvements in endurance exercise performance following both IMT (Gething et al. 2004; Griffiths and McConnell 2007; Johnson et al. 2007; Romer et al. 2002a; Volianitis et al. 2001) and VIH (Leddy et al. 2007).
The mechanisms underlying such performance improvements remain speculative but may include reduced perception of effort (Downey et al. 2007; Gething et al. 2004; Griffiths and McConnell 2007; Romer et al. 2002a; Verges et al. 2007; Volianitis et al. 2001) and possibly reductions in both diaphragm fatigue (Verges et al. 2007) and an associated metaboreflex that attenuates limb blood flow (McConnell and Lomax 2006; Witt et al. 2007). The notion that genuine physiological adaptation explains, in part, RMT-mediated improvements in endurance exercise performance is further supported by the frequently observed reduction in blood lactate concentration ([lac−]B) during whole-body exercise following both IMT (Griffiths and McConnell 2007; McConnell and Sharpe 2005; Romer et al. 2002b; Volianitis et al. 2001) and VIH (Leddy et al. 2007; Spengler et al. 1999). Furthermore, correlations have been reported between reductions in [lac−]B and performance improvements following RMT (Romer et al. 2002b; Spengler et al. 1999), with up to 52% of the variation in performance being attributed to the reduced [lac−]B (Romer et al. 2002b).
The mechanism(s) by which RMT reduces [lac−]B remains equivocal. An RMT-mediated change in minute ventilation \( \left( {\dot V_{{\text{E}}} } \right), \) which may conceivably alter both the work of breathing and acid base balance, is an unlikely mechanism since reductions in [lac−]B following RMT have been observed irrespective of whether \( \dot V_{{\text{E}}} \) is lower (Leddy et al. 2007), unchanged (McConnell and Sharpe 2005; Spengler et al. 1999; Volianitis et al. 2001), or increased (Kohl et al. 1997). The concept that RMT-mediated respiratory muscle adaptations explain, in part, that the reductions observed in [lac−]B remains contentious: the small size of these muscles and observations, that loading and unloading of the respiratory muscles during exercise fails to influence systemic [lac−]B, argue against this premise (Wetter and Dempsey 2000). However, volitional hyperpnoea increases [lac−]B both at rest (Martin et al. 1984; Verges et al. 2007) and during exercise (Johnson et al. 2006) suggesting that the respiratory muscles are capable of net lactate release. Furthermore, VIH appears to attenuate such net release during volitional hyperpnoea (Verges et al. 2007). However, this study did not rigorously control isocapnia that is essential for the interpretation of changes in [lac−]B. Also, the use of a breathing challenge based on maximum voluntary ventilation (MVV) limits external validity as both the breathing pattern and work of breathing are unreflective of that seen during exercise (Coast et al. 1993). Since many of the muscle adaptations associated with endurance-orientated training (i.e. VIH) are different from those associated with strength-orientated training (i.e. IMT), it also remains uncertain whether IMT would reduce [lac−]B during volitional hyperpnoea.
Therefore, to investigate this issue further the present study examined the hypothesis that 6 weeks of IMT would attenuate the increase in [lac−]B caused by mimicking, at rest, the breathing pattern observed during high-intensity endurance exercise.
Methods
Subjects
Following approval from Nottingham Trent University’s ethics committee, 22 non-smoking, recreationally active males provided written informed consent to participate in the study. Throughout the study, subjects were instructed to adhere to their usual training regimen and not to engage in strenuous exercise the day before test days, during which subjects refrained from ingesting caffeine and arrived at the laboratory 2 h post-prandial. Descriptive characteristics of the subjects are presented in Table 1.
Experimental procedure
Baseline pulmonary function and maximal inspiratory mouth pressure (MIP) were measured during the first laboratory visit. On subsequent visits separated by at least 48 h, subjects performed a maximal incremental cycling test, and two 10 min isocapnic volitional hyperpnoea tests (the first being a familiarisation test). The volitional hyperpnoea tests were performed at the \( \dot V_{{\text{E}}} , \) tidal volume (V T), breathing frequency (f R) and duty cycle (T I/T TOT) associated with 85% maximal exercise \( \dot V_{{\text{E}}} \left( {\dot V_{{\text{E}}} \max } \right). \) During volitional hyperpnoea tests, blood samples were taken every 2 min from 0 to 10 min, inclusive, and respiratory variables were measured breath by breath and averaged over 2 min intervals. Subjects were subsequently matched for 85% \( \dot V_{{\text{E}}} \max \) and divided into an IMT group (n = 11) or a control (no IMT) group (n = 11). Not more than a week, following a 6 week intervention MIP was measured and at least 48 h following this, subjects repeated the volitional hyperpnoea test. Each subject completed a 24 h diet record prior to the criterion pre-intervention volitional hyperpnoea test and this was then replicated during the 24 h prior to the post-intervention volitional hyperpnoea test.
Pulmonary function, maximal inspiratory pressure, and respiratory measurements
Pulmonary function was assessed using a pneumotachograph (ZAN 600USB, Nspire Health, Oberthulba, Germany), calibrated using a 3-L syringe. Each measurement was repeated three times and the highest recorded value was used for subsequent analysis (Quanjer et al. 1993). A hand-held mouth pressure metre (Ferraris Respiratory Europe, Hertford, UK) measured MIP as an index of global inspiratory muscle strength. The mouthpiece assembly incorporated a 1 mm orifice to prevent glottic closure during inspiratory efforts. Manoeuvres were performed in an upright standing posture, were initiated from residual volume, and sustained for at least 1 s. Repeat measurements separated by 30 s were taken until three values within 5 cmH2O of each other were produced (McConnell 2007). The highest recorded value was used for subsequent analysis. Throughout the maximal exercise test and volitional hyperpnoea, subjects wore a facemask (model 7940, Hans Rudolph, Kansas City, Missouri) connected to a pneumotachograph and respiratory variables were measured breath by breath (ZAN 600USB, Nspire Health, Oberthulba, Germany). During volitional hyperpnoea tests, a two-way non-rebreathing valve (model 2730, Hans Rudolph, Kansas City, Missouri) and a 1.5 m length of corrugated tubing was attached distally to the pneumotachograph allowing additional CO2 to be added to the inspirate.
Blood sampling and analysis
Arterialised venous blood was sampled from a dorsal hand vein via an indwelling cannula (Forster et al. 1972; McLoughlin et al. 1992). Arterialisation was ensured by immersing the hand in water at ~40°C for 10 min prior to cannulation and by warming the hand during volitional hyperpnoea tests using an infrared lamp. Blood samples were drawn into a 2 ml pre-heparinised syringe (PICO 50, Radiometer, Copenhagen, Denmark) and analysed immediately for blood gases (ABL520, Radiometer, Copenhagen, Denmark), including the partial pressure of carbon dioxide (PCO2) and pH, and [lac−]B (Biosen C_line Sport, EKF Diagnostics, Barleben, Germany). Plasma bicarbonate concentration ([HCO3 −]) was calculated from PCO2 and pH values using the Henderson Hasselbalch equation:
[HCO3 −] was then subsequently incorporated into the Siggaard–Anderson equation to calculate base excess of the extracellular fluid (BEECF) (Siggaard-Anderson and Fogh-Anderson 1995):
Maximal exercise test
Subjects performed a maximal incremental cycling test on an electromagnetically-braked cycle ergometer (Excalibur Sport, Lode, Groningen, The Netherlands). Cycling began at 0 W and power was subsequently increased by 10 W every 15 s in order to result in exercise intolerance within ~10 min. This rapid incremental protocol was selected to maximise \( \dot V_{{\text{E}}} \) at the cessation of the test and therefore reflect intense endurance exercise. The power at which exercise intolerance ensued defined maximal power output \( \left( {\dot W{ \max }} \right), \) and the highest oxygen uptake, \( \left( {\dot V{\text{O}}_{ 2} } \right) \) and \( \dot V_{{\text{E}}} , \) recorded in any 30 s period defined \( \dot V{\text{O}}_{ 2} \)max and \( \dot V_{{\text{E}}} \max , \) respectively.
Volitional hyperpnoea
Volitional hyperpnoea was performed whilst seated on the cycle ergometer in an body position identical to that adopted during the maximal exercise test. Subjects were instructed to increase \( \dot V_{{\text{E}}} \) and f R in a square wave manner to a level commensurate with 85% \( \dot V_{{\text{E}}} \max , \) which during pilot work was shown to represent the maximum square wave response that could be maintained for 10 min. An audio metronome paced f R and real-time visual feedback of \( \dot V_{{\text{E}}} \) was provided throughout the test. The prescribed breathing pattern (\( \dot V_{{\text{E}}} \), V T, f R and T I/T TOT) during volitional hyperpnoea was identical pre- and post-intervention and was chosen to provide a breathing challenge reflective of the work of breathing associated with exercise hyperpnoea. This methodology is deemed superior to an arbitrary %MVV as it more closely reflects the work of breathing during whole-body exercise: for a given \( \dot V_{{\text{E}}} \) greater than approximately 60 L min−1 the work of breathing of exercise hyperpnoea can be overestimated by as much as 25% when a spontaneous breathing pattern is adopted during volitional hyperpnoea (Coast et al. 1993). Isocapnia was maintained during volitional hyperpnoea by adding CO2 into the inspiratory circuit in order to maintain resting PCO2.
Intervention
IMT was performed using an inspiratory pressure-threshold device (POWERbreathe®, Gaiam, UK). The IMT group performed 30 dynamic inspiratory efforts twice daily for 6 weeks against a pressure-threshold load of ~50% MIP. Thereafter, subjects periodically increased the load to a level that would permit them to only just complete 30 manoeuvres. Each inspiratory manoeuvre was initiated from residual volume and subjects strove to maximise V T. This protocol is known to be effective in eliciting an adaptive response (Johnson et al. 2007; McConnell and Lomax 2006; McConnell and Sharpe 2005; Romer et al. 2002a, b; Volianitis et al. 2001). Subjects completed a training diary to record IMT adherence and habitual training, which the control group also recorded. The control group did not perform sham IMT since the duration of the volitional hyperpnoea test and breathing pattern employed was identical pre- and post-intervention, thus responses would not be influenced by either motivation or expectation.
Statistical analyses
Statistical analyses were performed using SPSS for Windows (SPSS, Chicago, Illinois, USA). Within group changes over time during volitional hyperpnoea were determined using one-way ANOVA for repeated measures and Tukey’s HSD post hoc analysis. Within and between group interaction effects were determined using two-way ANOVA for repeated measures. Pearson product-moment correlation coefficients were calculated to assess the relationship between selected variables. Statistical significance was set at P ≤ 0.05. Results are presented as mean ± SD.
Results
Pulmonary function and maximal inspiratory pressure
Baseline pulmonary function and MIP were all within normal limits (Table 1). The IMT group demonstrated excellent training compliance (91% adherence) and subjects’ habitual training remained unchanged in both IMT and control groups. MIP increased from 147 ± 27 to 189 ± 27 cmH2O (+31 ± 22%) following IMT (P < 0.01). No change was observed in the control group (pre- vs. post-: 163 ± 19 vs. 166 ± 20 cmH2O).
Responses to volitional hyperpnoea
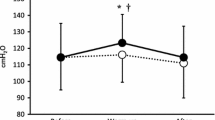
Group mean values for ventilatory and acid base responses to 10 min volitional hyperpnoea, pre- and post-intervention are shown in Table 2. Before and after the intervention, \( \dot V_{{\text{E}}} \), V T, f R, T I/T TOT and measures of acid base balance were not different between groups and remained unchanged over time during volitional hyperpnoea. The mean \( \dot V_{{\text{E}}} \) during volitional hyperpnoea represented 72 ± 8 and 81 ± 19% of MVV10 in control and IMT groups, respectively. PCO2 was maintained at resting levels throughout volitional hyperpnoea, prior to and following the intervention and was not different between groups (Fig. 1).
Prior to the intervention, significant increases in [lac−]B above rest were observed following 10 min of volitional hyperpnoea in IMT and control groups (P < 0.05) (Fig. 2) and such changes were not different between the groups. Following the intervention, the [lac−]B response to volitional hyperpnoea was unchanged in the control group. Conversely, [lac−]B during volitional hyperpnoea was reduced following IMT with 17 ± 37 and 25 ± 34% reductions being observed at 8 and 10 min, respectively (P < 0.05). These reductions exceeded changes observed in the control group (P < 0.05).
Correlations amongst variables
Prior to the intervention, increases in [lac−]B during volitional hyperpnoea did not correlate with any measure of pulmonary function, MIP, endurance training status \( \left( {\dot V{\text{O}}_{2} \max ,\,\dot W\max } \right), \) or ventilatory responses to volitional hyperpnoea. Increases in [lac−]B during volitional hyperpnoea did not correlate with absolute \( \dot V_{{\text{E}}} \) nor when expressed as %MVV. The attenuated increase in [lac−]B during volitional hyperpnoea after IMT was not correlated with increases in MIP. However, baseline MIP was negatively correlated with relative IMT-induced increases in MIP (r = −0.70, P < 0.05).
Discussion
The main finding of this study was that, 10 min of volitional hyperpnoea approximately doubled resting [lac−]B, and that 6 weeks of pressure-threshold IMT attenuated this increase by 25%. These findings strongly support the notion that the respiratory muscles are capable of increasing [lac−]B and are the first to show that this can be attenuated through specific IMT. This observation may help to explain some of the IMT-mediated reductions in [lac−]B, previously observed during whole-body exercise.
We report an increased [lac−]B of 0.96 ± 0.58 mmol L−1 (n = 22; range: 0.20–2.50 mmol L−1) from rest during 10 min of intense volitional hyperpnoea at 85% \( \dot V_{{\text{E}}} \max \) (130.7 ± 19.7 L min−1, 77 ± 15% MVV10; n = 22). Comparable increases in [lac−]B have been reported whilst breathing at similar (72% MVV, Martin et al. 1984; 70% MVV, Verges et al. 2007), but not at lower (62% MVV, Spengler et al. 2000), relative intensities. Therefore it is apparent that when \( \dot V_{{\text{E}}} \) surpasses a certain level, the respiratory muscles are capable of net lactate release. However, the potential for respiratory alkalosis to elevate [lac−]B is well documented (Davies et al. 1986; LeBlanc et al. 2002). Consequently, we were careful to maintain, with considerable accuracy, resting PCO2 throughout the 10 min of volitional hyperpnoea (see Fig. 1). Other measures of acid base status also remained unchanged from rest during volitional hyperpnoea in both the groups, pre- and post-intervention. We are thus confident that the increase in [lac−]B during volitional hyperpnoea was a consequence of increased lactate efflux from the respiratory muscles rather than respiratory alkalosis.
The attenuated increase in [lac−]B during volitional hyperpnoea following IMT is similar to that observed in healthy subjects performing an exhaustive respiratory endurance test at ~70% MVV following VIH training, although this reduction did not exceed that of a control group (Verges et al. 2007). Given the aforementioned importance of maintaining isocapnia, it is also unfortunate that end-tidal CO2 and/or PCO2 was not controlled during the respiratory endurance test. Furthermore, subjects were prescribed a pre-determined arbitrary breathing pattern which has previously received criticism for failing to accurately represent the work of breathing during exercise hyperpnoea (Coast et al. 1993). Notwithstanding this, VIH- and IMT-mediated reductions in [lac−]B observed during volitional hyperpnoea are similar to the reductions often observed during submaximal, whole-body exercise (Griffiths and McConnell 2007; Leddy et al. 2007; McConnell and Sharpe 2005; Romer et al. 2002b; Spengler et al. 1999; Volianitis et al. 2001); however, whether these observations during volitional hyperpnoea and exercise share a common mechanistic explanation is unclear.
RMT-mediated reductions in [lac−]B, occur (e.g. see Leddy et al. 2007; McConnell and Sharpe 2005; Spengler et al. 1999; Volianitis et al. 2001) when net lactate production from the respiratory muscles is probably negligible given the relatively low \( \dot V_{{\text{E}}} \) and minimal activation of less efficient accessory muscles (Martin et al. 1984; Johnson et al. 2006). Hence, under such conditions it seems more likely that reductions in [lac−]B result from increased uptake and metabolism of lactate by the trained respiratory muscles (Griffiths and McConnell 2007; Spengler et al. 1999) rather than a decrease in net lactate release. Conversely, during high-intensity exercise where \( \dot V_{{\text{E}}} \) relative to MVV, approaches/exceeds levels achieved in the breathing challenge of this study (e.g. see Edwards and Cooke 2004; Kohl et al. 1997; Spengler et al. 1999), it is possible that RMT-mediated respiratory muscle adaptation contributes to lowering [lac−]B through affecting both lactate clearance by and efflux from the trained respiratory muscles.
The plasticity of the inspiratory muscles has been well documented (McConnell and Romer 2004; Powers et al. 1997). It is thus attractive to suggest that changes in inspiratory muscle morphology may explain, in part, the attenuated hyperpnoea-mediated increase in [lac−]B following IMT. An approximate 10% increase in diaphragm thickness (Downey et al. 2007; Enright et al. 2006), and a 21% increase in the size of type II muscle fibres in the external intercostal muscles (Ramírez-Sarmiento et al. 2002), has been reported following 6 and 5 weeks of IMT, respectively. Increasing inspiratory muscle fibre cross-sectional area and subsequent strength decreases the relative intensity for a given absolute work load, which may reduce/delay fast twitch fibre recruitment and thus lactate production (Marcinik et al. 1991). A decrease in the relative workload per muscle fibre may also decrease blood flow occlusion, which may influence lactate production and/or clearance (Marcinik et al. 1991).
Increased muscle monocarboxylate transport (MCT) protein content, which facilitates inter- and intra-cellular lactate shuttling in sarcolemmal and mitochondrial membranes, respectively (Brooks et al. 1999; Dubouchaud et al. 2000), has been reported following endurance (Baker et al. 1998; Burgomaster et al. 2007) and strength (Juel et al. 2004) based training regimens. It is thus possible (cf. McConnell and Sharpe 2005) that similar adaptations would occur in the respiratory muscles following both IMT (strength-orientated) and VIH (endurance-orientated) training and may explain, in part, the decrease in [lac−]B observed during whole-body exercise and volitional hyperpnoea following these dissimilar training stimuli.
Finally, the attenuated [lac−]B response to volitional hyperpnoea following IMT (and VIH training) may also reside in a training-induced increase in the oxidative capacity of the inspiratory muscles. In support of this notion, Ramírez-Sarmiento et al. (2002) reported a 38% increase in the number of type I muscle fibres in the external intercostals following 5 weeks IMT. Moderate intensity, high repetition strength training, similar to the IMT protocol used in the present study, can increase oxidative enzyme activity (Costill et al. 1979; Sale et al. 1990) thereby reducing net lactate production (Holloszy and Coyle 1984). Since similar oxidative adaptations would be expected to occur following VIH (endurance-orientated) training (Holloszy and Coyle 1984), this also offers an attractive explanation for the decrease in [lac−]B observed during whole body exercise (Griffiths and McConnell 2007; Kohl et al. 1997; Leddy et al. 2007; McConnell and Sharpe 2005; Romer et al. 2002b; Spengler et al. 1999; Volianitis et al. 2001) and volitional hyperpnoea (present study; Verges et al. 2007).
Conclusions
In summary, the present study provides novel evidence that increases in [lac−]B when mimicking the breathing pattern observed during heavy exercise can be attenuated following IMT. These data suggest that the inspiratory muscles were the source of at least part of this reduction, and provide a possible explanation for at least some of the IMT-mediated reductions in [lac−]B, previously observed during whole-body exercise. The precise mechanisms that underpin these changes remain unknown, but an IMT-mediated increase in the oxidative and/or lactate transport capacity of the inspiratory muscles is an attractive possibility that merits further investigation.
References
Baker SK, McCullagh KJ, Bonen A (1998) Training intensity-dependent and tissue-specific increases in lactate uptake and MCT-1 in heart and muscle. J Appl Physiol 84:987–994
Brooks GA, Brown M, Butz CE et al (1999) Cardiac and skeletal muscle mitochondria have a monocarboxylate transporter MCT1. J Appl Physiol 87:1713–1718
Burgomaster KA, Cermak NM, Phillips SM et al (2007) Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am J Physiol Regul Integr Comp Physiol 292:R1970–R1976
Coast JR, Rasmussen SA, Krause KM et al (1993) Ventilatory work and oxygen consumption during exercise and hyperventilation. J Appl Physiol 74:793–798
Costill DL, Coyle EF, Fink WF et al (1979) Adaptations in skeletal muscle following strength training. J Appl Physiol 46:96–99
Davies SF, Iber C, Keene SA et al (1986) Effect of respiratory alkalosis during exercise on blood lactate. J Appl Physiol 61:948–952
Downey AE, Chenoweth LM, Townsend DK et al (2007) Effects of inspiratory muscle training on exercise responses in normoxia and hypoxia. Respir Physiol Neurobiol 156:137–146
Dubouchaud H, Butterfield GE, Wolfel EE et al (2000) Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab 278:E571–E579
Edwards AM, Cooke CB (2004) Oxygen uptake kinetics and maximal aerobic power are unaffected by inspiratory muscle training in healthy subjects where time to exhaustion is extended. Eur J Appl Physiol 93:139–144
Enright SJ, Unnithan VB, Heward C et al (2006) Effect of high-intensity inspiratory muscle training on lung volumes, diaphragm thickness, and exercise capacity in subjects who are healthy. Phys Ther 86:345–454
Forster HV, Dempsey JA, Thomson J et al (1972) Estimation of arterial PO2, PCO2, pH, and lactate from arterialized venous blood. J Appl Physiol 32:134–137
Gething AD, Williams M, Davies B (2004) Inspiratory resistive loading improves cycling capacity: a placebo controlled trial. Br J Sports Med 38:730–736
Griffiths LA, McConnell AK (2007) The influence of inspiratory and expiratory muscle training upon rowing performance. Eur J Appl Physiol 99:457–466
Holloszy JO, Coyle EF (1984) Adaptations of skeletal muscles to endurance exercise and their metabolic consequences. J Appl Physiol 56:831–838
Johnson MA, Sharpe GR, McConnell AK (2006) Maximal voluntary hyperpnoea increases blood lactate concentration during exercise. Eur J Appl Physiol 96:600–608
Johnson MA, Sharpe GR, Brown PI (2007) Inspiratory muscle training improves cycling time trial performance and anaerobic work capacity but not critical power. Eur J Appl Physiol 101:761–770
Juel C, Holten MK, Dela F (2004) Effects of strength training on muscle lactate release and MCT1 and MCT4 content in healthy and type 2 diabetic humans. J Physiol 556:297–304
Kohl J, Koller EA, Brandenberger M et al (1997) Effect of exercise-induced hyperventilation on airway resistance and cycling endurance. Eur J Appl Physiol 75:305–311
LeBlanc PJ, Parolin ML, Jones NL et al (2002) Effects of respiratory alkalosis on human skeletal muscle metabolism at the onset of submaximal exercise. J Physiol 544:303–313
Leddy JJ, Limprasertkul A, Patel S et al (2007) Isocapnic hyperpnea training improves performance in competitive male runners. Eur J Appl Physiol 99:556–676
Marcinik EJ, Potts J, Schlabach G et al (1991) Effects of strength training on lactate threshold and endurance performance. Med Sci Sports Exerc 23:739–743
Martin BJ, Chen HI, Kolka MA (1984) Anaerobic metabolism of the respiratory muscles during exercise. Med Sci Sports Exerc 16:82–86
McConnell AK (2007) Lung and respiratory muscle function. In: Winter EM, Jones AM, Davison RCR et al. (eds) Sport and exercise physiology testing guidelines, the British Association of Sport and Exercise Sciences Guide. Routledge, Oxford, UK
McConnell AK, Lomax M (2006) The influence of inspiratory muscle work history and specific inspiratory muscle training upon human limb muscle fatigue. J Physiol 577:445–457
McConnell AK, Romer LM (2004) Respiratory muscle training in healthy humans: resolving the controversy. Int J Sports Med 25:284–293
McConnell AK, Sharpe GR (2005) The effect of inspiratory muscle training upon maximum lactate steady-state and blood lactate concentration. Eur J Appl Physiol 94:277–284
McLoughlin P, Popham P, Linton RA et al (1992) Use of arterialized venous blood sampling during incremental exercise tests. J Appl Physiol 73:937–940
Powers SK, Coombes J, Demirel H (1997) Exercise training-induced changes in respiratory muscles. Sports Med 24:120–131
Quanjer PH, Tammeling GJ, Cotes JE et al (1993) Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, European community for steel and coal. Official statement of the European respiratory society. Eur Respir J 16:5–40
Ramírez-Sarmiento A, Orozco-Levi M, Güell R et al (2002) Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. Am J Respir Crit Care Med 166:1491–1497
Romer LM, McConnell AK, Jones DA (2002a) Effects of inspiratory muscle training on time-trial performance in trained cyclists. J Sports Sci 20:547–562
Romer LM, McConnell AK, Jones DA (2002b) Effects of inspiratory muscle training upon recovery time during high-intensity, repetitive sprint activity. Int J Sports Med 23:353–360
Sale DG, Macdougall JI, Garner S (1990) Interaction between strength and endurance training. J Appl Physiol 68:260–270
Siggaard-Anderson O, Fogh-Anderson N (1995) Base excess or buffer base (strong ion difference) as a measure of non-respiratory acid-base disturbance. Acta Anaesthesiol Scand 107:267–271
Spengler CM, Roos M, Laube SM et al (1999) Decreased exercise blood lactate concentrations after respiratory endurance training in humans. Eur J Appl Physiol 79:299–305
Spengler CM, Knöpfli-Lenzin C, Birchler K et al (2000) Breathing pattern and exercise endurance time after exhausting cycling or breathing. Eur J Appl Physiol 81:368–374
Verges S, Lenherr O, Haner AC et al (2007) Increased fatigue resistance of respiratory muscles during exercise after respiratory muscle endurance training. Am J Physiol Regul Integr Comp Physiol 292:R1246–R1253
Volianitis S, McConnell AK, Koutedakis Y et al (2001) Inspiratory muscle training improves rowing performance. Med Sci Sports Exerc 33:803–809
Wetter TJ, Dempsey JA (2000) Pulmonary system and endurance exercise. In: Shephard RJ, Astrand P-O (eds) Endurance in Sport. Blackwell Science, London, UK
Wilson SH, Cooke NT, Edwards RHT et al (1984) Predicted normal values for maximal respiratory pressures in Caucasian adults and children. Thorax 39:535–538
Witt JD, Guenette JA, Rupert JL et al (2007) Inspiratory muscle training attenuates the human respiratory muscle metaboreflex. J Physiol 584:1019–1028
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brown, P.I., Sharpe, G.R. & Johnson, M.A. Inspiratory muscle training reduces blood lactate concentration during volitional hyperpnoea. Eur J Appl Physiol 104, 111–117 (2008). https://doi.org/10.1007/s00421-008-0794-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-008-0794-7