Abstract
In an aged human female cadaver a left accessory aberrant colic artery (LAACA) was observed and studied. It originated from the superior mesenteric artery at 3 cm proximal to the middle colic artery, at the inferior border of pancreas, passing over Treitz’s muscle and continued covered by the superior duodenal fold where it crossed the inferior mesenteric vein. Further, it continued with a satellite vein anterior to the left renal vein and the anterior branch of the renal artery. The LAACA divided into an ascending branch and a descending one, anastomosed with the middle colic and proper left colic arteries; between its two primary branches and the splenic flexure of colon, a hypovascular area was observed. The surgical relevance of the LAACA detailed anatomy mainly relates to specific procedures performed in left colectomies and nephrectomies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The unpaired visceral branches of the abdominal aorta are the celiac trunk and the mesenteric arteries, superior and inferior, derived from the vitelline arteries [13]. The superior mesenteric artery is by far the most important of the arteries to the alimentary tract, as it supplies the whole of the small intestine from the superior part of the duodenum to the midtransverse colon; its branches with colic distribution are the ileocolic artery, the artery to right colic flexure and the middle colic artery. The inferior mesenteric artery supplies the left third of the transverse colon, all the descending colon, sigmoid colon and most of the rectum; the left colic artery emerges from the inferior mesenteric artery and passes to the descending colon—its ascending branch runs in the transverse mesocolon and it is also known as ascending (intermesenteric) artery. On the left side of the ascending part of the duodenum, the duodenal and paraduodenal folds are formed by the parietal peritoneum; usually, the inferior mesenteric vein corresponds to the superior duodenal fold [5, 15]. Laterally, to the ascending part of the duodenum, the inferior mesenteric vein and the left colic artery usually constitute Treitz’s arch, anterior to the left renal pedicle.
Case report
Routine dissections were performed on a human adult cadaver, aged (76 year), female, on the left side of the mesentery in order to gain access on the left renal pedicle.
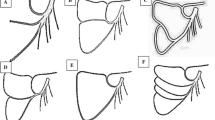
The intestinal loops were displaced towards the right side and the ascending part of the duodenum was evidenced, together with the duodenal folds: superior, inferior and paraduodenal and the corresponding recesses (Fig. 1a, b).
Stages of dissection of the LAACA originated from the superior mesenteric artery. a Evidence of the inferior mesenteric artery and the left (proper) colic artery: 1 first jejunal loop; 2 superior duodenal fold; 3 paraduodenal fold; 4 duodenum (ascending); 5 inferior duodenal fold; 6 abdominal aorta; 7 inferior mesenteric artery; 8 LAACA; 9 inferior mesenteric artery; 10 colon (descending); 11 middle left colic artery. b Dissection of the duodenal folds evidencing the LAACA covered by the superior duodenal fold: 1 transverse colon; 2 arcade of Haller–Riolan, transverse mesocolon; 3 first jejunal loop; 4 superior duodenal fold; 5 retroduodenal fossa; 6 duodenum (ascending); 7 inferior duodenal fold; 8 left ovarian artery; 9 left ovarian vein; 10 LAACA and its satellite vein; 11 left renal vein; 12 inferior mesenteric artery; 13 colon (descending). c Evidence of the LAACA and its satellite vein passing over the muscle of Treitz. 1 pancreas (body); 2 duodenojejunal angle; 3 muscle of Treitz, suspensor ligament of duodenum; 4 duodenum (ascending); 5 left ovarian artery; 6 posterior parietal peritoneum (reflected); 7 LAACA and the satellite vein; 8 left renal vein; 9 left kidney; 10 left ovarian vein; 11 inferior mesenteric vein; 12 colon (descending). d Evidence of the origin of the LAACA from the superior mesenteric artery. 1 transverse colon; 2 arcade of Haller–Riolan; 3 transverse mesocolon; 4 superior mesenteric vein; 5 middle colic artery; 6 superior mesenteric artery; 7 first jejunal loop; 8 body of pancreas; 9 LAACA and its vein; 10 left colic angle; 11 muscle of Treitz, suspensor ligament of duodenum; 12 inferior mesenteric vein; 13 descending colon. * hypovascular area between the branches of the LAACA and the left colic flexure
We started to dissect the inferior mesenteric vein above the level of the origin of the inferior mesenteric artery; the dissection of that vein was continued upwards, at the level of the paraduodenal fold. We evidenced a thick perivascular nerve satellite to the inferior mesenteric vein, on its medial aspect (Fig. 1a), but the inferior mesenteric vein was the only element of Treitz’s vascular arch and no artery was satellite to it.
We continued with the dissection of the inferior mesenteric artery; the superior rectal artery and a colosigmoid trunk were the only two primary branches of that artery. The colosigmoid trunk sent the left colic artery of 2-mm caliber and continued as a proper sigmoid trunk. The left colic artery proper, crossed behind the inferior mesenteric vein to reach the marginal arcade at the junction of the middle and inferior thirds of the descending colon (Fig. 1a). At the level of the marginal arcade a left colic vein added to the left colic artery; the vein drains into the inferior mesenteric vein.
The peritoneum on the left side of the ascending part of the duodenum was removed and the following elements were identified (Fig. 1b):
-
The left renal vein projected into the interval between the superior and inferior duodenal folds;
-
The left ovarian vein emptying into the left renal vein, located posterior to the inferior mesenteric vein;
-
The left ovarian artery arching inferior to the left renal vein continued medially to the left ovarian vein;
-
The inferior mesenteric vein passed anterior to the left renal vein and it continued arching from lateral to medial, beneath the superior duodenal fold:
-
at that level it was crossed over by an left accessory aberrant colic artery (LAACA);
-
the LAACA was followed by a periarterial nervous plexus and a satellite vein that drained into the inferior mesenteric vein, also located beneath the superior duodenal fold.
-
The dissection was continued and the left accessory aberrant colic artery (LAACA) was identified immediately postero-superior to the duodenojejunal flexure, crossing over the suspensory muscle of the duodenum or Treitz’s muscle, inferior to the body of the pancreas. From that level nerves and lymphatics were evidenced to continue inferior, over the left renal vein (Fig. 1c).
By displacing the duodenojejunal flexure and the body of the pancreas, the superior mesenteric vessels were identified. The LAACA was found emerging from the left side of the superior mesenteric artery, at the level of the parietal attachment of Treitz’s muscle. Below the origin of the LAACA, the middle colic artery also originated from the superior mesenteric artery. The inferior mesenteric vein crossed behind the left accessory colic artery and then over the superior mesenteric artery, above the origin of the LAACA. It was receiving the middle colic vein and joined the superior mesenteric vein into a common mesenteric trunk (Fig. 1d).
Approaching the splenic flexure of the colon, the LAACA crossed the anterior branch of the left renal artery (of 3-mm caliber) and at the level of the anterior lip of the left renal hilum it divided into an ascending and a descending branch. The former continued into the transverse mesocolon to participate in the arch of Haller and Riolan and the latter continued as the marginal artery, in immediate contact with the descending colon, to join the proper left colic artery. Between the two T-divided primary branches of the LAACA and the splenic colic flexure, a vascular poor-area could be identified (Fig. 1d).
At the origin from the superior mesenteric artery, the distance between the LAACA and the middle colic artery was 3 cm. The caliber of the LAACA was 2 mm. The length of the LAACA from the origin to the bifurcation was 6.6 cm, while its satellite vein measured 2.3 cm from the origin to the termination into the inferior mesenteric vein.
Discussion
According to Tandler, the definitive digestive arterial trunks (the coeliac trunk, the superior mesenteric artery, the inferior mesenteric artery) arise from several primitive arteries, which accounts for some variations induced by the craniocaudal shift along the aortic tube. During the lengthening of the digestive tube, the absorption and diversion will allow some primitive branches to disappear and respectively, develop. During their evolution, these primitive arteries progressively constitute the longitudinal anterior anastomosis of Tandler ahead of the aorta that will be correlated in adult with the coeliomesenteric and intermesenteric anastomoses (such as the arches of Riolan, Villemin and Drummond) [4]. Presumably, the absorbtion of the longitudinal anterior anastomosis of Tandler proximal to the left colic artery will make that one evolving as a branch of the inferior mesenteric artery, while the interruption of the anastomosis of Tandler distally to the left colic artery will make it become a branch of the superior mesenteric artery but this correlation needs to be demonstrated in embryos.
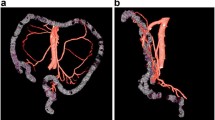
There are few reports that mention the existence of a left accessory colic artery (also described as l’artère colique accessoire d’Hovelacque) aberrantly originating from the superior mesenteric artery; the frequency of appearance is variable. Unfortunately for the surgical interest, a detailed anatomical and topographical description of such variant artery could not be found in the references we investigated. We present here the general schematic representation of the LAACA we report (Fig. 2).
Schema of the LAACA reported. 1 superior mesenteric a.; 2 middle colic artery; 3 superior mesenteric v.; 4 LAACA and its satellite vein; 5 arch of Riolan, in this case a superior intramesenteric anastomosis; 6 paucivascular area between the primary branches of the LAACA and the splenic flexure of colon; 7 inferior mesenteric v.; 8 left (proper) colic a.; 9 inferior mesenteric a
For example, in a study of Amonoo-Kuofi (1995) in two instances from a total of 27 cadavers, the LAACA was found originating from the superior mesenteric artery, meaning a rate of 0.54% for this variant. In the subjects with accessory left colic arteries, the superior mesenteric artery played a dominant role in the formation of the marginal artery by contributing the accessory left colic artery, which supplied the splenic flexure and the proximal part of the descending colon. The arterial variations underscore the importance of performing vascular studies prior to major abdominal surgery [1]. Also a volume-rendered 3D-CT may be helpful to assess the vascular branching anatomy for laparoscopic colorectal surgery [9].
Koizumi and Horiguchi communicated that an accessory colic artery, which arose from the superior mesenteric artery more proximally than the first jejunal artery and met the marginal artery at the splenic flexure was observed in 32 of 65 subjects (49.2%). Considering its constant morphological features, this accessory colic artery was given the new nomenclature “superior left colic artery” (arteria colica sinistra superior) [10]. The accessory/aberrant features of that artery and the topographical landmarks represented by Treitz’s muscle in our case, the superior duodenal fold and the inferior mesenteric vein are not mentioned in the report of the two authors.
The left accessory colic artery is much rarer than the right one. Its frequency varies from 4% by Nelson, 5% by Vandamme and 7.5% by Amonoo-Kuofi, to surprisingly high 25% by Calas, 35% of cases by Martin and 49.2% of cases by Koizumi and Horiguchi. Sonneland ranks the arteria colica sinistra accessoria to be an aberrant branch from the arteria mesenterica superior in only 0.4%; Vandamme in 0.6% of cases. Vandamme states that the artery leaves the arteria mesenterica superior at a higher place than the arteria colica media or other branches supplying the colon transversum, such as in our case presented here. It accompanies the vena mesenterica inferior in the base of the mesocolon transversum, heading towards the flexura coli sinistra, as in our case or, more frequently, to the colon descendens to join the arteria marginalis coli. Its calibre is reported to be 1.97 mm in average by Martin who proposed a classification of its origin site. It can originate from the arteria mesenterica superior trunk, the arteria colica media, the common trunk for the arteria colica dextra and media (arteria colica dextromedia), the arteria colica dextra, the arteria splenica or the arteria hepatica. According to Griffiths, any extramesocolic vessel running to the colon descendens has to be considered as the arteria colica sinistra accessoria. It is necessary to complete this view by a differentiation of the vessel origin. In case the vessel originates anywhere but in its own network (from the arteria mesenterica inferior or its branches), it is suggested to term it aberrant [8, 10].
We advocate that the left colic artery emerging from a superior mesenteric artery should be termed “accessory aberrant” to be distinguished from an accessory left colic artery that leaves the inferior mesenteric artery, as Kachlik and Hoch (2008) [8] also suggested.
Vandamme and the co-workers offered a consistent study of the inferior mesenteric artery branches, performed on156 abdominal preparations; the authors discuss that in the presence of a LAACA, the (proper) left colic artery is absent, atrophic or displaced [14]. In our case, the (proper) left colic artery appeared displaced.
We have to discuss the differences between the LAACA and Villemin’s arch (arcade). The latter is a large intermesenteric anastomosis, present in approximately 10% of the cases. First drawn by Mascagni, mentioned by Corsy, first name adopted by Villemin as “anastomose centrale intermésentérique”, it was widely described by Pikkieff (1931) who was the only one to use the term of “anastomosis accessoria”. Later authors called it “intermesenteric arcade”. This vessel connects either superior and inferior mesenteric trunk or, more frequently, their branches in the mesentery, close to its root, never in its peripheral margin [7]. It becomes clear that Villemin’s arcade cannot be confused with the LAACA.
The surgical anatomy of the colic vessels was also determined during surgery for esophageal replacement with colon (ERC), on 582 patients; in 97.3% of cases the left colic artery stemmed from the inferior mesenteric artery, with an absence rate of 0.7% [2]. For the esophageal replacements with colon it was advocated the use of the ascending branch of the left colic artery for blood supply and the transverse colon for replacement of the esophagus as the procedure of choice [2, 3]. Obviously, the presence of an undiagnosed LAACA will compromise that procedure.
Lorenzini and his co-workers mention in a study the angular artery of Donati as a distinctive supplier of the splenic colonic flexure originating from the superior mesenteric artery or from the splenic artery [11]. Within such limited territory, Donati’s artery cannot be confused with the LAACA.
One of the surgical relevancies of paying attention to a LAACA originating from the superior mesenteric artery refers to the resections of the colon and the respective ligatures—the segmentary hemicolectomy Dixon will visualize the respective colic vessels but a left hemicolectomy with ligation of the inferior mesenteric artery (as in the treatment of primary cancer of the colon) must check for the possible existence of such a LAACA [6]. Depending on the tumor location, the LAACA may come with a supplemental blood supply that must be considered for the surgical approach.
A surgical approach of the pancreas and mesenteric vessels must also take into account the possible existence of a LAACA hidden behind a middle colic vein that drains into the inferior mesenteric vein. By avoiding its damage, the surgeon will keep the splenic flexure of colon and the adjoining colic segments irrigated.
Radical nephrectomy is the gold standard for treatment of renal cell carcinoma and the early ligature using direct access to the renal artery at Treitz’s ligament permits the surgeon to follow the classic steps and principles of radical nephrectomy [12]. The laparoscopic procedure begins with the identification of the fourth portion of the duodenum and the inferior mesenteric vein, continues with the incision of peritoneal duodenum ligaments and further the ligament/muscle of Treitz is evidenced and incised. Considering the specific surgical procedure, we emphasize the importance for the surgeon to be aware of the detailed topography of a variant LAACA that will cross the field and will be exposed during the surgical approach of the renal pedicle.
References
Amonoo-Kuofi HS, el-Badawi MG, el-Naggar ME (1995) Anomalous origins of colic arteries. Clin Anat 8(4):288–293
Cheng BC, Chang S, Huang J, Mao ZF, Wang ZW, Lu SQ, Wang TS, Wu XJ, Hu H, Xia J, Kang GJ, Xiao YG, Lin HQ (2006) Surgical anatomy of the colic vessels in Chinese and its influence on the operation of esophageal replacement with colon. Zhonghua Yi Xue Za Zhi 86(21):1453–1456
Cheng BC (1989) Clinical study of colic vessels with respect to their significance in the replacement of the esophagus by the colon. Zhonghua Wai Ke Za Zhi 27(9):566–568, 575–576
Douard R, Chevallier JM, Delmas V, Cugnenc PH (2006) Clinical interest of digestive arterial trunk anastomoses. Surg Radiol Anat 28(3):219–227
Feneis H, Dauber W (2000) Pocket atlas of human anatomy (Based on the international nomenclature), 4th edn. Thieme, Stuttgart, pp 178, 220
Herfarth C, Runkel N (1994) Surgical standards in primary colon cancer. Chirurg 65(6):514–523
Kachlik D, Baca V (2006) Macroscopic and microscopic intermesenteric communications. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 150(1):121–124
Kachlik D, Hoch J (2008) The blood supply of the large intestine. Vydavatelstvi Karolnium, Praha
Kobayashi M, Morishita S, Okabayashi T, Miyatake K, Okamoto K, Namikawa T, Ogawa Y, Araki K (2006) Preoperative assessment of vascular anatomy of inferior mesenteric artery by volume-rendered 3D-CT for laparoscopic lymph node dissection with left colic artery preservation in lower sigmoid and rectal cancer. World J Gastroenterol 12(4):553–555
Koizumi M, Horiguchi M (1990) Accessory arteries supplying the human transverse colon. Acta Anat (Basel) 137(3):246–251
Lorenzini L, Bertelli L, Lorenzi M (1999) Arterial supply in the left colonic flexure. Ann Ital Chir 70(5):691–698
Porpiglia F, Renard J, Bilia M, Morra I, Scoffone C, Cracco C, Tarabuzzi R, Terrone C, Scarpa RM (2006) Left laparoscopic radical nephrectomy with direct access to the renal artery: technical advantages. Eur Urol 49(6):1004–1010
Sadler TW (1995) Langman’s medical embryology, 7th edn. Williams and Wilkins, Baltimore pp 216
Vandamme JP, Bonte J, van der Schueren G (1982) Re-evaluation of the colic irrigation from the inferior mesenteric artery. Acta Anat (Basel) 112(1):18–30
Williams Pl, Bannister LH, Berry MM, Collins P, Dyson M, Dussek JE, Ferguson MWJ (eds.) (1995) Gray’s anatomy, 38th edn. Churchill Livingstone, New York, pp 772–773
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rusu, M.C., Vlad, M., Voinea, L.M. et al. Detailed anatomy of a left accessory aberrant colic artery. Surg Radiol Anat 30, 595–599 (2008). https://doi.org/10.1007/s00276-008-0362-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-008-0362-1