Abstract
Purpose
The aim of this study is to reveal the vascular branching variation in SFC (splenic flexure cancer) patients using the preoperative three-dimensional computed tomography angiography with colonography (3D-CTAC).
Methods
We retrospectively analyzed patients with SFC who underwent preoperative 3D-CTAC between January 2014 and December 2019.
Results
Among 1256 colorectal cancer (CRC) patients, 96 (7.6%) manifested SFC. The arterial branching from the superior mesenteric artery (SMA) was classified into five patterns, as follows: (type 1A) the left branch of middle colic artery (LMCA) diverged from middle colic artery (MCA) (N = 47, 49.0%); (2A) the LMCA diverged from the MCA and the accessory middle colic artery (AMCA) (N = 26, 27.1%); (3A) the LMCA independently diverged from the SMA (N = 16, 16.7%); (4A) the LMCA independently diverged from the SMA and AMCA (N = 3, 3.1%); (5A) only the AMCA and the LMCA was absent (N = 4, 4.1%). Venous drainage was classified into four patterns, as follows: (type 1V) the SFV flows into the inferior mesenteric vein (IMV) then back to the splenic vein (N = 50, 52.1%); (2V) the SFV flows into the IMV then back to the superior mesenteric vein (SMV) (N = 19, 19.8%); (type 3V) the SFV independently flows into the splenic vein (N = 3, 3.1%); (type 4V) the SFV is absent (N = 24, 25.0%).
Conclusion
3D-CTAC could reveal accurate preoperative tumor localization and vascular branching. These classifications should be helpful in performing accurate complete mesocolic excision and central vessel ligation for SFC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surgery for colon carcinoma of the splenic flexure, otherwise known as splenic flexure cancer (SFC), requires special attention [1, 2]. This is because feeding arteries arise from both the superior mesenteric artery (SMA) and inferior mesenteric artery (IMA) [3, 4]. Japanese D3 resection and European complete mesocolic excision (CME) with central vascular ligation (CVL) for colon cancer are both based on oncologic principles. According to the principle of CME with CVL, lymph node dissection should be performed along with both arteries on SFC. Recently, another feeding artery toward the splenic flexure, called the accessory middle colic artery (AMCA), has gained attention in CME and CVL on SFC [5]. In the previous literature, AMCA was detected in 30.7–36.4% of patients [5, 6]. Considering the concept of CME and CVL, AMCA plays a significant role in SFC surgery. However, little is known about the vascular branching of SFC, and the optimal area of lymph node dissection has not been established for SFC. The current guidelines of the Japanese Society for Cancer of the Colon and Rectum did not classify AMCA as a feeding artery [7]. In addition, using indocyanine green fluorescence imaging, Watanabe et al. found that 61.3% of SFC patients had lymphatic flow directed around the inferior mesenteric vein (IMV) [8]. Therefore, it is also necessary to evaluate the venous anatomy of SFC and not the arterial systems alone.
A three-dimensional computed tomography angiography with computed tomography colonography (3D-CTAC) is an advanced technique that visualizes vessels and effectively evaluates the entire colon for clinically relevant lesions [9,10,11,12]. It enables accurate preoperative diagnosis and intraoperative imaging of vascular anatomy and tumor localization. This study aimed to evaluate the branching pattern of both the artery and vein and tumor localization in SFC patients using the preoperative 3D-CTAC.
Material and methods
Patients
Subjects of the study were selected patients with colorectal cancer from the Department of Surgery, Saiseikai Yokohamashi Nanbu Hospital, between January 2014 and December 2019. Anatomically, splenic flexure was the junction of the descending and transverse colons. In this study, SFC was defined as a colonic adenocarcinoma with a tumor center located at the distal one-third of the transverse colon and proximal one-third of the descending colon. The tumor localization of SFC was divided into three parts: (1) transverse colon side of SFC (proximal SFC), (2) central SFC, and (3) descending colon side of SFC (distal SFC). Patients who had previously undergone colectomy and patients who could not perform enhanced computed tomography (CT) examination due to renal dysfunction or iodine allergy comorbidity were excluded in this study.
Protocol of 3D-CTAC
In our hospital, preoperative 3D-CTAC for colon cancer is routinely performed. It was performed preoperatively and used in reality for preoperative planning of surgery. All patients have undergone preoperative colonoscopy. Tumor localization was marked by colonoscopy using an Indian tattoo ink and mucosal clipping in all cases. After colonoscopy, the patients underwent immediate enhanced CT examination in both arterial and venous phases using the bolus tracking technique. The region of interest was set in the abdominal aorta at the bifurcation level of the celiac artery. Imaging commenced when the threshold increased by 100 Hounsfield units from the CT value prior to imaging. The CT examination was performed on a 0.5-mm slice (Aquilion ONE; Toshiba medical systems, Tokyo, Japan). All CT images were analyzed and reconstructed into 3D-CT angiography (3D-CTA) with CT colonography (CTC) images using the workstation system to evaluate vascular traffic and tumor localization (Aquarius iNtuition client viewer; TeraRecon. Int., USA). CTA and CTC were constructed using the same CT images.
Definition of the artery
The MCA is typically defined as a vessel originating from the SMA. When the MCA has an origin from another source (celiac trunk, IMA), it is referred to as an aberrant MCA. In the present study, the MCA was defined as a branch from the SMA, which runs to the right and feeds the transverse colon. The AMCA typically originates from the proximal SMA toward the left side of the transverse colon [5, 13]. However, there has been no global consensus on the definition of AMCA. Therefore, we defined AMCA as meeting all of the following criteria in this study: (1) the artery which branches toward the left from the SMA, (2) the artery which branches from proximal to MCA, and (3) the artery which runs toward the splenic flexure.
Venous drainage pattern
We also evaluated the venous drainage pattern of the IMV and the splenic flexure vein (SFV). The SFV was defined as vein flowing from the SFC to the surroundings of the pancreas [14].
Ethics
This study was approved by the Saiseikai Yokohamashi Nanbu Hospital’s Institutional Review Board, and written informed consent was obtained from study patients for the use of their data from medical records (NANBU2019-D34). The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
Results
Evaluation of preoperative tumor localization and vessel anatomy using 3D-CTAC
A total of 1256 CRC patients underwent surgery at our hospital between January 2014 and December 2019. Among those, 96 patients (7.6%) with SFC were extracted based on the inclusion criteria. Patient characteristics and vascular anatomy of SFC were summarized in Table 1. The rate of proximal SFC was 41.7%, central SFC was 13.5%, and distal SFC was 44.8%. Intraoperative findings showed that tumor localization was exactly the same as preoperative diagnosis in all cases. Preoperative 3D-CTAC enabled accurate tumor localization and evaluation of feeding artery and venous variations of SFC in all patients (Fig. 1). The blood supply from the SMA to the splenic flexure was through the left branch of MCA (LMCA) or AMCA. The AMCA was recognized in 33 patients (34.4%). The right branch of MCA (RMCA) and LMCA diverged from the main trunk of MCA in 73 patients (76.0%), and the RMCA and LMCA independently diverged from the SMA in 19 patients (19.8%). LMCA was deficient in four patients (4.2%). Meanwhile, AMCA was present in all patients. The blood supply from the IMA to the splenic flexure was through the left colic artery (LCA) in all cases. The LCA diverged from IMA in all patients, and there were 19 patients (19.8%) whose LCA formed a common trunk with the first sigmoid colon artery. The IMV returned to the SMV in 28 patients (29.2%) and to the splenic vein in 68 patients (70.8 %). The SFV was present in 72 patients (75.0%). The SFV returned to the IMV in 69 patients (71.9 %) and to the splenic vein in three patients (3.1 %).
Representative case using 3D-CTAC of tumor localization and vascular anatomy in patients with SFC. a 3D-CT angiography. The AMCA and the MCA were branched from SMA. The LCA was branched from IMA. Preoperative colonoscopy using mucosal clipping reveals tumor localization (arrow). b 3D-CT angiography with colonography. CT colonography showed an apple-core stenosing tumor in the distal transverse colon (arrow). By combining CT angiography and colonography, surgeons could evaluate tumor localization and vessel running preoperatively
Arterial variations of SFC focused on MCA and AMCA
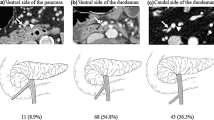
Arterial variations of SFC were presented in Fig. 2 and Table 2. Based on our results, arterial blood supply to the SFC was classified into five types: (type 1A) the LMCA diverged from the main trunk of the MCA (N = 47, 49.0%); (type 2A) the LMCA diverged from the main trunk of the MCA and the AMCA (N = 26, 27.1%); (type 3A) the LMCA independently diverged from the SMA (N = 16, 16.7%); (type 4A) the LMCA independently diverged from the SMA and the AMCA (N = 3, 3.1%); (type 5A) the AMCA and the LMCA were absent (N = 4, 4.1%). All patients lacking LMCA had a branching AMCA from SMA.
Venous variations of SFC focused on the IMV and the SFV
The venous drainage pattern from SFC was classified into four types (Fig. 3 and Table 2): (type 1V) the SFV flows into the IMV then back to the splenic vein (N = 50, 52.1%); (type 2V) the SFV flows into the IMV then back to the SMV (N = 19, 19.8%); (type 3V) the SFV independently flows into the splenic vein (N = 3, 3.1%); (type 4V) the SFV is absent (N = 24, 25.0%).
Discussion
This study revealed the vascular anatomy of SFC under 3D-CTAC. The main findings of the study were as follows: first, SFC was present in 7.6% of CRC patients, and all patients were preoperatively diagnosed through accurate tumor localization and vascular anatomy by the 3D-CTAC. Second, the AMCA was manifested in 34.4% of SFC patients, and arterial variations of SFC were classified into five types. Finally, the SFV was evident in 75.0% of SFC patients, and we classified venous drainage variations into four types.
SFC surgery was first described in the early 1900s. Jamieson et al. advocated the resection of the lymph area in colorectal cancer surgery and reported that surgery for SFC would require blockage of blood supply from the MCA and the LCA [15]. The existence of the AMCA has been known since then, and the recognition rate of the AMCA has been increasing due to the improvement of diagnostic imaging technology [16,17,18,19]. Tanaka et al. reported the arterial anatomy of the splenic flexure using 3D-CT [6]. They analyzed 88 patients, where the AMCA was detected in 27 patients (30.7%). This result was consistent with the present study. On the other hand, Fukuoka et al. revealed that the incidence of the AMCA evaluated by 3D-CTA was only 6.7%, which was almost the same as reports from Western countries [20, 21]. This difference may be due to varied protocols of 3D-CT imaging and ethnic differences in blood vessel variation. With regard to the blood supply of SFC from the SMA, our study suggested that surgeons should recognize whether the LMCA is derived from the common trunk of the MCA (Type1A + Type2A; 76.1%), an independent origin from the SMA (Type3A + Type4A; 19.8%), or absent (Type5A; 4.1%). In particular, there were some cases where the LMCA was absent [22, 23]. Hence, surgeons should know that the AMCA exists in such cases. In addition, there were some reports of blood flow to the pancreas from the AMCA. Ito et al. reported cases in which the dorsal pancreatic artery (DPA) and the inferior pancreaticoduodenal artery (IPDA) diverged from the AMCA and flowed into the pancreas, with a frequency of 8.1% [24]. The DPA and IPDA had thin blood vessels and were not evaluated in our study.
In the current study, the SFV was present in 75% of SFC patients, and the SFV returned to either the splenic vein or the IMV. Arimoto et al. reported that there was a 2% pattern of return to the middle colonic vein, but whether this vein should be called the SFV is controversial [14]. Considering the concepts of CME with CVL and the presence of lymph flow around IMV, these venous variations should be recognized prior to surgery to determine the area of lymph node dissection and the optimal central venous ligation.
This study is the first report that utilized 3D-CTAC to assess tumor localization of the SFC and to visualize and classify both arterial and venous anatomies as far as possible. Considering the anatomical features and abundance of the vascular variations in SFC, it is considered that accurate preoperative evaluation of tumor localization greatly contributes to the identification of the optimal areas of CME and CVL.
This study has some limitations. First, the small sample size and single-center setting may limit the generalization of results. Since 3D-CTAC examination can be performed by usual CT and colonoscopy, it may be possible to perform it in general hospitals. However, immediate CT imaging after colonoscopy and reconstruction of images from the surgeon’s point of view are required, and multidisciplinary teamwork including radiology technologists is needed. Second, in this study, the AMCA was defined as branching from the SMA, and blood vessels branching from the coeliac artery were excluded. Previously, there were reports that the feeding arteries to SFC originated from the branches of the coeliac artery, such as the hepatic artery, splenic artery, and gastroduodenal artery. Murono et al. reported that the frequency of the AMCA derived from the coeliac flow was 10.9% [13]. Whether blood vessels branching from the coeliac artery should be called the AMCA is still controversial. Furthermore, from the concept of CME with CVL, if the feeding artery of SFC is derived from the coeliac system, it is worth considering whether lymph node dissection should be performed around it. In the future, it is necessary to evaluate the lymphatic flow around the coeliac artery under indocyanine green fluorescence imaging to reveal the frequency of lymph node metastasis and the efficacy of lymph node dissection around the coeliac artery, if the SFC possesses the AMCA derived from the coeliac flow. Furthermore, in addition to the vascular variation clarified in this study, it will be possible to clarify the optimal lymph node dissection range of SFC by evaluating the metastasis rate of each lymph node station. Future studies are necessary and will help improve long-term survival in SFC patients.
Conclusions
In conclusion, the accurate preoperative assessment of tumor localization and vascular branching of SFC could be taken by 3D-CTAC. The AMCA was detected in 34.4% of SFC patients, while the SFV was detected in 75.0%. Arterial anatomy was classified into five types, while venous anatomy was classified into four types. Understanding the vascular branching of SFC is helpful in performing accurate CME and CVL.
Data availability
All data analyzed during this study are included in this published article.
References
Matsumura N, Tokumura H, Saijo F, Katayose Y (2018) Strategy of laparoscopic surgery for colon cancer of the splenic flexure: a novel approach. Surg Endosc 32:2559
Pisani Ceretti A, Maroni N, Sacchi M, Bona S, Angiolini MR, Bianchi P, Opocher E, Montorsi M (2015) Laparoscopic colonic resection for splenic flexure cancer: our experience. BMC Gastroenterol 15:76
Okuda J, Yamamoto M, Tanaka K, Masubuchi S, Uchiyama K (2016) Laparoscopic resection of transverse colon cancer at splenic flexure: technical aspects and results. Updat Surg 68:71–75
Manceau G, Mori A, Bardier A, Augustin J, Breton S, Vaillant JC, Karoui M (2018) Lymph node metastases in splenic flexure colon cancer: is subtotal colectomy warranted? J Surg Oncol 118:1027–1033
Miyake H, Murono K, Kawai K, Hata K, Tanaka T, Nishikawa T, Otani K, Sasaki K, Kaneko M, Emoto S, Nozawa H (2018) Evaluation of the vascular anatomy of the left-sided colon focused on the accessory middle colic artery: a single-centre study of 734 patients. Color Dis 20:1041–1046
Tanaka T, Matsuda T, Hasegawa H, Yamashita K, Nakamura T, Suzuki S, Kakeji Y (2019) Arterial anatomy of the splenic flexure using preoperative three-dimensional computed tomography. Int J Color Dis 34:1047–1051
Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y et al (2018) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 23:1–34
Watanabe J, Ota M, Suwa Y, Ishibe A, Masui H, Nagahori K (2017) Evaluation of lymph flow patterns in splenic flexural colon cancers using laparoscopic real-time indocyanine green fluorescence imaging. Int J Color Dis 32:201–207
Mari FS, Nigri G, Pancaldi A, De Cecco CN, Gasparrini M, Dall’Oglio A et al (2013) Role of CT angiography with three-dimensional reconstruction of mesenteric vessels in laparoscopic colorectal resections: a randomized controlled trial. Surg Endosc 27:2058–2067
Obaro AE, Burling DN, Plumb AA (2018) Colon cancer screening with cT colonography: logistics, cost-effectiveness, efficiency and progress. Br J Radiol 91(1090):20180307
De Haan MC, Pickhardt PJ, Stoker J (2015) CT colonography: accuracy, acceptance, safety and position in organised population screening. Gut 64:342–350
Bian L, Wu D, Chen Y, Zhang Z, Ni J, Zhang L, Xia J (2019) Clinical value of multi-slice spiral CT angiography, colon imaging, and image fusion in the preoperative evaluation of laparoscopic complete mesocolic excision for right colon cancer: a prospective randomized trial. J Gastrointest Surg
Murono K, Miyake H, Hojo D, Nozawa H, Kawai K, Hata K, Tanaka T, Nishikawa T, Shuno Y, Sasaki K, Kaneko M, Emoto S, Ishii H, Sonoda H, Ishihara S (2020) Vascular anatomy of the splenic flexure, focusing on the accessory middle colic artery and vein. Color Dis 22:392–398
Arimoto A, Matsuda T, Hasegawa H, Yamashita K, Nakamura T, Sumi Y, Suzuki S, Kakeji Y (2019) Evaluation of the venous drainage pattern of the splenic flexure by preoperative three-dimensional computed tomography. Asian J Endosc Surg 12:412–416
Jamieson JK, Dobson JF (1909) Lymphatics of the colon: with special reference to the operative treatment of cancer of the colon. Ann Surg 50:1077–1090
Robillard GL, Shapiro AL (1947) Variational anatomy of the middle colic artery: its significance in gastric and colonic surgery. J Int Coll Surg 10:157–169
Griffiths JD (1956) Surgical anatomy of the blood supply of the distal colon. Ann R Coll Surg Engl 19:241–256
Sonneland J, Anson BJ, Beaton LE (1958) Surgical anatomy of the arterial supply to the colon from the superior mesenteric artery based upon a study of 600 specimens. Surg Gynecol Obstet 106:385–398
Koizumi M, Horiguchi M (1990) Accessory arteries supplying the human transverse colon. Cells Tissues Organs 137:246–251
Fukuoka A, Sasaki T, Tsukikawa S, Miyajima N, Ostubo T (2017) Evaluating distribution of the left branch of the middle colic artery and the left colic artery by CT angiography and colonography to classify blood supply to the splenic flexure. Asian J Endosc Surg 10:148–153
Rusu MC, Vlad M, Voinea LM, Curcǎ GC, Şişu AM (2008) Detailed anatomy of a left accessory aberrant colic artery. Surg Radiol Anat 30:595–599. https://doi.org/10.1007/s00276-008-0362-1
Nesgaard JM, Stimec BV, Bakka AO, Edwin B, Ignjatovic D, Oresland T et al (2015) Navigating the mesentery: a comparative pre- and per-operative visualization of the vascular anatomy. Color Dis 17:810–818
Alsabilah J, Kim WR, Kim NK (2017) Vascular structures of the right colon: incidence and variations with their clinical implications. Scand J Surg 106:107–115
Ito K, Takemura N, Inagaki F, Mihara F, Kurokawa T, Kokudo N (2019) Arterial blood supply to the pancreas from accessary middle colic artery. Pancreatology. 19:781–785
Acknowledgments
The authors would like to express their heartfelt gratitude to all pathologists and radiologists at the Saiseikai Yokohamashi Nanbu Hospital who had always provided carefully considered and constructive feedback and valuable comments.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Kenta Iguchi, Hiroyuki Mushiake, Seiji Hasegawa, and Tadao Fukushima. The first draft of the manuscript was written by Kenta Iguchi and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval/consent to participate
This study was approved by the institutional review board of Saiseikai Yokohamashi Nanbu Hospital, and a written informed consent for the use of medical records was obtained from the patients (NANBU D-23). The study was conducted based on the ethical guidelines of the Declaration of Helsinki.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iguchi, K., Mushiake, H., Hasegawa, S. et al. Evaluation of vascular anatomy for colon cancer located in the splenic flexure using the preoperative three-dimensional computed tomography angiography with colonography. Int J Colorectal Dis 36, 405–411 (2021). https://doi.org/10.1007/s00384-020-03773-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-020-03773-x