Abstract
Purpose
The treatment of splenic flexural colon cancer is not standardized because the lymphatic drainage is variable. The aim of this study is to evaluate the lymph flow at the splenic flexure.
Methods
From July 2013 to January 2016, consecutive patients of the splenic flexural colon cancer with a preoperative diagnosis of N0 who underwent laparoscopic surgery were enrolled. Primary outcome is frequency of the direction of lymph flow from splenic flexure. We injected indocyanine green (2.5 mg) into the submucosal layer around the tumor and observed lymph flow using the laparoscopic near-infrared camera system in 30 min after injection.
Results
Thirty-one patients were enrolled in this study. The lymph flow was visualized in 31 patients (100 %) without any complications. No case exhibited lymph flow in both the left colic artery (LCA) and left branch of the middle colic artery (lt-MCA) areas. There were 19 cases (61.3 %) with lymph flow directed to the area of the root of the inferior mesenteric vein (IMV), regardless of the presence of the left accessory aberrant colic artery. Lymph node metastases were observed in six cases (19.4 %), and all of the involved lymph nodes existed in lymph flow areas determined by real-time indocyanine green fluorescence imaging.
Conclusions
The findings of the lymph flow pattern of splenic flexure suggest that lymph node dissection at the root of the IMV area is important, and it may be not necessary to ligate both the lt-MCA and LCA, at least in cases without widespread lymph node metastases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many clinical trials have demonstrated the benefits and safety of laparoscopic surgery for colon cancer compared with open surgery [1–4]. Moreover, the long-term outcomes after laparoscopic surgery for colon cancer are equal to that of open surgery [5–7]. Therefore, laparoscopic surgery for colon cancer has become an alternative to open surgery. Complete mesocolic excision (CME) and central vascular ligation (CVL) are current state-of-the-art colon cancer treatments that may provide improved oncologic outcomes [8, 9]. However, laparoscopic surgery of colon cancer located in the splenic flexure is not standardized. Patients with tumors in this location have been excluded from randomized controlled trials because of difficulty in deciding on the appropriate operative procedure, as well as technical difficulties with laparoscopic lymph node dissection. The lymphatic drainage at this site is variable, and the exact site of lymphatic dissection is uncertain [10–16].
It is considered that carcinoma at this site has several lymphatic drainage roots. The lymphatic drainage roots may be the left branch of the middle colic artery (lt-MCA) and left colic artery (LCA) areas [12], in addition to the left accessory aberrant colic artery (LAACA) area when present [17]. Moreover, aberrant metastatic pathways to the infrapancreatic lymph node region and the splenic hilum have been reported [11, 16]. However, to date, there have been no systematic data in the literature regarding the frequency of lymphatic drainage roots at this site.
In recent years, the usefulness of indocyanine green fluorescent imaging (ICG-FI) has been reported in various surgical oncology studies, such as the identification of sentinel lymph nodes in breast cancer [18]. In the colorectal cancer field, many reports have evaluated the blood flow, whereas reports on the lymph flow have been scarce [19–23].
The aim of this study was to evaluate the lymph flow patterns in splenic flexural colon cancers using laparoscopic real-time ICG-FI.
Materials and methods
Patients
This study protocol was approved by the Ethics Advisory Committee of Yokosuka Kyosai Hospital before initiation and was registered in the UMIN Clinical Trials Registry as UMIN-CTR000013047 [http://www.umin.ac.jp/ctr/index.htm]. The procedure used during this study was explained, and written informed consent was obtained from all of the patients. From July 2013 to January 2016, consecutive patients with a preoperative diagnosis of colon cancer at the splenic flexure with a preoperative diagnosis of N0 who underwent laparoscopic surgery with lymph node dissection in Yokosuka Kyosai Hospital were enrolled in this study. The splenic flexure was defined as the junction between the distal third of the transverse colon and the first part of the descending colon [14, 24]. The cancer site was diagnosed using colonoscopy and a barium enema. The clinical stage was diagnosed using contrast-enhanced computed tomography (CT) of the chest and abdomen. A lymph node with a minor axis measuring >10 mm on CT was defined as a metastatic lymph node in this study. The exclusion criteria were a past history of colonic surgery, extended colorectal resection, allergic hypersensitivity to indocyanine green (ICG), or allergic hypersensitivity to iodine. The primary outcome was the frequency of the direction of the lymph flow of splenic flexural colon cancer. Secondary outcomes were identification of the lymph flow (in percentage), number of fluorescent lymph nodes, and total number of dissected lymph nodes.
Indocyanine green fluorescent imaging
We performed tumor marking with India ink under colonoscopic vision <96 h before surgery. A stock solution of ICG (Diagnogreen; Daiichi Pharmaceuticals, Tokyo, Japan) was prepared by dissolving 25 mg of powdered ICG in 10.0 ml of sterilized water; 1.0 ml of the suspension was used for each injection. The laparoscopic NIR camera system was provided by Karl Storz (D-Light P; Tuttlingen, Germany). For the detection of the lymph flow, we laparoscopically injected ICG (2.5 mg/1.0 ml) into the subserosal-submucosal layer around the tumor at two points with a 23-gauge localized injection after trocar insertion, guided the tumor marking with India ink, and observed the lymph flow laparoscopically using the near-infrared camera system 30 min after ICG injection.
Operative procedure
A single surgeon (JW) who was qualified by the endoscopic surgical skill qualification system of the Japan Society for Endoscopic Surgery (JSES) [25] performed CME with CVL. The operative procedure was performed with five ports: a 12-mm port in the umbilical region; 5-mm ports in the upper-right, left, and lower-left quadrants; and a 12-mm port in the lower-right quadrant. A 12-mm umbilical trocar was used as a camera port for a rigid scope. CVL and colon mobilization were performed laparoscopically. Lymph node dissection was performed without regard to the fluorescence results. The lymphatic drainage roots of the lt-MCA and LCA and the root of the inferior mesenteric vein (IMV) areas were dissected by ligating the lt-MCA, LCA, IMV (at the inferior border of pancreas), and the LAACA (when present). The specimen was extracted through the umbilical port, which extended to about 2–5 cm. To avoid contamination, a wound protector was used in every case. Functional end-to-end anastomosis (FEEA) was performed using the Endo GIA™ Universal or Echelon™60 stapling system extracorporeally. After the specimen was extracted through the umbilical incision, we excised the lymph nodes from the mesentery on the back table and observed the lymph nodes extracorporeally using the near-infrared camera system (HyperEye Medical System, Mizuho Corporation, Tokyo, Japan) to evaluate the number of fluorescent lymph nodes.
Statistical analysis
The data are presented as the mean and standard deviation or as the median and range. Statistical analyses were performed using the SPSS statistical software program (SPSS Inc., Chicago, IL, USA).
Results
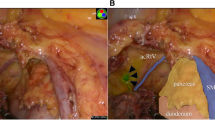
A total of 31 consecutive patients were enrolled in this study. The clinicopathological characteristics and surgical outcomes of the patients are presented in Table 1. These patients included nine females and had a mean age of 67.5 years and a mean body mass index of 23.6 kg/m2. The mean operative time was 208 min, and the mean blood loss was 52 ml. The lymph flow was visualized in 31 patients (100 %) intraoperatively (Fig. 1). All procedures were safely performed without any complications, and there were no issues with outshining lymph nodes near the tumor or spillage of ICG.
The presence of LAACA rate was 38.7 % (12 cases). In these cases, the prevalent direction of the lymph flow was the LAACA and LCA areas in five cases (16.1 %), the LAACA area in four cases (12.9 %), and the LAACA and lt-MCA areas in three cases (9.7 %) (Fig. 2). Regarding cases in which the LAACA was absent, the prevalent direction of the lymph flow was the LCA area in eight cases (25.8 %), the lt-MCA area in six cases (19.4 %), and the root of IMV which did not accompany the artery in five cases (16.1 %). Among eight cases in which the lymph flow ran along the LCA, we observed two cases (6.5 %) that had lymph flow running along the LCA at the beginning, followed by branching to the root of the LCA and to the root of the IMV (Fig. 3). Overall, no case exhibited lymph flow in both the LCA and lt-MCA areas. There were 19 cases (61.3 %) with lymph flow directed to the area of the root of IMV, regardless of the presence of the LAACA. The total number of retrieved lymph nodes was 17.5, and fluorescent lymph nodes were 10.4 (Fig. 4). Lymph node metastases were observed in six cases (19.4 %), and all of the involved lymph nodes existed in lymph flow areas determined by the fluorescence lymphatic flow evaluation.
Regarding cases in which the LAACA was absent, the prevalent direction of the lymph flow was the LCA area in eight cases (25.8 %), the lt-MCA area in six cases (19.4 %), and the root of IMV which did not accompany the artery in five cases (16.1 %). Among eight cases in which the lymph flow ran along the LCA, we observed two cases (6.5 %) that had lymph flow running along the LCA at the beginning, followed by branching to the root of the LCA and to the root of the IMV
Discussion
In CME for splenic flexural colon cancer, dissection of both the transverse and descending mesocolon must be considered [12]. In addition, due to blood vessel variation and complicated lymph flow, including aberrant lymph flow in the splenic flexure sites [10–16], lymph node dissection may be required depending on each individual case; however, it is quite difficult to determine the necessity during surgery. The blood supply to the splenic flexure has been shown to be variable; it is predominantly carried by the inferior mesenteric artery (IMA) via the LCA in 89 % of cases, while the remaining cases (11 %) are carried by the superior mesenteric artery (SMA) via the middle colic artery (MCA) [26]. Moreover, a LAACA can aberrantly originate from the SMA more proximal to the MCA, at the inferior border of the pancreas, which supplies the splenic flexure and the proximal part of the descending colon [17]. The frequency of appearance has been reported to be 4–49.2 % [17]. In this study, the frequency of the presence of LAACA was 38.7 % (12 cases). With respect to the selection of the operative procedure for CME for splenic flexural colon cancer, left hemicolectomy with CVL, ligating both the MCA at its origin, the ascending branch of the LCA, and, if it exists, the LAACA to remove the lymph nodes likely involved, has been advocated [10, 12]. However, there have been no systematic data in the literature on the choice of operative procedure at this site. Thus, understanding the lymph flow pattern of splenic flexural colon cancer would be useful. In this study, no case exhibited a lymph flow directed to the areas of both the LCA and lt-MCA, and 19 cases (61.3 %) demonstrated a lymph flow directed to the area of the root of IMV, regardless of the presence of the LAACA. With regard to the distal third of the transverse colon cancer (six cases), the lymph flow ran along the lt-MCA in all cases. With regard to the first part of the descending colon cancer (seven cases), the lymph flow ran along the LCA in all cases. Thus, in the case of the distal third of the transverse colon cancer, we should perform CME with lymph node dissection of the lt-MCA and the root of the IMV areas; in the case of the first part of the descending colon cancer, we should perform CME with lymph node dissection of the LCA and the root of the IMV areas. In the case of just splenic flexural colon cancer, the lymph flow could run in various directions. Therefore, the use of laparoscopic real-time ICG-FI may help to identify appropriate central vessels to be dissected, as well as determine the appropriate separation line of mesentery. We speculate that it is not necessary to ligate both the MCA and LCA, at least in cases without widespread lymph node metastasis, because no case with lymph flow directed to both the LCA and lt-MCA was observed in this study.
Direct lymphatic drainage to the splenic hilum and along the pancreatic tail has been demonstrated in anatomical studies of lymphatic drainage of the splenic flexure, and routine splenectomy with distal pancreatectomy has been advocated for splenic flexural colon cancer [24]. On the other hand, it has been reported that splenectomy and distal pancreatectomy for splenic flexural colon cancer did not improve the long-term survival [15]. In this study, no lymph flow directed to the pancreatic tail and the splenic hilum was observed. Therefore, we believe that routine distal pancreatectomy and splenectomy are not necessary, at least in cases without widespread lymph node metastasis.
ICG-FI has been developed for the use of perioperative blood flow assessment and identification of sentinel lymph nodes in coronary artery bypass grafting and melanoma and breast cancer surgery [18, 27, 28]. In the field of colorectal cancer, it has been used for marking tumors [29], blood flow assessment for anastomotic sites [19–21, 23], and identification of sentinel lymph nodes [30]. In the colorectal cancer field, regarding false-negative results (low sensitivity), an optimum biopsy method for the sentinel lymph nodes has not yet been established, and further clinical trials are required for such a biopsy to be applied in actual clinical settings [31–33]. ICG-FI is capable of assessing not only lymph nodes but also the lymph flow in real time [22]. However, lymph flow assessments using ICG-FI have been scarcely reported [22]. In this study, the lymph flow was visualized in 31 patients (100 %) intraoperatively without any complications. This lymph flow evaluation differs from sentinel lymph node mapping in terms of the selective removal of the mesocolon to drain the tumor. Furthermore, it is possible to clinically apply this method in combination with the concept of CME without causing problems such as a false-negative result for the sentinel lymph nodes, helping to optimize and individualize CME. Indeed, in two patients histopathologically diagnosed with lymph node metastases, no metastases were detected in the fluorescent sentinel lymph nodes. These two patients were found to have skip metastases. However, all of the involved lymph nodes in these patients existed in the lymph flow areas observed on the fluorescence lymphatic flow evaluation.
Sentinel lymph node mapping of patients with colorectal cancer is a difficult technique, and a learning curve of 20–30 cases has been described. However, this lymph flow evaluation is better suited for clinical application than sentinel lymph node biopsy and has less of a learning curve. Before conducting the present study, we evaluated the intraoperative lymphatic flow using ICG in ten patients. Spillage was observed during injection of ICG in 1 of the 10 patients. In the present study, no ICG spillage occurred.
In this study, two patients had a body mass index >30 kg/m2 (31.63 and 31.14 kg/m2). The lymph flow was visualized in both patients intraoperatively. We therefore believe that ICG fluorescence imaging is acceptable for patients with high BMI.
In cases with widespread lymph node metastasis as the lymph flow may be bypassed to other areas as a result of lymphatic vessel occlusion, this method may possibly misinterpret the dissection range [34]. For these reasons, the present study included cases without any obvious lymph node metastasis observed in the preoperative CT scan. However, a preoperative CT scan is not standard worldwide. The applicability of these findings is therefore limited to institutions where a preoperative CT scan is routinely performed.
There are some limitations associated with this study. Only a small number of patients were included, and the follow-up period was short. Further studies with a larger population and a long-term follow-up are needed to confirm our findings.
In conclusion, the findings on the analysis of the lymph flow pattern using ICG-FI during laparoscopic splenic flexural colon cancer surgery suggest that lymph node dissection of the root of the IMV area is important, and it may not be necessary to ligate both the lt-MCA and LCA, at least in cases without widespread lymph node metastases. The comprehension of this lymph flow pattern may be helpful for identifying appropriate central vessels to be dissected.
References
The Clinical Outcomes of Surgical Therapy Study Group (2004) A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 350(20):2050–2059
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365(9472):1718–1726
Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Pahlman L, Cuesta MA, Msika S, Morino M, Lacy AM (2005) Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 6(7):477–484
Yamamoto S, Inomata M, Katayama H, Mizusawa J, Etoh T, Konishi F, Sugihara K, Watanabe M, Moriya Y, Kitano S (2014) Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: Japan Clinical Oncology Group Study JCOG 0404. Ann Surg 260(1):23–30
Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Pahlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ (2009) Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 10(1):44–52
Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ (2010) Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 97(11):1638–1645
Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, Pique JM (2008) The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg 248(1):1–7
West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P (2010) Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 28(2):272–278
West NP, Kobayashi H, Takahashi K, Perrakis A, Weber K, Hohenberger W, Sugihara K, Quirke P (2012) Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol 30(15):1763–1769
Pisani Ceretti A, Maroni N, Sacchi M, Bona S, Angiolini MR, Bianchi P, Opocher E, Montorsi M (2015) Laparoscopic colonic resection for splenic flexure cancer: our experience. BMC Gastroenterol 15:76
Perrakis A, Weber K, Merkel S, Matzel K, Agaimy A, Gebbert C, Hohenberger W (2014) Lymph node metastasis of carcinomas of transverse colon including flexures. Consideration of the extramesocolic lymph node stations. Int J Color Dis 29(10):1223–1229
Nakagoe T, Sawai T, Tsuji T, Jibiki M, Ohbatake M, Nanashima A, Yamaguchi H, Yasutake T, Kurosaki N, Ayabe H, Ishikawa H (2001) Surgical treatment and subsequent outcome of patients with carcinoma of the splenic flexure. Surg Today 31(3):204–209
Nakagoe T, Sawa T, Tsuji T, Jibiki M, Nanashima A, Yamaguchi H, Yasutake T, Ayabe H, Ishikawa H (2000) Carcinoma of the splenic flexure: multivariate analysis of predictive factors for clinicopathological characteristics and outcome after surgery. J Gastroenterol 35(7):528–535
Steffen C, Bokey EL, Chapuis PH (1987) Carcinoma of the splenic flexure. Dis Colon rectum 30(11):872–874
Khafagy MM, Stearns MW Jr (1973) Carcinoma of the splenic flexure. Dis Colon rectum 16(6):504–507
Jamieson JK, Dobson JF (1909) The lymphatics of the colon. Proc R Soc Med 2(Surg Sect):149–174
Rusu MC, Vlad M, Voinea LM, Curca GC, Sisu AM (2008) Detailed anatomy of a left accessory aberrant colic artery. Surg Radiol Anat 30(7):595–599
Mieog JS, Troyan SL, Hutteman M, Donohoe KJ, van der Vorst JR, Stockdale A, Liefers GJ, Choi HS, Gibbs-Strauss SL, Putter H, Gioux S, Kuppen PJ, Ashitate Y, Lowik CW, Smit VT, Oketokoun R, Ngo LH, van de Velde CJ, Frangioni JV, Vahrmeijer AL (2011) Toward optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann Surg Oncol 18(9):2483–2491
Jafari MD, Lee KH, Halabi WJ, Mills SD, Carmichael JC, Stamos MJ, Pigazzi A (2013) The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc 27(8):3003–3008
Jafari MD, Wexner SD, Martz JE, McLemore EC, Margolin DA, Sherwinter DA, Lee SW, Senagore AJ, Phelan MJ, Stamos MJ (2015) Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg 220(1):82–92 e81
Kudszus S, Roesel C, Schachtrupp A, Hoer JJ (2010) Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbeck’s Arch Surg 395(8):1025–1030
Nishigori N, Koyama F, Nakagawa T, Nakamura S, Ueda T, Inoue T, Kawasaki K, Obara S, Nakamoto T, Fujii H, Nakajima Y (2016) Visualization of lymph/blood flow in laparoscopic colorectal cancer surgery by ICG fluorescence imaging (lap-IGFI. Ann Surg Oncol 23(Suppl 2):266–274
Sherwinter DA, Gallagher J, Donkar T (2013) Intra-operative transanal near infrared imaging of colorectal anastomotic perfusion: a feasibility study. Color Dis 15(1):91–96
Aldridge MC, Phillips RK, Hittinger R, Fry JS, Fielding LP (1986) Influence of tumour site on presentation, management and subsequent outcome in large bowel cancer. Br J Surg 73(8):663–670
Mori T, Kimura T, Kitajima M (2010) Skill accreditation system for laparoscopic gastroenterologic surgeons in Japan. Minim Invasive Ther Allied Technol 19(1):18–23
Griffiths JD (1956) Surgical anatomy of the blood supply of the distal colon. Ann R Coll Surg Engl 19(4):241–256
Korn JM, Tellez-Diaz A, Bartz-Kurycki M, Gastman B (2014) Indocyanine green SPY elite-assisted sentinel lymph node biopsy in cutaneous melanoma. Plast Reconstr Surg 133(4):914–922
Reuthebuch O, Haussler A, Genoni M, Tavakoli R, Odavic D, Kadner A, Turina M (2004) Novadaq SPY: intraoperative quality assessment in off-pump coronary artery bypass grafting. Chest 125(2):418–424
Nagata J, Fukunaga Y, Akiyoshi T, Konishi T, Fujimoto Y, Nagayama S, Yamamoto N, Ueno M (2016) Colonic marking with near-infrared, light-emitting, diode-activated indocyanine green for laparoscopic colorectal surgery. Dis Colon rectum 59(2):e14–e18
Cahill RA, Anderson M, Wang LM, Lindsey I, Cunningham C, Mortensen NJ (2012) Near-infrared (NIR) laparoscopy for intraoperative lymphatic road-mapping and sentinel node identification during definitive surgical resection of early-stage colorectal neoplasia. Surg Endosc 26(1):197–204
Doekhie FS, Peeters KC, Kuppen PJ, Mesker WE, Tanke HJ, Morreau H, van de Velde CJ, Tollenaar RA (2005) The feasibility and reliability of sentinel node mapping in colorectal cancer. Eur J Surg Oncol 31(8):854–862
Des Guetz G, Uzzan B, Nicolas P, Cucherat M, de Mestier P, Morere JF, Breau JL, Perret G (2007) Is sentinel lymph node mapping in colorectal cancer a future prognostic factor? A meta-analysis. World J Surg 31(6):1304–1312
van der Pas MH, Meijer S, Hoekstra OS, Riphagen II, de Vet HC, Knol DL, van Grieken NC, Meijerink WJ (2011) Sentinel-lymph-node procedure in colon and rectal cancer: a systematic review and meta-analysis. Lancet Oncol 12(6):540–550
Kelder W, Braat AE, Karrenbeld A, Grond JA, De Vries JE, Oosterhuis JW, Baas PC, Plukker JT (2007) The sentinel node procedure in colon carcinoma: a multi-centre study in The Netherlands. Int J Color Dis 22(12):1509–1514
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study protocol was approved by the Ethics Advisory Committee of Yokosuka Kyosai Hospital before initiation and was registered in the UMIN Clinical Trials Registry as UMIN-CTR000013047 [http://www.umin.ac.jp/ctr/index.htm]. The procedure used during this study was explained, and written informed consent was obtained from all of the patients.
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Watanabe, J., Ota, M., Suwa, Y. et al. Evaluation of lymph flow patterns in splenic flexural colon cancers using laparoscopic real-time indocyanine green fluorescence imaging. Int J Colorectal Dis 32, 201–207 (2017). https://doi.org/10.1007/s00384-016-2669-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-016-2669-4