Abstract
Purpose
Hyperventilation, implemented during recovery of repeated maximal sprints, has been shown to attenuate performance decrement. This study evaluated the effects of hyperventilation, using strength exercises, on muscle torque output and EMG amplitude.
Methods
Fifteen power-trained athletes underwent maximal isokinetic knee extensions consisting of 12 repetitions × 8 sets at 60°/s and 25 repetitions × 8 sets at 300°/s. The inter-set interval was 40 s for both speeds. For the control condition, subjects breathed spontaneously during the interval period. For the hyperventilation condition, subjects hyperventilated for 30 s before each exercise set (50 breaths/min, PETCO2: 20–25 mmHg). EMG was recorded from the vastus medialis and lateralis muscles to calculate the mean amplitude for each contraction.
Results
Hyperventilation increased blood pH by 0.065–0.081 and lowered PCO2 by 8.3–10.3 mmHg from the control values (P < 0.001). Peak torque declined with repetition and set numbers for both speeds (P < 0.001), but the declining patterns were similar between conditions. A significant, but small enhancement in peak torque was observed with hyperventilation at 60°/s during the initial repetition phase of the first (P = 0.032) and fourth sets (P = 0.040). EMG amplitude also declined with set number (P < 0.001) for both speeds and muscles, which was, however, not attenuated by hyperventilation.
Conclusion
Despite a minor ergogenic effect in peak torque at 60°/s, hyperventilation was not effective in attenuating the decrement in torque output at 300°/s and decrement in EMG amplitude at both speeds during repeated sets of maximal isokinetic knee extensions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During intense activities, the accumulation of hydrogen ions (H+) and resulting fall in pH have been considered major factors contributing to muscle fatigue, although complex interplays among other metabolic by-products and central mechanisms may coexist (MacLaren et al. 1989; Green 1997). The proposed mechanisms of performance decrement under intramuscular acidic milieu include reduced activities of glycolytic enzymes (e.g., phosphofructokinase) with compromised glycolytic energy supply (Ui 1966; McCartney et al. 1986) and impaired excitation/contraction mechanisms (Fuchs et al. 1970; Nakamaru and Schwartz 1970; Orchardson 1978; Green 1997).
Over the last several decades, conflicting results have been reported, depending on exercise modes, for the efficacy of sodium bicarbonate (NaHCO3)-induced metabolic alkalosis that may delay performance decrement by reversing the unfavorable effects of acidosis (Lavender and Bird 1989; Webster et al. 1993; Kozak-Collins et al. 1994; Bishop and Claudius 2005). These inconsistent reports may be ascribed to concomitant negative hemodynamic effects of alkalosis, namely reduction in blood flow (through vasoconstriction or precluded vasodilation) and in diffusion of O2 into active muscles (resulting from the left shift of oxyhemoglobin dissociation curve) (Chin et al. 2007; Forbes et al. 2007). The ergogenic aid of metabolic alkalosis, therefore, becomes less promising for exercise tasks that rely considerably on aerobic energy metabolism (Kozak-Collins et al. 1994). The exercise tasks that have most consistently gained benefits from the ingestion of NaHCO3 are intermittent activities such as repeated short sprints performed especially by highly fit individuals, where a substantial whole-body anaerobic metabolic challenge is incurred (Lavender and Bird 1989; Webster et al. 1993; Bishop et al. 2004; Bishop and Claudius 2005). The benefits of NaHCO3 ingestion are, however, debatable given the adverse gastrointestinal effects (bloating and retching) (Kozak-Collins et al. 1994) and controversies over optimal dosage and timing (Webster et al. 1993; McNaughton et al. 1999).
Hyperventilation is an alternative method to induce body alkalosis (respiratory alkalosis). Previous studies of hyperventilation, implemented before and/or during exercise, have yielded no ergogenic effects or impaired performance because the exercise tests consisted of a single bout of maximal exercise or long-lasting aerobic exercise, which were unsuitable to investigate the buffering effects (Davies et al. 1986; Morrow et al. 1988; Walsh et al. 2006). Studies of hyperventilation implemented intermittently during recovery of repeated short bouts of exercise have been limited (Hilbert et al. 2012; Sakamoto et al. 2014). Hilbert et al. (2012) examined the influence of respiratory alkalosis via hyperventilation on muscle performance of untrained subjects during intermittent hand grip exercise at maximum speed and frequency (15 s × 10 sets with 45 s recovery interval). Despite stabilized magnitude of the compound action potentials (M-wave), hyperventilation implemented prior to the first set of exercise and throughout each of the recovery intervals produced no improvement in muscle performance compared to spontaneous breathing (control). In contrast, Sakamoto et al. (2014) reported performance improvement when a 30 s hyperventilation was implemented during the last half of a 60 s recovery before each set of 10 s standing sprint pedaling in highly trained athletes. Hyperventilation, as verified by the fall in blood PCO2 and rise in pH, significantly attenuated the decrement in power output. The improved performance was attributed to an enhanced anaerobic energy supply via PCr and glycolysis (Ui 1966; Sahlin et al. 1979; Davies et al. 1986; Lindinger et al. 1990; McMahon and Jenkins 2002; Forbes et al. 2007) and delayed failure in excitation/contraction coupling (Fuchs et al. 1970; Nakamaru and Schwartz 1970; Orchardson 1978; Green 1997) through the accelerated buffering effects. Given the substantial whole-body metabolic challenge during the exercise task, the authors proposed the contribution of the “Setchenov phenomenon,” where the volitional activation of respiratory muscles acted as a diverting activity that countered the development of central fatigue (Asmussen 1979; MacLaren et al. 1989). As hyperventilation does not require an exogenous agent and can be initiated and terminated instantly without major adverse effects, unless implemented excessively (paresthesia and dizziness may result from reduced blood flow to the periphery and the brain), the study of Sakamoto et al. (2014) has demonstrated hyperventilation as a potential replacement for NaHCO3 ingestion.
Nonetheless, the study of hyperventilation warrants further investigations to identify exercise modes and conditions under which hyperventilation would be efficacious, as has been undertaken for metabolic alkalosis research. The current novel proposal extends our previous work on sprint pedaling (Sakamoto et al. 2014) to a strength-oriented exercise, repeated maximal isokinetic knee extensions, by targeting a larger muscle group than the finger flexors (Hilbert et al. 2012). Assuming that the recruitment of motor units in the active muscles is near maximum during contractions at maximal isokinetic effort, EMG amplitude would be expected to gradually fall as the exercise is repeated over time. The decline in EMG amplitude during maximal effort has been well documented and ascribed to the peripheral excitation–transmission failure and/or central fatigue depending on the types of contraction (Kawakami et al. 2000; Nordlund et al. 2004). The decline in EMG amplitude, however, may be countered by hyperventilation, since hyperventilation increases the neuronal and muscle excitabilities at rest or prevents the fall in excitability during exercise (Katz and Wolf 1964; Seyal et al. 1998; Hilbert et al. 2012). Moreover, if the contribution of the Setchenov phenomenon was true, the reduction in central command outflow associated with perceived exertion would be attenuated, which in turn attenuates the EMG amplitude decline.
Taken together, this study aimed to document the effect of hyperventilation, performed before each set of exercise during the recovery period, on the decrements in torque output and EMG amplitude over repeated sets of maximal repetitive isokinetic contractions. The isokinetic dynamometer, rather than inertial resistance condition such as free-weight lifting, was used to ascertain a constant range of motion and speed for both the spontaneous breathing and hyperventilation condition. This exercise condition avoids confounding EMG amplitude effects due to change in skin-electrode geometry (Rainoldi et al. 2000) and contraction speed (Sakamoto and Sinclair 2012).
Methods
Subjects
Fifteen power-trained university athletes (10 males and 5 females), who were well accustomed to high-intensity resistance training, participated in this study. Their sports, physical characteristics, experience and training frequency at the time of the experiment are summarized in Table 1.
All subjects reported to the laboratory on four separate occasions (two non-experimental days and two experimental days). On the first non-experimental day, they were informed of any risks associated with the experiment, read the guidelines and received verbal instructions for the experimental procedures, and gave informed consent. On the second non-experimental day, within 7 days, the subjects were accustomed to the hyperventilation method and maximal isokinetic knee extensions involving a few exercise sets. Then, they performed a complete train of exercise procedures at both speeds (described below) under the hyperventilation condition. This practice session assured subjects’ capabilities of withstanding the fatiguing nature of the exercise and the methodological hyperventilation without major adverse symptoms such as paresthesia or dizziness (Macefield and Burke 1991).
Within another 7 days after the second practice session, the experimental sessions began. On each of the two experimental days (control or hyperventilation condition), subjects were instructed to eat a carbohydrate-rich meal 2 h prior to the experiment and consume plenty of water. No subjects consumed alcoholic or caffeinated drinks during the experimental period. To minimize diurnal and longitudinal performance variations, the two experiments were conducted at the same time of day and completed in 48–72 h. Subjects were instructed to assume constant training routines throughout the experimental period and to avoid intense lower body workouts immediately prior to the exercise testing. This study was approved by the Human Ethics Committee of Juntendo University, Graduate School of Health and Sports Science.
Experimental procedures
This was a randomized, crossover counterbalanced measure design, where subjects underwent both control and hyperventilation conditions (held 48 or 72 h apart). The experiment was conducted between August 2012 and February 2013. All trials were performed in a temperature and humidity controlled room at 23 °C and 59 %, respectively.
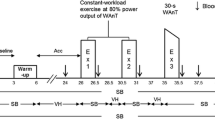
Figure 1 presents an overview of the experimental procedure. On each of the experimental days, subjects performed maximal voluntary isokinetic concentric knee extensions with one leg for 12 repetitions (reps) × 8 sets at 60°/s, followed by 25 reps × 8 sets at 300°/s with the other after recessing for approximately 45 min. The inter-set recovery was 40 s for both speeds. This exercise protocol was developed based on our pilot tests to result in progressive reduction in torque output with repetition and set, even for trained athletes, without spinning out the exercise duration. Moreover, the total work outputs, during the first exercise set before fatigue set in, were similar between the two speeds (χ = 2667.0 to 2669.0 J). No voluntary contractions of the knee flexors were involved, but the lever was lowered by the gravity during which the knee extensors relaxed and prepared for the subsequent contraction. The first assigned breathing condition (control or hyperventilation) and the numbers of dominant and non-dominant legs between the two speed conditions were counterbalanced between subjects. Two contraction speeds were selected so as to identify optimal situations for the hyperventilation effects: strength-oriented or speed-oriented torque production.
An overview of the experimental flow. Blood pH and PCO2 were measured, at rest (baseline), and then before the first, fourth and seventh sets (pre-exercise set), and after the second, fifth and eighth sets (post-exercise set). Blood [La−] was measures at rest (baseline) and then after every second set
Before the commencement of each speed condition, subjects warmed up for at least 10 min including specific practice on the dynamometer to elicit maximum performance, during which a 500-mL standard mineral water drink was given to ensure euhydration (before the test at 60°/s) or rehydration (before the test at 300°/s). Within 5 min after the completion of the warm-up, the isokinetic exercise began. The exercise test was, however, preceded by a EMG normalization set (Fig. 1), which consisted of three maximal knee extensions before the 60°/s, or 5 extensions before the 300°/s condition, performed at respective speeds. For each speed condition, two contractions that produced the first and second highest peak torques were determined, and the mean EMG amplitudes of these two contractions were averaged. This value was used to normalize the EMG amplitude recorded during the subsequent exercise test to offset day-to-day differences. A rest duration of 40 s took place between the normalization set and the first experimental set (Fig. 1).
For the control condition, subjects breathed spontaneously during the 40-s break between sets, whereas for the hyperventilation condition, subjects initially performed spontaneous breathing for the first 10 s of recovery followed by 30 s of methodological hyperventilation (50 breaths/min with end-tidal partial pressure of CO2 maintained at 20–25 mmHg) until the start of the next set (Fig. 1). The first set was also preceded by 30 s of hyperventilation in order to examine the preparatory effect of respiratory alkalosis and altered neural drive. Strong verbal encouragement was avoided, since its motivational nature may lessen the development of central fatigue. Only the number of reps performed was read out rep-by-rep with a casual tone within each set, with no emphasis on the remaining reps or set numbers so that subjects remained in a self-motivated situation.
The peak torque achieved in each rep was used to detect performance decrement over eight sets. EMG was recorded from the vastus lateralis and medialis, and signal amplitudes served to estimate the combined levels of excitability and central motor command output. Blood samples were collected from the earlobe for blood lactate concentration to reflect glycolytic metabolism and for pH and PCO2 to verify respiratory alkalosis resulting from hyperventilation. Expired gases including minute ventilation, respiratory rate, expired tidal volume and end-tidal partial pressure of CO2 were monitored breath-by-breath to control for the methodological hyperventilation and to document altered ventilation patterns and ventilatory demands. Details about each of these sampling techniques are described below.
Equipment
Isokinetic dynamometer
Subjects were seated and strapped to an isokinetic dynamometer (Biodex System 4, Biodex Medical System Inc., Shirley, NY, USA) with the knee joint (the lateral epicondyle of the femur) aligned with the axis of lever rotation. The lever pad was securely strapped around the ankle of the exercising leg. The range of lever motion was 90° with the knee extension limit set at 5–10° lower than the full extension, depending on the flexibility of the hamstrings. The seat and dynamometer positions, lever length and the lever limit position were determined during the familiarization session and kept constant throughout the subsequent experiments. The gravity effect torque was measured at an angle of 20° below the extension limit for each leg (60 and 300°/s). This value measured before the first experimental test was applied in subsequent test sessions, assuming that the gravity effect torque stayed constant within 48–72 h. Any slight variation in the gravity effect torque was likely due to the relaxation state of hamstrings, rather than changes in limb mass. The auxiliary smoothing and isokinetic filters were applied to the recorded peak torque values for later data processing.
EMG
Silver/silver chloride surface electrodes, 9 mm in diameter (Ambu Blue Sensor P, Ambu A/S, Denmark), were placed on the skin over the bellies of vastus medialis (VM) and lateralis (VL). The inter-electrode distance and impedance were, respectively, 34 mm and less than 3 kΩ after skin preparation. The electrode positions were marked using a semi-permanent pen to reproduce the same electrode placements for the subsequent test. EMG signals were amplified using a Telemyo 2400R G2 telemetry system (Noraxon USA Inc., Scottsdale, Arizona, USA) with high- and low-pass filters set at 10 and 500 Hz, respectively, and collected at 1500 Hz using the auxiliary software installed on a personal computer (PC). The isokinetic dynamometer was linked to the EMG device, and the analogue data of the lever position and velocity were digitalized (1500 Hz), synchronized with the EMG data and recorded on the same PC screen. Using the lever position and velocity data, the concentric phase of the knee extension was identified for each rep, over which mean EMG amplitude was calculated after full-wave rectification. All EMG amplitude data were normalized with the value recorded during the EMG normalization set within respective speed condition and defined as follows: normalized EMG amplitude (AMPnorm) = (mean EMG amplitude over each exercise rep/average of the two mean EMG amplitudes that produced the first and second highest peak torque during the normalization set) × 100 %.
Aeromonitor
Expired gas through a fitted facial mask was monitored breath-by-breath on a PC screen during the experiment using an aeromonitor (AE300 s, Minato Medical Science, Osaka, Japan) for minute ventilation (\( {\dot{V}}{\text{E}} \)), respiratory rate (RR), expired tidal volume (VT) and end-tidal partial pressure of CO2 (PETCO2). The airflow sensor was calibrated using a 2-L syringe, and O2 and CO2 sensors with known O2 and CO2 gas concentrations in accordance with the manufacturer’s instructions before each experiment. During the hyperventilation intervention (30 s until the start of each exercise set), RR was set at 50 breaths/min using a metronome (set at 100 bpm: inspiration or expiration per beat) according to our pilot tests for \( {\dot{V}}{\text{E}} \) and RR to be necessarily greater than the control, while being able to maintain the target PETCO2 value of 20–25 mmHg until the last set so as to induce respiratory alkalosis (Davies et al. 1986). VT was adjusted constantly (breathed shallower or deeper) according to the PETCO2 value read out by an investigator.
Blood gas and lactate analyzers
Whole capillary blood (approximately 60 µL) was collected into a heparinized tube from a hyperemized earlobe and analyzed for blood gases (pH and PCO2) using Cobas b221 System (Roche Diagnostics, Tokyo, Japan). Blood was sampled at rest, then 15 s before the first, fourth and seventh sets (pre-exercise set), and immediately after the second, fifth and last sets (post-exercise set) (seven times in total, Fig. 1). From the contralateral earlobe, 20 µL of blood was collected to calculate blood lactate concentration ([La−]), at rest and immediately after every second set (second, fourth, sixth and last set) (five times in total, Fig. 1) using a lactate analyzer (Biosen S-Line, EFK Diagnostics, Barleben, Germany). Limited number of blood samples was collected for blood gases due to the time required by the device for self-cleaning for the subsequent measurement (about 90–120 s). Occasionally, the measurements were unsuccessful due to: (1) unexpectedly long time taken for the self-cleaning process resulting in clotting of the pending blood sample; (2) failure to collect blood sample with sufficient amount, especially during pre-exercise set sampling; or (3) unidentified device errors.
Statistical analyses
Small fluctuations in peak torque values and EMG AMPnorm data, due to device sensitivity, were managed by averaging the data over every three reps for 60°/s and every five reps for 300°/s before statistical analyses. Likewise, the breath-by-breath data of \( {\dot{V}}{\text{E}} \), RR, VT and PETCO2 were averaged over 10 s before (pre-exercise set) and after (post-exercise set) each exercise set.
Statistical tests were performed using SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA). Test–retest repeatability of the peak torque was evaluated by means of intra-class correlation (ICC) using the average of the first and second highest values recorded during the EMG normalizing set (pre-test value). The ICCs for 60°/s and 300°/s were, respectively, 0.958 (95 % CI 0.884–0.986) and 0.961 (95 % CI 0.892–0.987). Paired t tests were used to compare the pretest value of peak torque, and resting blood pH, PCO2 and [La−] to ensure nonsignificant differences in the baseline values between the two conditions.
Linear mixed model analyses were applied to the blood pH and PCO2 data with occasional missing data to test the main effects of condition (control vs. hyperventilation) and exercise set (separate analyses for the pre-exercise set and post-exercise set samples), and their interaction for each speed condition.
Three-way repeated-measures ANOVAs were used on peak torque and AMPnorm (VM and VL separately) to study the main effects of condition (control vs. hyperventilation), set (first–eighth set) and rep (every three reps for 60°/s and every five reps for 300°/s), and their interactions. Two-way repeated-measures ANOVAs were used for \( {\dot{V}}{\text{E}} \), RR, VT and PETCO2 (separate analyses for 10 s pre- and post-exercise set), as well as [La−] to test the main effects of condition and exercise set, and their interaction. For those cases violating the assumption of sphericity, significance was corrected using the Greenhouse–Geisser adjustment.
Where necessary, post hoc comparisons were performed using the Bonferroni method. P values of equal to or less than 0.05 were considered statistically significant, and all values are presented as mean ± SD.
Results
There was no significant differences between control and hyperventilation conditions for pre-test values of peak torque (60°/s: 234.0 ± 58.4 vs. 237.1 ± 56.8 N m, 300°/s: 131.3 ± 36.8 vs. 131.9 ± 33.6 N m), resting blood pH (60°/s: 7.401 ± 0.017 vs. 7.399 ± 0.014, 300°/s: 7.407 ± 0.008 vs. 7.404 ± 0.015), PCO2 (60°/s: 39.4 ± 2.8 vs. 39.8 ± 2.9 mmHg, 300°/s: 37.8 ± 2.1 vs. 38.0 ± 3.2 mmHg) and [La−] (60°/s: 1.2 ± 0.3 vs. 1.3 ± 0.3 mM, 300°/s: 1.5 ± 0.6 vs. 1.5 ± 0.5 mM).
Peak torque
The main effects of set and rep, and set × rep interaction on peak torque were significant for both 60°/s and 300°/s (Fig. 2a, b), demonstrating a progressive decrease in peak torque with rep and set, but the declining patterns with rep varied among sets. The main effect of condition was not significant for both speeds. A significant condition × rep interaction, however, existed for 60°/s (P = 0.019, Fig. 2a). Post hoc tests revealed that significant falls occurred in torque output between the initial rep phase (first–third rep) and the second rep phase (fourth–sixth rep) for the hyperventilation condition (P = 0.002). No significant differences, however, existed in torque output between these rep phases for the control condition (Fig. 2a). Further post hoc analyses indicated that the different repetition effect between the two conditions resulted from greater torque achieved with hyperventilation than the control in the initial rep phase. Statistical evidence for this trend was, however, confirmed for the first and fourth sets only (P = 0.032 and P = 0.040, respectively) (Fig. 2a).
Changes in peak torque at 60°/s (a) and 300°/s (b), normalized EMG amplitude (AMPnorm) of the vastus medialis (VM) at 60°/s (c) and 300°/s (d), and AMPnorm of the vastus lateralis (VL) at 60°/s (e) and 300°/s (f) for control versus hyperventilation conditions (n = 15). Values were averaged over every three reps (60°/s) or every five reps (300°/s) for statistical analyses. *Significant main effect or interaction according to three-way repeated-measures ANOVA (condition × set × rep). ‡Significant difference in peak torque between control and hyperventilation conditions in the initial rep phase (first–third rep, a)
AMPnorm
For the 60°/s condition, the main effects of set and rep, and set × rep interaction on AMPnorm were significant for both VM and VL, showing that AMPnorm decreased with set, but the effect of rep was dependent on set numbers (Fig. 2c, e). In early exercise sets, AMPnorm progressively increased with rep number followed by a fall toward the last rep phase. This rep effect was, however, reduced in later exercise sets with AMP norm becoming relatively constant over the rep phases (Fig. 2c, e). No significant condition effect, condition × set or condition × rep interaction, was evident on AMPnorm of both VM and VL for the 60°/s condition (Fig. 2c, e).
For the 300°/s condition, AMPnorm also decreased significantly with set for both VM and VL (P < 0.001, Fig. 2d, f). The main effect of rep was observed only for VM (P = 0.045), exhibiting an AMPnorm change similar to that observed in the 60°/s condition (gradual rise followed by a fall with rep phase). This rep effect, however, may have been present also for VL in early exercise sets, given a significant set × rep interaction (P = 0.03, Fig. 2f). Again, the main effect of condition was not significant for both VM and VT for the 300°/s condition (Fig. 2d, f). A noteworthy finding was that there was a significant condition × set interaction for VL (P = 0.019). Post hoc analyses revealed that for the control condition, there was a significant reduction in AMPnorm (the average within each set) between the first and second sets. The AMPnorm of the remaining sets stayed lower than the first set, however, by similar degrees without any further decrement (Fig. 2f), whereas AMPnorm of the hyperventilation condition decreased until the fifth set and then remained similar thereafter (Fig. 2f).
Blood pH, PCO2 and [La−]
Blood samples successfully obtained for blood gas analyses (pH and PCO2) were 94 out of 105 (15 subjects × 7 time points) for both control and hyperventilation conditions (89.5 %). The missing data points occurred at random with 0–4 out of 15 subjects per designated timing. No reading error occurred for the blood gas values at rest.
A significant condition effect was observed for both pH and PCO2 with no condition × set interaction regardless of pre- or post-exercise set, or speed condition (Fig. 3a–d), indicating an elevated blood pH and lowered PCO2 with hyperventilation at any measurement time point, although the effect was greater for the pre-exercise set samples. The main effect of set was also significant for all cases showing a progressive fall in blood pH and PCO2 toward the end of exercise, except for post-exercise set pH in the 60°/s condition (Fig. 3a–d).
Changes in blood pH at 60°/s (a) and 300°/s (b), PCO2 at 60°/s (c) and 300°/s (d), and [La−] at 60°/s (e) and 300°/s (f) for control and hyperventilation conditions (n = 15). Pre-exercise set values for blood pH and PCO2 (before the first, fourth and seventh sets) are shown on the left, and the post-exercise set values (after the second, fifth and eighth sets) are shown on the right (a–d). Blood [La−] was measured at rest (baseline) and then after every second set (post-exercise set). *Significant main effect or interaction according to two-way repeated-measures ANOVA (condition × set)
Blood [La−] significantly increased after the onset of exercise for both speeds (P < 0.001, Fig. 3e, f). The 60°/s condition showed a significant condition effect (P = 0.009) as well as condition × set interaction (P < 0.001), indicating that [La−] was higher with hyperventilation, especially for later exercise sets (Fig. 3e). [La−] was not affected by the breathing condition for 300°/s (Fig. 3f).
\( {\dot{V}}{\text{E}} \), RR, VT and PETCO2
As can be seen from the values recorded at the pre-exercise set, the target RR (50 breaths/min) and PETCO2 (20–25 mmHg) were well achieved by the hyperventilation method (Fig. 4c, d, g, h, pre-exercise set), resulting in significantly greater \( {\dot{V}}{\text{E}} \), RR and VT, and lower PETCO2 than the control (P < 0.001, Fig. 4a–h, pre-exercise set). Significant set effects as well as condition × set interactions were observed for pre-exercise set \( {\dot{V}}{\text{E}} \), RR and PETCO2 (Fig. 4a–d, g, h, pre-exercise set), showing spontaneous exercise responses of these variables (progressive increase in \( {\dot{V}}{\text{E}} \) and RR, and decrease in PETCO2 with exercise set) under the control condition, whereas these variables stayed relatively constant throughout the exercise for the hyperventilation condition under methodological control. VT also demonstrated a significant set effect for both speeds (P < 0.001, Fig. 4e, f, pre-exercise set), but pair-wise comparisons revealed that this effect was attributable to the initial rise, after which the values remained relatively constant.
Changes in minute ventilation (\( {\dot{V}}{\text{E}} \)) at 60°/s (a) and 300°/s (b), respiratory rate (RR) at 60°/s (c) and 300°/s (d), expired tidal volume (VT) at 60°/s (e) and 300°/s (f), and end-tidal partial pressure of CO2 (PETCO2) at 60°/s (g) and 300°/s (h) for control and hyperventilation conditions (n = 15). Breath-by-breath data were averaged over s before (pre-exercise set, left) and after each exercise set (post-exercise set, right). *Significant main effect or interaction according to two-way repeated-measures ANOVA (condition × set)
All respiratory variables, measured at the post-exercise set, demonstrated a significant main effect of set for both speeds (Fig. 4a–h, post set), indicating a progressive increase in \( {\dot{V}}{\text{E}} \) and RR, and decrease in PETCO2 with set number, while VT was more variable among sets (Fig. 4e, f, post-exercise set). As opposed to the pre-exercise set, post-exercise set \( {\dot{V}}{\text{E}} \) was lower for the hyperventilation condition for both speeds (P < 0.05) due to lower post-exercise set VT (P < 0.005, Fig. 4a, b, e, f, post-exercise set), although the post-exercise set RR held at similar (60°/s, P = 0.217) or higher values (300°/s, P = 0.038) compared to the control (Fig. 4c, d, post-exercise set). Post-exercise set PETCO2 of the hyperventilation condition remained lower than the control for both speeds (P < 0.05, Fig. 4g, h, post-exercise set).
Discussion
The hyperventilation implemented in this study (30-s duration, RR set at 50 breaths/min and PETCO2 maintained within 20–25 mmHg) successfully induced respiratory alkalosis as indicated by the elevated pre-exercise set blood pH by 0.069–0.081 for 60°/s and 0.065–0.075 for 300°/s, and lowered PCO2 by 8.3–10.3 mmHg for 60°/s and 9.0–9.7 mmHg for 300°/s from the control values (Fig. 3a–d, pre-exercise set). For the post-exercise set, the observed differences in blood pH and PCO2 between the two conditions were smaller but remained significant compared to the pre-exercise set (Fig. 3a–d, post-exercise set). None of the subjects complained of any major adverse effects such as paresthesia or dizziness with the hyperventilation method. Despite evidence of respiratory alkalosis, the presence of the expected ergogenic effects was limited.
The present exercise protocol elicited a significant fall in torque output with set and rep (Fig. 2a, b) and in AMPnorm with set number (Fig. 2c-f), suggestive of muscle fatigue, a potential cause being a combined effect of peripheral and central factors (Kawakami et al. 2000; Nordlund et al. 2004). Within an exercise set, AMPnorm followed a convex-up manner with rep phases (Fig. 2c–f). The rise in AMPnorm in early rep phases may be explained by the myoelectrical potentiation (Jubeau et al. 2010), or recruitment of additional motor units that were inadvertently not stimulated in the earlier rep phase, since the level of activation of a muscle may range between 80 and 100 % even during maximal voluntary effort (Kawakami et al. 2000; Nordlund et al. 2004). However, this convex-up rep effect was not sustained, but reduced in later sets.
A significant condition × rep interaction existed in torque output at 60°/s, implying a small but significant enhancement with hyperventilation in peak torque recorded in the initial rep phase (first–third rep), notably the first and fourth sets (Fig. 2a). The observed ergogenic effect could be partly attributable to less compromised rates of glycolysis and glycogenolysis, as well as PCr breakdown through accelerated buffering of H+ (Ui 1966; Sahlin et al. 1979; Davies et al. 1986; Lindinger et al. 1990; McMahon and Jenkins 2002; Forbes et al. 2007). Blood [La−] values were, indeed, higher for the hyperventilation condition than the control at 60°/s (Fig. 3e), suggestive of a greater lactate production and a greater efflux of lactate through a greater lactate gradient, although other mechanisms may coexist (e.g., slowed activity of pyruvate dehydrogenase, a greater transporter activity for lactate and/or reduced muscle and hepatic lactate uptake through reduced blood flow) (McCartney et al. 1983; Davies et al. 1986; Morrow et al. 1988; Lindinger et al. 1990; Druml et al. 1991; Marx et al. 2002; Bishop et al. 2004; Chin et al. 2007). The greater [La−] results were, however, more evident in later sets of exercise (after the fourth set), where no enhancement in torque output occurred (Fig. 2a). It was, therefore, unlikely that an increased rate of glycolysis accounted for the greater torque observed, especially during the initial three reps of the first set, since anaerobic fuel sources were readily available at exercise onset.
An alternative explanation for the small enhancement in torque output could be a greater central readiness or drive with hyperventilation prior to the exercise set. The “Setchenov phenomenon,” expected to derive from volitional increase in the activation of respiratory and trunk muscles (a diverting activity), involves stimulation of the facilitatory part of the reticular formation. In turn, the phenomenon promotes arousal, resulting in greater levels of voluntary activation or central command outflows (Asmussen 1979; MacLaren et al. 1989). Furthermore, hyperventilation increases the sensitivity of excitable neurons, nerves and/or membranes within both the central (corticospinal) and peripheral nervous systems (Katz and Wolf 1964; Macefield and Burke 1991; Seyal et al. 1998; Sparing et al. 2007; Hilbert et al. 2012). However, hyperventilation did not result in greater AMPnorm in either of the knee extensor muscles (VM or VL) when greater torque outputs were recorded at the initial rep phase of the first and fourth sets at 60°/s (Fig. 2c, e). This implies that no evidence has been recorded for augmented activation or excitability of the exercising muscles. It was possible that surface EMG was not sensitive enough to detect and signal augmented voluntary activation and neural excitability in the current experimental preparation. At present, however, we are unable to uncover the exact mechanisms for the torque output enhancement.
Despite an enhanced torque output at 60°/s, the torque output at 300°/s and AMPnorm at both speeds were not significantly altered with hyperventilation. Moreover, the torque values at 60°/s, other than those in the initial rep phase of the first and fourth sets, were similar between the breathing conditions (Fig. 2). Hence, the evidence for ergogenic effects of hyperventilation was not convincing. A possible explanation for the lack of effects of hyperventilation may be that the exercise task was not as metabolically challenging as required. The fatiguing nature of the workout was evidenced by the progressive falls in torque and AMPnorm with repeated sets. It may be, therefore, expected that the pH within the active muscles and adjacent interstitial spaces was exposed to significant acidosis. In spite of this, the exercise in the present study may have provided an opportunity for repletion of blood flow, since the exercise involved unilateral single-joint concentric-only contractions with the muscles relaxing during the lowering phase. Notably, under the control condition, the changes in blood pH and respiratory demand parameters (\( {\dot{V}}{\text{E}} \) and RR) of the present study (Figs. 3a, b, 4a–d) were smaller compared to those observed in our previous repeated sprint study (Sakamoto et al. 2014), in which positive ergogenic effects of hyperventilation were seen (blood pH: 7.366 or 7.351 vs. 7.151, \( {\dot{V}}{\text{E}} \): 44.5 or 52.1 vs. 127.8 l/min, RR: 31.1 or 37.2 vs. 61.2 breaths/min) (60 or 300°/s vs. repeated sprint, respectively). These differences imply that the present isokinetic exercise incurred a much less anaerobic metabolic challenge, although maximally performed repeatedly over multiple sets. Previous work of metabolic alkalosis has shown that exhaustive resistance exercise (the number of reps able to be performed during leg press) was not enhanced by the ingestion of NaHCO3 (Webster et al. 1993; Portington et al. 1998). The authors proposed that relatively localized muscle actions rendered the anaerobic metabolism not as demanding as the whole-body maximum effort and thus not ideal for the investigation of buffering effects. Additionally, the current exercise task may have failed to impose significant central stresses on highly trained athletes, although a self-motivated situation was systemically assigned (no verbal encouragement). The prospect of central drive being recovered through the Setchenov phenomenon, therefore, could not be overtly manifested by either the torque or AMPnorm findings.
It should be noted that a significant condition × set interaction (P = 0.019) was observed for the VL in 300°/s condition, showing that the AMPnorm decrement was greater with hyperventilation as opposed to our hypothesis of attenuation of decrements (Fig. 2f). The increased neuronal excitability with hyperventilation reported previously was observed by means of transcutaneous electrical or transcranial magnetic pulse stimulation (Macefield and Burke 1991; Seyal et al. 1998; Sparing et al. 2007; Hilbert et al. 2012). In contrast, under voluntary efforts, the hyperventilation-induced reduction in cerebral blood flow may have a deleterious effect on central motor command outflow (Duarte et al. 1995; Nybo and Rasmussen 2007). Implementing hyperventilation without sufficient metabolic and central stresses may exaggerate the consequences of reduced cerebral blood, which in turn, compensate for the expected ergogenic effects. These mechanisms could possibly explain the lower AMPnorm observed with hyperventilation at 300°/s in later exercise sets, without exacerbation of decrement in torque output (Fig. 2b, f).
Carr et al. (2013) have reported that NaHCO3 administration increased the number of reps able to be lifted when multiple resistance exercises are performed (squat, leg press and knee extension). It could be argued that hyperventilation may prove to be beneficial for resistance exercise that involves multiple muscle groups of both the upper and lower body parts, and/or multiple-joint exercises. Muscle actions with both concentric and eccentric actions, rather than concentric-only contractions may be necessary to bring out higher metabolic demands and central stresses.
The limitations of the present study include the possibility that the variation in training experience, training frequency, sporting discipline and gender in the current sample may have a direct or indirect impact on the findings. Moreover, we are unable to answer why the minor enhancement occurred exclusively at 60°/s, whereas a greater reduction in AMPnorm was apparent at 300°/s with hyperventilation, despite relatively similar changes in blood and respiratory parameters with exercise between the two speeds (Figs. 3, 4). Further research is warranted to better ascertain factors that influence the consequences of hyperventilation strategies.
In conclusion, a minor ergogenic effect was observed at 60°/s in the initial rep phase of the first and fourth sets when hyperventilation was applied between sets of repeated maximal isokinetic knee extensions. Hyperventilation, on the other hand, demonstrated no benefits at 300°/s. The EMG data did not corroborate any evidence of enhanced muscle activations with hyperventilation at both speeds. The current findings suggest that future studies should examine resistance exercises that involve multiple joints, body parts and concentric–eccentric repetitions to achieve significant metabolic and central stresses, which may help establish a more ideal setting for the investigation of ergogenic effects of hyperventilation.
Abbreviations
- AMPnorm :
-
Normalized EMG amplitude
- ANOVA:
-
Analysis of variance
- EMG:
-
Electromyography/electromyogram
- [La−]:
-
Blood lactate concentration
- NaHCO3 :
-
Sodium bicarbonate
- PCO2 :
-
Partial pressure of carbon dioxide (blood)
- PCr:
-
Creatine phosphate
- PETCO2 :
-
End-tidal partial pressure of carbon dioxide (expired air)
- RR:
-
Respiratory rate
- \( {\dot{V}}E \) :
-
Minute ventilation
- VL:
-
Vastus lateralis muscle
- VM:
-
Vastus medialis muscle
- VT:
-
Expired tidal volume
References
Asmussen E (1979) Muscle fatigue. Med Sci Sports Exerc 11:313–321
Bishop D, Claudius B (2005) Effects of induced metabolic alkalosis on prolonged intermittent-sprint performance. Med Sci Sports Exerc 37:759–767
Bishop D, Edge J, Davis C, Goodman C (2004) Induced metabolic alkalosis affects muscle metabolism and repeated-sprint ability. Med Sci Sports Exerc 36:807–813
Carr BM, Webster MJ, Boyd JC, Hudson GM, Scheett TP (2013) Sodium bicarbonate supplementation improves hypertrophy-type resistance exercise performance. Eur J Appl Physiol 113:743–752
Chin LM, Leigh RJ, Heigenhauser GJ, Rossiter HB, Paterson DH, Kowalchuk JM (2007) Hyperventilation-induced hypocapnic alkalosis slows the adaptation of pulmonary O2 uptake during the transition to moderate-intensity exercise. J Physiol 583:351–364
Davies SF, Iber C, Keene SA, McArthur CD, Path MJ (1986) Effect of respiratory alkalosis during exercise on blood lactate. J Appl Physiol 61:948–952
Druml W, Grimm G, Laggner AN, Lenz K, Schneeweiss B (1991) Lactic acid kinetics in respiratory alkalosis. Crit Care Med 19:1120–1124
Duarte J, Markus H, Harrison MJ (1995) Changes in cerebral blood flow as monitored by transcranial Doppler during voluntary hyperventilation and their effect on the electroencephalogram. J Neuroimaging 5:209–211
Forbes SC, Kowalchuk JM, Thompson RT, Marsh GD (2007) Effects of hyperventilation on phosphocreatine kinetics and muscle deoxygenation during moderate-intensity plantar flexion exercise. J Appl Physiol 102:1565–1573
Fuchs F, Reddy Y, Briggs FN (1970) The interaction of cations with the calcium-binding site of troponin. Biochim Biophys Acta 221:407–409
Green HJ (1997) Mechanisms of muscle fatigue in intense exercise. J Sports Sci 15:247–256
Hilbert M, Shushakov V, Maassen N (2012) The influence of respiratory acid-base changes on muscle performance and excitability of the sarcolemma during strenuous intermittent hand grip exercise. J Appl Physiol 112:571–579
Jubeau M, Gondin J, Martin A, Van Hoecke J, Maffiuletti NA (2010) Differences in twitch potentiation between voluntary and stimulated quadriceps contractions of equal intensity. Scand J Med Sci Sports 20:e56–e62
Katz RL, Wolf CE (1964) Neuromuscular and electromyographic studies in man: effects of hyperventilation, carbon dioxide inhalation and d-Tubocurarine. Anesthesiology 25:781–787
Kawakami Y, Amemiya K, Kanehisa H, Ikegawa S, Fukunaga T (2000) Fatigue responses of human triceps surae muscles during repetitive maximal isometric contractions. J Appl Physiol 88:1969–1975
Kozak-Collins K, Burke ER, Schoene RB (1994) Sodium bicarbonate ingestion does not improve performance in women cyclists. Med Sci Sports Exerc 26:1510–1515
Lavender G, Bird SR (1989) Effect of sodium bicarbonate ingestion upon repeated sprints. Br J Sports Med 23:41–45
Lindinger MI, Heigenhauser GJ, Spriet LL (1990) Effects of alkalosis on muscle ions at rest and with intense exercise. Can J Physiol Pharmacol 68:820–829
Macefield G, Burke D (1991) Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 114:527–540
MacLaren DPM, Gibson H, Parry-Billings M, Edwardhe RHT (1989) A review of metabolic and physiological factors in fatigue. Exerc Sport Sci Rev 17:29–66
Marx JO, Gordon SE, Vos NH, Nindl BC, Gomez AL, Volek JS, Pedro J, Ratamess N, Newton RU, French DN, Rubin MR, Hakkinen K, Kraemer WJ (2002) Effect of alkalosis on plasma epinephrine responses to high intensity cycle exercise in humans. Eur J Appl Physiol 87:72–77
McCartney N, Heigenhauser GJ, Jones NL (1983) Effects of pH on maximal power output and fatigue during short-term dynamic exercise. J Appl Physiol 55:225–229
McCartney N, Spriet LL, Heigenhauser GJF, Kowalchuk JM, Sutton JR, Jones NL (1986) Muscle power and metabolism in maximal intermittent exercise. J Appl Physiol 60:1164–1169
McMahon S, Jenkins D (2002) Factors affecting the rate of phosphocreatine resynthesis following intense exercise. Sports Med 32:761–784
McNaughton L, Backx K, Palmer G, Strange N (1999) Effects of chronic bicarbonate ingestion on the performance of high-intensity work. Eur J Appl Physiol Occup Physiol 80:333–336
Morrow JA, Fell RD, Gladden LB (1988) Respiratory alkalosis: no effect on blood lactate decline or exercise performance. Eur J Appl Physiol Occup Physiol 58:175–181
Nakamaru Y, Schwartz A (1970) Possible control of intracellular calcium metabolism by [H+]: sarcoplasmic reticulum of skeletal and cardiac muscle. Biochem Biophys Res Commun 41:830–836
Nordlund MM, Thorstensson A, Cresswell AG (2004) Central and peripheral contributions to fatigue in relation to level of activation during repeated maximal voluntary isometric plantar flexions. J Appl Physiol 96:218–225
Nybo L, Rasmussen P (2007) Inadequate cerebral oxygen delivery and central fatigue during strenuous exercise. Exerc Sport Sci Rev 35:110–118
Orchardson R (1978) The generation of nerve impulses in mammalian axons by changing the concentrations of the normal constituents of extracellular fluid. J Physiol 275:177–189
Portington KJ, Pascoe DD, Webster MJ, Anderson LH, Rutland RR, Gladden LB (1998) Effect of induced alkalosis on exhaustive leg press performance. Med Sci Sports Exerc 30:523–528
Rainoldi A, Nazzaro M, Merletti R, Farina D, Caruso I, Gaudenti S (2000) Geometrical factors in surface EMG of the vastus medialis and lateralis muscles. J Electromyogr Kinesiol 10:327–336
Sahlin K, Harris RC, Hultman E (1979) Resynthesis of creatine phosphate in human muscle after exercise in relation to intramuscular pH and availability of oxygen. Scand J Clin Lab Invest 39:551–558
Sakamoto A, Sinclair PJ (2012) Muscle activations under varying lifting speeds and intensities during bench press. Eur J Appl Physiol 112:1015–1025
Sakamoto A, Naito H, Chow CM (2014) Hyperventilation as a strategy for improved repeated sprint performance. J Strength Cond Res 28:1119–1126
Seyal M, Mull B, Gage B (1998) Increased excitability of the human corticospinal system with hyperventilation. Electroencephalogr Clin Neurophysiol 109:263–267
Sparing R, Dafotakis M, Buelte D, Meister IG, Noth J (2007) Excitability of human motor and visual cortex before, during, and after hyperventilation. J Appl Physiol 102:406–411
Ui M (1966) A role of phosphofructokinase in pH-dependent regulation of glycolysis. Biochim Biophys Acta 124:310–322
Walsh ML, Takeda C, Takahashi A, Ikeda Y, Endo M, Miura A, Kan A, Fukuba Y (2006) Volitional hyperventilation during ramp exercise to exhaustion. Appl Physiol Nutr Metab 31:211–217
Webster MJ, Webster MN, Crawford RE, Gladden LB (1993) Effect of sodium bicarbonate ingestion on exhaustive resistance exercise performance. Med Sci Sports Exerc 25:960–965
Acknowledgments
This study was supported by Juntendo University, Institute of Health and Sports Science and Medicine (k1212), and by JSPS KAKENHI Grant Number 25750333. We would like to thank all the subjects for their participation and effort. We also thank the laboratory members at Juntendo University, Graduate School of Health and Sports Science for their assistance in data collection.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
Sakamoto, A., Naito, H. & Chow, C.M. Hyperventilation-induced respiratory alkalosis falls short of countering fatigue during repeated maximal isokinetic contractions. Eur J Appl Physiol 115, 1453–1465 (2015). https://doi.org/10.1007/s00421-015-3134-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3134-8