Abstract
Purpose
Splenic infarction may occur if the splenic branches are injured or ligated accidentally during gastrectomy. We used three-dimensional computed tomography (3D-CT) imaging to distinguish the vascular anatomy of the splenic hilum in individual patients, focusing on the splenic polar branches and the gastric branches.
Methods
The subjects of this study were 104 patients who underwent computed tomography (CT) with intravenous contrast before gastrectomy. SYNAPSE 3D® (Fujifilm Medical, Tokyo, Japan) was used to generate the 3D-CT images. The total spleen volume and the area supplied by the superior polar artery (SPA) in each patient were estimated using the “liver analysis” function.
Results
The SPA without the gastric branch (supplying only the spleen), the SPA with the gastric branch (supplying both the stomach and the spleen), and the posterior gastric artery (supplying only the stomach) were present in 14, 45, and 18% of the patients, respectively. The SPA supplied 12% of the total spleen volume on average; however, it supplied over 30% in two patients.
Conclusion
We identified the vascular anatomy around the splenic hilum in over 100 patients. Based on our findings, we recommend preservation of the SPA when it is supplying a large area of the spleen. Preoperative 3D-CT analysis provides useful information to optimize safe gastrectomy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The role of splenectomy during total gastrectomy for gastric cancer has long been a topic of debate. In 2016, the Japan Clinical Oncology Group trial JCOG0110 concluded that splenectomy should be avoided in total gastrectomy for proximal gastric cancer unless the primary tumor is located in the greater curvature [1]. Consequently, the practice of performing splenectomy with total gastrectomy is expected to decrease, while spleen-preserving surgery with gastrectomy is expected to increase. The splenic artery usually originates from the celiac trunk, running along the superior edge of the pancreas, with some branches into the stomach and the pancreas, and terminating at the spleen. The branches to the stomach should be transected, whereas those to the spleen and pancreas should be preserved during the procedure. Splenic infarction may occur if the splenic branches are injured or ligated accidentally. In practice, we sometimes encounter ischemic color changes in the spleen during gastrectomy, especially in the polar segments.
Splenic infarction usually heals spontaneously; however, it can be fatal if it leads to splenic abscess, hemorrhage, or rupture [2, 3]. Despite the risk, splenic infarction is not considered a common postoperative complication of gastric cancer surgery [4, 5], and few reports document its incidence [6, 7]. Akin et al. [6] investigated the frequency of splenic infarction following gastric cancer surgery. They identified splenic infarction in 5 (25%) of 20 symptomatic patients for whom postoperative computed tomography (CT) was performed within 3 months. As the symptoms of splenic infarction, such as left upper pain or fever, are non-specific, these authors suggested that previous studies may have underestimated the incidences. The largest study on this subject, conducted recently by Jung et al. [7], demonstrated that 36 of 877 patients (4.10%) had splenic infarction after gastrectomy. It is noteworthy that a large volume of splenic infarction was associated with the development of complications in their study. Similarly, a high splenic infarction ratio or a large infarct volume have been reported as risk factors for complications after partial splenic arterial embolization, used widely for patients with hypersplenism and cirrhosis, [8,9,10].
Recent developments in diagnostic imaging technology have made it possible to obtain clear three-dimensional (3D) images from CT scanning data. The course of the vessels and their anatomic relationship with the surrounding organs are clarified using the 3D-CT images. There are various branching patterns of the splenic artery; therefore, preoperative 3D-CT simulation can be valuable for identifying the branch that terminates at the stomach or the spleen, to identify the branch that should be transected and the one that should be preserved during spleen-preserving surgery with gastrectomy. Accordingly, in this study, we aimed to identify the vascular anatomy of the splenic hilum with focus on the splenic polar branches and the gastric branches.
Methods
Patients
This study included 104 adult patients with gastric cancer, who underwent CT with intravenous contrast, prior to gastrectomy, between October, 2016 and May, 2017.
Names of the splenic artery branches and the splenic vein tributaries
Although several reports involving cadaver dissection have described the splenic artery anatomy, the definition of splenic artery branches varies in each report. According to most studies, including in vivo studies, the splenic artery divides into two or three terminal branches at the splenic hilum [11,12,13,14]. We defined these branches as the superior terminal artery (STA), the middle terminal artery (MTA) if present, and the inferior terminal artery (ITA). In some patients, a branch from the splenic artery terminates at the superior or inferior pole of the spleen before bifurcating or trifurcating to the terminal arteries at the splenic hilum. We defined these branches as the superior polar artery (SPA) or the inferior polar artery (IPA), irrespective of whether they branch into the stomach. The splenic artery was divided into the distal and proximal portions according to the Japanese classification of gastric carcinoma [15]. The proximal splenic artery is the part from its origin to halfway between the origin and the pancreatic tail end. The distal splenic artery is the part from halfway between the origin and the pancreatic tail end to the end of the pancreatic tail. The posterior gastric artery (PGA) was defined as the branch arising from the splenic artery and supplying only the stomach. The splenic vein tributaries were defined in a similar manner. Figure 1 is an illustration of the splenic artery and its branches with their names, and shows the splenic artery dividing into two branches, the STA and the ITA, at the splenic hilum; thus, the MTA is absent. The superior pole and inferior pole are supplied by two other branches arising from a site more proximal to the bifurcation at the splenic hilum. These branches are named “the SPA” and “the IPA”.
CT protocol and three-dimensional reconstruction
CT was carried out using the dual-energy mode of a dual-source CT scanner (SOMATOM Definition Flash; Siemens Healthcare, Munich, Germany). Nonionic iodinated contrast material (Iopamiron 370 or 300; Bayer, Osaka, Japan) was administered rapidly at a flow rate of 600 mg I/kg for 25 s, using an automatic injector through a 20-G plastic intravenous catheter. The injection of contrast material was followed by the injection of a saline bolus of 20 mL at a rate of 2 mL/s. The bolus tracking technique was used to acquire the arterial phase scanning data. The region of interest was placed in the abdominal aorta below the diaphragmatic dome, and the trigger threshold level was set to the CT value of 150 HU with an 8 s delay. Portal phase scanning and the equilibrium phase was started 20 s after the arterial phase scanning.
We used SYNAPSE 3D® (Fujifilm Medical, Tokyo, Japan) to generate 3D-CT images. Pancreas 3D imaging and arteriography were developed from the arterial phase scanning data. Spleen 3D imaging and portography were developed from the portal phase scanning data and 3D-CT images were obtained by fusing these. The “liver analysis” function of SYNAPSE 3D® was used to estimate the total spleen volume and the area supplied by the SPA.
Results
Patients
The study population of 104 patients comprised 65 men and 39 women, with a median age of 72 years (range 43–89 years). No patients had a history of upper abdominal surgery.
Terminal branches
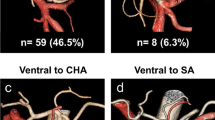
The splenic artery originated from the celiac trunk in all patients (n = 104). At the splenic hilum, it bifurcated into the STA and the ITA in most patients (n = 96, 92%) and trifurcated into the STA, MTA, and ITA in the others (n = 8, 8%). The bifurcation or trifurcation of the splenic artery was located ventral to the splenic vein in 69 patients (66%) and dorsal to the splenic vein in the other 35 patients (34%; Fig. 2).
Polar branches and the posterior gastric artery
The SPA was found in 61 patients (59%). In 14 patients (14%), the SPA did not branch into the stomach (SPA without a gastric branch; Fig. 3a). In the remaining 47 patients (45%), the SPA had one or more branches into the stomach (SPA with gastric branches; Fig. 3b). The PGA was detected in 19 patients (18%, Fig. 3c), while 24 patients (23%) had neither the SPA nor the PGA (Fig. 3d). The SPA originated from the proximal splenic artery in 26 patients and from the distal splenic artery in the other 35. The PGA origin was the proximal splenic artery in five patients and the distal splenic artery in 14 patients.
Illustration depicting the distribution of the superior polar artery and the posterior gastric artery. a SPA without a gastric branch: supplying only the superior pole of the spleen. b SPA with a gastric branch: supplying both the stomach and the superior pole of the spleen. c PGA: supplying only the stomach. d None: No SPA or PGA. Splenic A splenic artery, SPA superior polar artery, PGA posterior gastric artery
The total spleen volume and the splenic area supplied by the SPA were estimated in the 61 patients with the SPA. The total spleen volume ranged from 32 to 464 mL, with a median of 102 mL. The volume of the area supplied by the SPA ranged from 5 to 47 mL, with a median value of 11 mL. The SPA supplied ≤ 10% of the total spleen volume in 26 patients, 11–20% in 26 patients, 21–30% in 7 patients, and > 30% in 2 patients (Fig. 4a). On average, it supplied 12% of the total spleen volume in 61 patients. Figure 4b, c show the 3D-CT images and the area supplied by the SPA in one patient. The SPA originating from the proximal splenic artery supplied 41% of the total spleen volume (83 mL) in this patient.
The splenic area supplied by the superior polar artery. a Histogram distribution of the ratio of the SPA supplying volume to the total spleen volume. b 3D-CT image of one patient who had a large SPA. The SPA originated from the proximal splenic artery and soon bifurcated into two branches, both of which terminated at the spleen. c The SPA supplied 41% of the total spleen in this patient. The area supplied by the SPA is shown in white. SPA superior polar artery, 3D-CT three-dimensional computed tomography
The IPA was detected in 22 patients (21%). While almost half of the SPAs (27/61, 44%) originated from the proximal splenic artery, the IPA rarely originated from the proximal splenic artery (1/22, 5%). Eleven patients had both the SPA and the IPA. Table 1 summarizes the incidence and the origin sites of the SPA, PGA, and IPA.
Left gastroepiploic artery and left gastroepiploic vein
Both the left gastroepiploic artery (LGEA) and the left gastroepiploic vein (LGEV) were found in all the patients. There were three branching types of the LGEA, according to their sites of origin (Fig. 5a). The LGEA originated from the ITA or its branch in most patients (n = 75, 72%). The LGEA originating directly from the distal splenic artery was present in few patients (n = 9, 9%). Twenty-two patients had an IPA, and the LGEA originated from the IPA in 20 of these patients (19%).
Illustration depicting three patterns of the left gastroepiploic artery and the left gastroepiploic vein. a Three types of LGEA based on the sites of origin. b Three types of LGEV based on the draining sites. LGEA left gastroepiploic artery, LGEV left gastroepiploic vein, Splenic A splenic artery, ITA inferior terminal artery, IPA inferior polar artery, Splenic V. splenic vein, ITV inferior terminal vein, IPV inferior polar vein
Similar to the LGEA, the LGEV also had three types, depending on their draining sites (Fig. 5b). The LGEV drained into the inferior terminal vein (ITV) or its tributary in 66 patients (64%), into the distal splenic vein in 15 (14%), and into the inferior polar vein (IPV) in 23 (22%).
Discussion
The branch running cranially from the middle of the splenic artery is commonly recognized as the PGA, and used as a landmark between the proximal and distal splenic arteries in clinical practice. However, our study demonstrated that some patients do not have this artery. We also found that this artery supplied the superior pole of the spleen in most of these patients. Several reports have described the incidence and anatomy of each PGA [16,17,18] and SPA [12,13,14]; however, few have focused on the correlations between the two arteries. Trubel et al. [19] investigated the incidence of the PGA, the SPA with a gastric branch (termed the “gastrosplenic artery” in their paper), and the SPA without a gastric branch in 126 cadavers. In accordance with our results, they found the PGA in 27.7%, the SPA with a gastric branch in 45.2%, and the SPA without a gastric branch in 3.2%.
Kinoshita et al. [20] also described the presence of the SPA (named the “upper branch” in their report) and suggested that injury of this artery may lead to superior polar infarction after gastrectomy. Although it is obvious that SPA preservation can reduce the risk of splenic infarction, this makes removal of the lymph node along the splenic artery challenging and time-consuming. Considering that a small splenic infarction is unlikely to cause complications [7,8,9,10], we hypothesized that the SPA could be transected with relative safety when it was supplying only a small volume. To estimate the area supplied by the SPA, we devised a novel method that used the “liver analysis” function of SYNAPSE 3D®. By using this method, we found that the SPA supplied an average 12% of the total spleen volume, although it supplied > 30% in two patients. Thus, preserving the SPA when it is supplying a large area of the spleen is advisable; however, further investigation is required to determine the optimal cut off value.
Attention should be paid not only to the arterial anatomy, but also to the venous anatomy. It was found that the splenic artery branches are not always associated with the splenic vein tributaries. Figure 6 shows the 3D-CT image in one patient, in whom the superior pole of the spleen was drained by the SPV, whereas arterial supply was from the branch of the STA. The SPA was absent in this patient. As veins are more fragile than arteries they can easily be damaged. We often encounter venous bleeding during identification of the LGEA and LGEV. This may be due to laceration of the tiny splenic tributaries caused by over-traction. Preoperative information about the vascular anatomy may help surgeons prevent bleeding, and thereby also prevent postoperative complications.
The present study demonstrates that the vascular anatomy around the splenic hilum is very complicated. Zheng et al. [21] investigated the influence of the splenic artery anatomy on laparoscopic spleen-preserving splenic lymphadenectomy. They divided the branching patterns of the splenic artery into two groups according to the distance between the arterial furcation and the splenic hilar region. The mean operative time and mean blood loss during the splenic hilar lymphadenectomy were both higher in the distant group. We intend to investigate this subject further to show the effectiveness of preoperative 3D-CT simulation and demonstrate how our findings influence the procedure of gastric surgery.
This study had some limitations. First, the anatomy described was evaluated retrospectively using only 3D-CT images. As tiny vessels may have been overlooked on 3D-CT, the images should be compared with the intraoperative findings. Second, the accuracy of our original method that predicts the splenic infarcted volume needs to be validated. Third, the correlation between the splenic infarcted volume and postoperative complications after gastrectomy needs to be analyzed further.
In conclusion, we identified the vascular anatomy around the splenic hilum using 3D-CT images. The incidences of SPA without a gastric branch, SPA with a gastric branch, and PGA were 14, 45, and 18%, respectively. The SPA was found more frequently than is commonly believed. On an average, the SPA supplied 12% of the total spleen volume; however, it supplied > 30% of the volume in two patients. Thus, it may be advisable to preserve the SPA when it is supplying a large area of the spleen. Preoperative 3D-CT analysis provides useful information for safe gastrectomy.
References
Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg. 2017;265:277–83.
Jaroch MT, Broughan TA, Hermann RE. The natural history of splenic infarction. Surgery. 1986;100:743–50.
Nores M, Phillips EH, Morgenstern L, Hiatt JR. The clinical spectrum of splenic infarction. Am Surg. 1998;64:182–8.
Watanabe M, Miyata H, Gotoh M, Baba H, Kimura W, Tomita N, et al. Total gastrectomy risk model: data from 20,011 Japanese patients in a nationwide internet-based database. Ann Surg. 2014;260:1034–9.
Kunisaki C, Miyata H, Konno H, Saze Z, Hirahara N, Kikuchi H, et al. Modeling preoperative risk factors for potentially lethal morbidities using a nationwide Japanese web-based database of patients undergoing distal gastrectomy for gastric cancer. Gastric Cancer. 2017;20:496–507.
Akin K, Kosehan D, Cengiz AY, Dener NC, Koktener A, Inan A, et al. Splenic infarction following conventional open gastrectomy in patients with gastric malignancy: a CT-based study. Abdom Imaging. 2012;37:609–15.
Jung YJ, Seo HS, Lee HH, Kim JH, Song KY, Choi MH, et al. Splenic infarction as a delayed febrile complication following radical gastrectomy for gastric cancer patients: computed tomography-based analysis. World J Surg. 2017. https://doi.org/10.1007/s00268-017-4401-0. [Epub ahead of print].
Lee CM, Leung TK, Wang HJ, Lee WH, Shen LK, Liu JD, et al. Evaluation of the effect of partial splenic embolization on platelet values for liver cirrhosis patients with thrombocytopenia. World J Gastroenterol. 2007;13619–22.
Hayashi H, Beppu T, Okabe K, Masuda T, Okabe H, Baba H. Risk factors for complications after partial splenic embolization for liver cirrhosis. Br J Surg. 2008;95:744–50.
Cai M, Huang W, Lin C, Li Z, Qian J, Huang M, et al. Partial splenic embolization for thrombocytopenia in liver cirrhosis: predictive factors for platelet increment and risk factors for major complications. Eur Radiol. 2016;26:370–80.
Liu DL, Xia S, Xu W, Ye Q, Gao Y, Qian J. Anatomy of vasculature of 850 spleen specimens and its application in partial splenectomy. Surgery. 1996;119:27–33.
Sow ML, Dia A, Ouedraogo T. Anatomic basis for conservative surgery of the spleen. Surg Radiol Anat. 1991;13:81–7.
Redmond HP, Redmond JM, Rooney BP, Duignan JP, Bouchier-Hayes DJ. Surgical anatomy of the human spleen. Br J Surg. 1989;76:198–201.
Zeon SK, Kim SG, Huyn JA, Kim YS. Angiographic branching patterns of the splenic artery. Int J Angiol. 1998;7:57–61.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Suzuki K, Prates JC, DiDio LJ. Incidence and surgical importance of the posterior gastric artery. Ann Surg. 1978;187:134–6.
Okabayashi T, Kobayashi M, Morishita S, Sugimoto T, Akimori T, Namikawa T, et al. Confirmation of the posterior gastric artery using multi-detector row computed tomography. Gastric Cancer. 2005;8:209 – 13.
Yu W, Whang I. Surgical implications of the posterior gastric artery. Am J Surg. 1990;159:420–22.
Trubel W, Rokitansky A, Turkof E, Firbas W. Correlations between posterior gastric artery and superior polar artery in human anatomy. Anat Anz. 1988;167:219–23.
Kinoshita T, Shibasaki H, Enomoto N, Sahara Y, Sunagawa H, Nishida T. Laparoscopic splenic hilar lymph node dissection for proximal gastric cancer using integrated three-dimensional anatomic simulation software. Surg Endosc. 2016;30:2613–9.
Zheng CH, Xu M, Huang CM, Li P, Xie JW, Wang JB, et al. Anatomy and influence of the splenic artery in laparoscopic spleen-preserving splenic lymphadenectomy. World J Gastroenterol. 2015;21:8389–97.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflicts of interest.
Ethical approval
All the study procedures were approved by the Ethical Committee of Saitama Cancer Center (approval number 767) and were therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all participants included in the study.
Rights and permissions
About this article
Cite this article
Ishikawa, Y., Ehara, K., Yamada, T. et al. Three-dimensional computed tomography analysis of the vascular anatomy of the splenic hilum for gastric cancer surgery. Surg Today 48, 841–847 (2018). https://doi.org/10.1007/s00595-018-1679-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-018-1679-y