Abstract
Background
To perform a safe and precise laparoscopic surgery for the splenic flexure cancer, it is important for surgeons to gain a preoperative understanding of the running of the feeding artery of the splenic flexure. We evaluated the blood supply to the splenic flexure by using preoperative three-dimensional computed tomography (3D-CT).
Method
We retrospectively analyzed a total of 88 patients with colorectal cancer who underwent preoperative 3D-CT at our institutions between April 2016 and June 2017.
Results
The arterial blood supply to the splenic flexure was divided into four patterns as follows: type 1, the left branch of the middle colic artery (MCA) with common trunk and the left colic artery (LCA) (n = 48, 54.5%); type 2, the left branch of the MCA with independent origin and the LCA (n = 8, 9.1%); type3, the accessory-MCA (A-MCA) and the LCA (n = 27, 30.7%); and type4, the LCA alone (n = 5, 5.7%). The MCA had the common trunk of the right and left branches in the majority of cases (85.2%). The right and left branches of the MCA arose separately from the superior mesenteric artery (SMA) in 8 of 88 patients (9.1%).
Conclusions
The arterial patterns of the splenic flexure were classified into four patterns by using preoperative 3D-CT. The A-MCA existed in 30% of the patients in this study. These information should be helpful to perform the optimal surgery for the splenic flexure cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lately, laparoscopic surgery has gained broad clinical acceptance as a minimally invasive technique for colorectal cancer. A safe and precise surgical intervention warrants all surgeons to gain a preoperative understanding of the tumor location, extent of lymph nodes, and main feeding artery [1,2,3]. In addition, preoperative awareness of the arterial branching or variations is necessary to help surgeons devise preoperative strategies and perform safe and rapid vessel ligation and lymph node dissection. Recently, three-dimensional computed tomography (3D-CT) has been recognized as a less invasive method to assess vascular anatomy [4,5,6]. The advantage of 3D-CT is that it enables viewing images of the tumor, lymph nodes, blood vessels, and colon from all angles [7].
As the embryological adhesion renders the anatomy around the splenic flexure complicated and difficult to understand, surgical intervention for splenic flexure cancer remains non-standardized [8]. In addition, this difficulty is attributed to the major factor of vascular variability around the middle colic vessels [9, 10]. Previously, several studies have investigated anatomical variations of the vessels in the right-sided colon using 3D-CT [7, 11, 12]; however, few studies have investigated the blood supply in splenic flexure cancer [13]. Thus, this study aims to investigate the arterial branching patterns running toward the splenic flexure using 3D-CT and provide practically useful classifications.
Materials and methods
Patient selection
In this study, we retrospectively enrolled 88 patients (43 males and 45 females; age: 35–92 years; mean age: 69.2 years) with colorectal cancer who underwent abdominal enhancement computed tomography (CT) scan at the Kobe University Hospital from April 2016 to June 2017. We obtained informed consent from all patients. Of note, we excluded patients with previous abdominal surgery, such as colectomy and abdominal aortic aneurysm surgery, from the analysis.
3D-CT angiography protocol
In all patients, CT was performed using a 192-slice CT scanner (SOMATOM Force; Siemens Healthcare, Forchheim, Germany) with the following parameters: tube voltage, 70 kV; tube current, 512 Ref. mAs rotation speed, 0.5 s/r; helical pitch, 1.3 mm/r; and slice thickness, 0.6 mm. The reconstruction intervals were set at 0.4 mm. For contrast-enhanced CT images, a nonionic contrast agent with an iodine concentration of 300, 320, 350, or 370 mg I/mL was infused at 25 s. The volume of the injected contrast agent was 550 mg I/kg. In addition, two radiological technologists performed the image processing analysis using a 3D volume rendering technique with the Ziostation (Zio Software, Tokyo, Japan).
Classification and measurements
Anatomically, the splenic flexure is the junction of the transverse colon and the descending colon. We defined the splenic flexure as the junction of the distal third of the transverse colon and the proximal third of the descending colon. In addition, we defined the middle colic artery (MCA) as the artery supplying the transverse colon, which is branching first from the right side of the superior mesenteric artery (SMA) and more distally than the first jejunal artery and then forks into the right and left branches.
We defined the right branch of the MCA as the branch running toward the right transverse colon and hepatic flexure. Conversely, the left branch of the MCA was defined as the branch running toward the left transverse colon and splenic flexure which originated from the common trunk of the MCA or from the independent origin distal to the first jejunal artery. The accessory MCA (A-MCA) was defined as a branch artery originating directly from the SMA and more proximally than the first jejunal artery, which supplied the splenic flexure. In addition, the distance from the SMA origin to the A-MCA bifurcation was measured on Ziostation. We defined the left colic artery (LCA) as the first branch of the inferior mesenteric artery (IMA) supplying the descending colon and splenic flexure.
Results
The feeder pattern of the splenic flexure
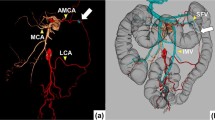
The anatomy of the arterial blood supply to the splenic flexure could be classified into the following four patterns: type 1, the left branch of the MCA originated from the common trunk and the LCA (n = 48; 54.5%); type 2, the left branch of the MCA with independent origin and the LCA (n = 8; 9.1%); type 3, the A-MCA and the LCA (n = 27; 30.7%); and type 4, the LCA alone (n = 5; 5.7%; Fig. 1). Although the LCA always supplied the splenic flexure in all patients, no artery from the SMA fed the splenic flexure in five patients (5.7%). Furthermore, the mean length from the root of the SMA to that of the A-MCA was 41.74 ± 11.3 mm. Figure 2 shows the representative data of each group.
Branching pattern of the MCA and the A-MCA
We recognized the A-MCA in 27 of 88 patients (30.7%). The right and left branches of the MCA originated from the common trunk in most patients (90.9%). In addition, the right and left branches of the MCA originated separately from different origins of the SMA in eight patients (9.1%). We encountered no case in which the A-MCA and the left branch of the MCA arising directly from the SMA coexisted (Fig. 3).
The branching pattern of the MCA. a The MCA had the common origin of the right and left branch but not the A-MCA. b The MCA had the common origin of both the right and left branches and the A-MCA. c The MCA had different origins of the right and left branches but not the A-MCA. d No patient had both the left and right branches of the MCA branching directly from the SMA and A-MCA
Discussion
Although laparoscopic surgery has become a widely recognized procedure for colorectal cancer, it occasionally requires conversion to open surgery intraoperatively for some reasons [14, 15]. One of those reasons is intraoperative bleeding. Laparoscopic surgery is considered technically challenging specifically for transverse and splenic flexure colon cancer because of the anatomical complexity, which might present a higher risk of intraoperative bleeding compared with colon cancer at other sites. If the feeding arteries and their branching variations are delineated preoperatively or intraoperatively, surgeons could perform laparoscopic surgery more safely and accurately. Recent advancements in multidetector-row CT have facilitated excellent visualization of the mesenteric vasculature. In addition, preoperative 3D imaging has been reported to be correlated with a marked reduction in the operative time and incidence of complications associated with complicated or erroneous identification of mesenteric vessels [16]. Hence, in this study, we used the 3D reconstruction technique to assess the arterial patterns of the splenic flexure.
Although the splenic flexure is described anatomically as the junction of the transverse colon and the descending colon, its blood supply has rarely been investigated. Fukuoka et al. reported six types of blood supplies to the splenic flexure, which were the MCA, A-MCA, and LCA of main feeder vessels [13]. However, in the present study, the branching patterns of the blood supply to the splenic flexure were merely classified into four groups for easy understanding.
Reportedly, the incidence of the A-MCA varies widely among Western patients (5–8%) and Japanese patients (49%) [2, 9, 13, 17, 18]. In this study, 30% of the patients had the A-MCA, indicating possible differences in the incidence of the A-MCA between the ethnic groups. The diameter of the A-MCAs ranges from 3 to 5 mm by 3D-CT, and the AMCAs were always dissected with clipping when they were recognized during surgery. In addition, the mean length from the root of the SMA to that of the A-MCA was 41.74 ± 11.3 mm, which could facilitate in detecting the A-MCA by using preoperative images such as 3D-CT. We observed that the leading pattern of the blood supply to the splenic flexure was type 1 (54.5%), followed by type 3 (30.7%), type 2 (9.1%), and type 4 (5.7%) (Fig. 1). Fukuoka et al. reported that there was no direct feeder originating from the IMA [13]. In contrast, the feeder artery to the splenic flexure from the IMA always existed in our patient cohort. In five patients (5.7%), the LCA originating from the IMA was the only feeder to the splenic flexure. Previously, the incidence of an independent right and left branch of the MCA has been reported in 8–41% of cases [19]. In this study, an independent right and left branch of the MCA occurred only in 9.1% of patients. Perhaps, these differences could be attributed to the difference in the CT protocol or the anatomical classification of the arteries. Hence, standardizing the CT protocol and unification of the terminology is mandatory.
In conclusion, this study presents four types of arterial patterns of the splenic flexure by using preoperative 3D-CT. The A-MCA existed in 30% of the patients in this study. We believe the information provided in this study should be helpful to perform the optimal surgery for splenic flexure cancer. Furthermore, this study proposes the use of the 3D reconstruction technique as a novel modality for the preoperative assessment and intraoperative navigation in laparoscopic colectomy.
References
Ke J, Cai J, Wen X, Wu X, He Z, Zou Y, Qiu J, He X, He X, Lian L, Wu X, Zhou Z, Lan P (2017) Anatomic variations of inferior mesenteric artery and left colic artery evaluated by 3-dimensional CT angiography: insights into rectal cancer surgery - a retrospective observational study. Int J Surg 41:106–111. https://doi.org/10.1016/j.ijsu.2017.03.012
Watanabe J, Ota M, Suwa Y, Ishibe A, Masui H, Nagahori K (2017) Evaluation of lymph flow patterns in splenic flexural colon cancers using laparoscopic real-time indocyanine green fluorescence imaging. Int J Color Dis 32(2):201–207. https://doi.org/10.1007/s00384-016-2669-4
Kawamoto A, Inoue Y, Okigami M, Yasuda H, Okugawa Y, Hiro J, Toiyama Y, Tanaka K, Uchida K, Mohri Y, Kusunoki M (2015) Preoperative assessment of vascular anatomy by multidetector computed tomography before laparoscopic colectomy for transverse colon cancer: report of a case. Int Surg 100(2):208–212. https://doi.org/10.9738/INTSURG-D-13-00232.1
Matsuda T, Iwasaki T, Sumi Y, Yamashita K, Hasegawa H, Yamamoto M, Matsuda Y, Kanaji S, Oshikiri T, Nakamura T, Suzuki S, Kakeji Y (2017) Laparoscopic complete mesocolic excision for right-sided colon cancer using a cranial approach: anatomical and embryological consideration. Int J Color Dis 32(1):139–141. https://doi.org/10.1007/s00384-016-2673-8
Ishikawa Y, Ehara K, Yamada T, Matsuzawa N, Arai S, Ban D, Kudo A, Tanabe M, Kawashima Y, Sakamoto H (2018) Three-dimensional computed tomography analysis of the vascular anatomy of the splenic hilum for gastric cancer surgery. Surg Today 48(9):841–847. https://doi.org/10.1007/s00595-018-1679-y
Miyake H, Murono K, Kawai K, Hata K, Tanaka T, Nishikawa T, Otani K, Sasaki K, Kaneko M, Emoto S, Nozawa H (2018) Evaluation of the vascular anatomy of the left-sided colon focused on the accessory middle colic artery: a single-Centre study of 734 patients. Color Dis 20(11):1041–1046. https://doi.org/10.1111/codi.14287
Hirai K, Yoshinari D, Ogawa H, Nakazawa S, Takase Y, Tanaka K, Miyamae Y, Takahashi N, Tsukagoshi H, Toya H, Totsuka O, Sunose Y, Takeyoshi I (2013) Three-dimensional computed tomography for analyzing the vascular anatomy in laparoscopic surgery for right-sided colon cancer. Surg Laparosc Endosc Percutan Tech 23(6):536–539. https://doi.org/10.1097/SLE.0b013e31828f66fb
Matsuda T, Sumi Y, Yamashita K, Hasegawa H, Yamamoto M, Matsuda Y, Kanaji S, Oshikiri T, Nakamura T, Suzuki S, Kakeji Y (2018) Anatomical and embryological perspectives in laparoscopic complete mesocoloic excision of splenic flexure cancers. Surg Endosc 32(3):1202–1208. https://doi.org/10.1007/s00464-017-5792-6
Hamabe A, Park S, Morita S, Tanida T, Tomimaru Y, Imamura H, Dono K (2018) Analysis of the vascular interrelationships among the first Jejunal vein, the superior mesenteric artery, and the middle colic artery. Ann Surg Oncol 25(6):1661–1667. https://doi.org/10.1245/s10434-018-6456-z
Matsuda T, Iwasaki T, Mitsutsuji M, Hirata K, Maekawa Y, Tsugawa D, Sugita Y, Sumi Y, Shimada E, Kakeji Y (2015) Cranially approached radical lymph node dissection around the middle colic vessels in laparoscopic colon cancer surgery. Langenbeck's Arch Surg 400(1):113–117. https://doi.org/10.1007/s00423-014-1250-2
Murono K, Kawai K, Ishihara S, Otani K, Yasuda K, Nishikawa T, Tanaka T, Kiyomatsu T, Hata K, Nozawa H, Yamaguchi H, Watanabe T (2016) Evaluation of the vascular anatomy of the right-sided colon using three-dimensional computed tomography angiography: a single-center study of 536 patients and a review of the literature. Int J Color Dis 31(9):1633–1638. https://doi.org/10.1007/s00384-016-2627-1
Ogino T, Takemasa I, Horitsugi G, Furuyashiki M, Ohta K, Uemura M, Nishimura J, Hata T, Mizushima T, Yamamoto H, Doki Y, Mori M (2014) Preoperative evaluation of venous anatomy in laparoscopic complete mesocolic excision for right colon cancer. Ann Surg Oncol 21(Suppl 3):S429–S435. https://doi.org/10.1245/s10434-014-3572-2
Fukuoka A, Sasaki T, Tsukikawa S, Miyajima N, Ostubo T (2017) Evaluating distribution of the left branch of the middle colic artery and the left colic artery by CT angiography and colonography to classify blood supply to the splenic flexure. Asian J Endosc Surg 10(2):148–153. https://doi.org/10.1111/ases.12349
Mari FS, Nigri G, Pancaldi A, De Cecco CN, Gasparrini M, Dall'Oglio A, Pindozzi F, Laghi A, Brescia A (2013) Role of CT angiography with three-dimensional reconstruction of mesenteric vessels in laparoscopic colorectal resections: a randomized controlled trial. Surg Endosc 27(6):2058–2067. https://doi.org/10.1007/s00464-012-2710-9
Sakorafas GH, Zouros E, Peros G (2006) Applied vascular anatomy of the colon and rectum: clinical implications for the surgical oncologist. Surg Oncol 15(4):243–255. https://doi.org/10.1016/j.suronc.2007.03.002
Moghadamyeghaneh Z, Masoomi H, Mills SD, Carmichael JC, Pigazzi A, Nguyen NT, Stamos MJ (2014) Outcomes of conversion of laparoscopic colorectal surgery to open surgery. JSLS 18(4):e2014.00230. https://doi.org/10.4293/JSLS.2014.00230
Bhama AR, Wafa AM, Ferraro J, Collins SD, Mullard AJ, Vandewarker JF, Krapohl G, Byrn JC, Cleary RK (2016) Comparison of risk factors for unplanned conversion from laparoscopic and robotic to open colorectal surgery using the Michigan surgical quality collaborative (MSQC) database. J Gastrointest Surg 20(6):1223–1230. https://doi.org/10.1007/s11605-016-3090-6
Rusu MC, Vlad M, Voinea LM, Curca GC, Sisu AM (2008) Detailed anatomy of a left accessory aberrant colic artery. Surg Radiol Anat 30(7):595–599. https://doi.org/10.1007/s00276-008-0362-1
Yada H, Sawai K, Taniguchi H, Hoshima M, Katoh M, Takahashi T (1997) Analysis of vascular anatomy and lymph node metastases warrants radical segmental bowel resection for colon cancer. World J Surg 21(1):109–115
Acknowledgements
We would like to thank Noriyuki Negi (Center for Radiology and Radiation Oncology, Department of Medical Technology Support, Kobe University Hospital) for technical assistance.
Funding
This study is not supported by research grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tanaka, T., Matsuda, T., Hasegawa, H. et al. Arterial anatomy of the splenic flexure using preoperative three-dimensional computed tomography. Int J Colorectal Dis 34, 1047–1051 (2019). https://doi.org/10.1007/s00384-019-03289-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-019-03289-z