Abstract
Laparoscopic cholecystectomy is one of the most common procedures performed each year and can be associated with various post-operative complications. Imaging is integral to diagnosis and management of patients with suspected cholecystectomy complications, and a thorough understanding of normal and abnormal biliary anatomy, risk factors for biliary injury, and the spectrum of adverse events is crucial for interpretation of imaging studies. Magnetic resonance cholangiography (MRC) enhanced with hepatobiliary contrast agent is useful in delineating biliary anatomy and pathology following cholecystectomy. In this article, we provide a protocol for contrast-enhanced MR imaging of the biliary tree. We also review the classification and imaging manifestations of post-cholecystectomy bile duct injuries in addition to other complications such as bilomas, retained/dropped gallstones, and vascular injuries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laparoscopic cholecystectomy is one of the most common surgical procedures with approximately 750,000 performed annually in the United States [1]. Compared to open cholecystectomy, laparoscopic cholecystectomy is associated with a shorter hospital stay and an earlier return to normal activity [2]. A major disadvantage of a laparoscopic approach is the increased incidence of bile duct injuries. The reported rate of bile duct injury with this approach is 0.3–0.5% as compared to an incidence of 0.1–0.2% with open cholecystectomy [3,4,5,6]. Major bile duct injury is associated with prolonged hospitalization, high costs for the health care system, and decreased quality of life [7]. Biliary leak and obstruction are the most frequent biliary injuries [8]. A variety of other complications such as retained and dropped gallstones, hematoma, and abscess formation may also occur after this procedure [9].
Multiple imaging modalities can be used to identify these complications and are selected based on the clinical situation. Findings on ultrasound (US) and computed tomography (CT) are often non-specific, showing fluid collections in the surgical bed or in the perihepatic space. Magnetic resonance imaging (MRI) can provide more specific anatomic and functional information and is increasingly being used for these indications [10]. To ensure adequate treatment planning, it is important to accurately determine the degree and location of bile duct injury based on established classification systems. While minor biliary injuries can be managed endoscopically, major injuries often require surgical treatment. The focus of this article is to review the classification and imaging manifestations of post-cholecystectomy bile duct injuries. Imaging features of other complications will also be discussed and illustrated.
Normal anatomy and variants
The hepatocystic triangle, known historically as Calot’s triangle, is the anatomic space identified during laparoscopic cholecystectomy. The hepatocystic triangle is bordered by the common hepatic duct (CHD) medially, the cystic duct laterally, and the inferior edge of the liver superiorly [11]. This space contains the cystic artery and may also contain an accessory hepatic artery or an anomalous bile duct [12]. Misidentification of contents in the hepatocystic triangle, especially in the setting of biliary variations, can contribute to bile duct injury during laparoscopic cholecystectomy [13, 14].
Low medial insertion of the cystic duct into the common bile duct (CBD) (Fig. 1) and a parallel course of the cystic duct and CBD (where they share a common fibrous sheath) are the most common variants of the cystic duct seen in about 20% of cases [15, 16]. This proximity of structures in this compact anatomic space can complicate laparoscopic cholecystectomy and may be a challenge especially with Mirizzi syndrome when the associated inflammation distorts the normal anatomy. The extrahepatic duct may be inadvertently ligated after being mistaken for the cystic duct, resulting in transection or stricture of the extrahepatic duct in 0.1% of cases [17, 18]. These cystic duct variants can also result in a long cystic duct remnant that increases the chance of retained stones in the cystic duct and post-cholecystectomy syndrome, a constellation of heterogeneous abdominal symptoms that recur or persist after cholecystectomy. The incidence of post-cholecystectomy syndrome ranges from 10 to 50% [19, 20].
Variations regarding the insertion of the right hepatic duct (RHD) are also common. In 15% of cases, the right anterior duct has a separate low insertion into the common hepatic duct [21]. The simultaneous insertion of the right anterior duct, right posterior duct, and left hepatic duct into the CHD (also known as triple confluence) is another variant seen in 9–14% of cases [21]. The right posterior duct can also separately insert into the CHD (3–4% of cases) [13, 22] or the cystic duct (0.2–2% of cases) [22, 23], a variation known as an aberrant right posterior hepatic duct (Fig. 2). An aberrant right posterior hepatic duct can increase the risk of inadvertent bile duct ligation due to its proximity to the hepatocystic triangle [24, 25].
Subvesical bile ducts, or ducts of Luschka, are accessory bile ducts seen in 4–10% of patients [13, 26, 27]. These bile ducts run in proximity with the gallbladder fossa and typically originate from the right hepatic lobe [17]. Injuries to the subvesical bile ducts can complicate 0.1–0.2% of cholecystectomies and are the second most common cause of post-cholecystectomy bile leaks after cystic duct leaks [28].
Recognition of normal post-cholecystectomy anatomy on imaging is important to differentiate expected post-operative findings from pathologic ones. Cystic duct remnants after cholecystectomy are often 1–2 cm and can be visualized on magnetic resonance cholangiography (MRC). Longer cystic duct remnants are typically due to the cystic duct sharing a common fibrous sheath with an extrahepatic duct, in the setting of variations such as a low medial cystic duct or parallel cystic duct [17]. Longer cystic duct remnants can predispose patients to residual calculi, which can migrate and lead to distal obstruction of the CBD or contribute to post-cholecystectomy syndrome [29]. The CBD can undergo compensatory dilation after cholecystectomy, ranging from a 1.6 to 2.3 mm increase in diameter. Although greater than 6–7 mm is usually considered a dilated CBD, the upper limit of normal tends toward 10 mm in patients with history of cholecystectomy [30,31,32]. Liver function tests (LFTs) and correlation with symptoms are often recommended in these cases to determine if further evaluation (such as MRCP, ERCP, or endoscopic ultrasound) is necessary.
Risk factors for bile duct injury
In addition to the anatomical variations discussed above, other factors (such as the nature of the underlying gallbladder pathology, patient characteristics, and operator-dependent factors) can increase the risk of bile duct injury during laparoscopic cholecystectomy. Obtaining a “critical view of safety” is imperative for prevention of bile duct injuries and can reduce the incidence of injury from 0.6 to 0.03% [33]. This is defined as well visualization of hepatocystic triangle contents, where the triangle is cleared of fat and fibrous tissue, the distal third of the gallbladder is separated from the liver, and only two tubular structures are visualized entering the gallbladder [34]. In a retrospective multicenter survey of 50,000 cholecystectomies, frequent causes of bile duct injury were poor identification of anatomical features within the hepatocystic triangle, inflammatory changes in the gallbladder, and technical errors such as improper use of electrocautery and issues with controlling hemorrhage [35]. The risk of iatrogenic bile duct injury is twofold in patients with acute cholecystitis as compared to the risk after elective cholecystectomy [1, 36]. Inflammation around the gallbladder and along the hepato-duodenal ligament prevents proper visualization of the hepatocystic triangle and timely identification of a bile duct injury [37,38,39].
Subtotal cholecystectomy is a temporizing measure often performed in cases of severe inflammation in order to prevent misidentification of biliary and vascular structures and injury to those structures. It is associated with an increased risk of post-operative bilomas and retained stones [40]. Other factors that increase the risk of bile duct injury are excessive fat within the hepato-duodenal ligament, male sex, older age, and surgeon inexperience [37, 41]. Additional less common causes of bile duct injury, which are related to surgical technique, include thermal injury to the CBD or slippage of surgical clips on the bile ducts [41, 42].
Clinical presentation
Injuries to the bile ducts are often missed intraoperatively, especially if the bile leaks originate from the cystic duct or small ducts of Luschka in the liver bed [1]. Depending on institution, 25–75% of bile duct injuries are recognized intraoperatively (using cholangiography or based on the presence of bile in the operative field) and may be repaired if a surgeon with proper expertise is available [35, 43]. If not recognized, patients will often present within a week with vague non-specific abdominal symptoms such as bloating, abdominal pain, anorexia, and malaise [41]. Clinical signs, when present, include jaundice, peritonitis, cholangitis, and continuous bilious output in those with surgically placed drains. Laboratory values are often non-specific, such as leukocytosis and bandemia due to inflammation and infection of bile. Patients with jaundice will present with hyperbilirubinemia and elevated alkaline phosphatase [1]. Pathologic levels of aminotransferases are seen with serious complications of unrecognized or untreated bile duct injuries resulting in secondary biliary cirrhosis [37].
Imaging modalities
Imaging is crucial in establishing the presence, and localization, of a bile duct injury (Table 1). CT or US is usually performed initially to establish the diagnosis, depending on the symptoms at presentation. For localized right upper quadrant pain, US is the preferred initial imaging modality to look for fluid collections and biliary ductal dilation. Fluid collections usually appear as well-circumscribed anechoic collections in the gallbladder fossa or perihepatic space. Collections may become complex with internal septa which are non-specific findings, usually attributed to superimposed infection or proteinaceous/hemorrhagic content. The sensitivity of US for detection of intra-abdominal fluid collections is 70% [44, 45]. The disadvantages of US include operator dependency and inability to distinguish between various types of fluid collections [1, 10, 41, 46].
Contrast-enhanced CT (CECT) is employed when patients present with diffuse abdominal pain or sepsis. CECT is able to identify bilomas, ascites, abscesses, and inflammation [1]. It has a sensitivity of 95% to detect intra-abdominal fluid collections [45], but as with US, it lacks the specificity to identify the source of fluid. If infected, the fluid collection often has an enhancing wall, local mass effect, and may contain locules of gas. CT also offers increased specificity in detecting arterial injuries compared to US [47]. Drip infusion CT cholangiography can provide a more detailed evaluation of biliary anatomy and localization of bile duct leakage, but the contrast agent used (meglumine iotroxate) is not available in most countries and is associated with infusion reactions [48].
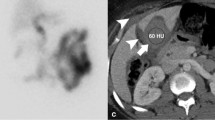
Hepatobiliary scintigraphy is a non-invasive modality that is more specific for bile leaks than US and CT and has a sensitivity that ranges from 64 to 100% [45, 49, 50]. It is limited by its poor spatial resolution and inability to confidently identify the bile duct involved, which is important for preoperative planning. The addition of single-photon emission computed tomography (SPECT–CT) improves localization of biliary leaks but also further increases radiation exposure [44, 51].
Endoscopic retrograde cholangiopancreatography (ERCP) is another useful imaging technique that not only enables localization of the bile duct leak, but also allows for interventional therapy, if needed. CBD stent placement decreases the pressure gradient between the biliary system and duodenum, allowing bile to flow away from the leak, which assists with the healing process and prevents stricture formation [37, 41]. Despite its advantages, ERCP is invasive and has 1.4% to 3.2% chances of complication including acute pancreatitis, hemorrhage, perforation, and infection [52]. In addition, ERCP is unable to evaluate the biliary tree proximal to a major transection or ligation and could be challenging in patients with altered bowel anatomy (such as Roux-en-Y gastric bypass or biliary-enteric anastomosis) [9, 10].
Percutaneous cholangiography (PTC) is useful in assessing proximal bile duct injuries, aberrant RHD transections, and common duct transections. External drainage has a therapeutic role by decreasing the pressure within the biliary tree [47]. PTC is invasive and cannot provide a complete assessment of the biliary tree in cases of complete obstruction [53].
Intraoperative cholangiogram (IOC) is another modality that can identify bile duct leaks at the time of injury. Although IOC has the potential to aid in prevention and intraoperative recognition of bile duct injuries, studies have shown conflicting evidence regarding its benefit [54]. A recent randomized controlled trial comparing outcomes between patients who underwent laparoscopic cholecystectomy alone and laparoscopic cholecystectomy with routine IOC showed no improvements in the rate of bile duct injury, CBD stone retention, or length of hospital stay, but demonstrated significantly longer operative time [55]. As such, less surgeons routinely perform IOC [54, 56].
MRC is a non-invasive modality for diagnosis of bile duct injury. Owing to its high spatial and contrast resolution, MRC can provide better delineation of the biliary tree and better recognition of level and length of injury. It also provides other valuable information necessary for patient management (such as delineation of underlying variant biliary anatomy). [44, 46, 47, 57]. Although routine T2-weighted MRC can delineate biliary tree anatomy well, it does not provide information regarding biliary excretion and cannot distinguish bilomas from other fluid collections [58]. The sensitivity of T2-weighted MRC for localization of biliary leakage is between 70 and 74% [59]. MRC enhanced with hepatobiliary contrast agents (contrast-enhanced magnetic MR cholangiography; CE-MRC) can distinguish between fluid collections of biliary and non-biliary origins and provides much higher spatial resolution compared to hepatobiliary scintigraphy [9, 60]. It has a sensitivity of 96% and a specificity of 100% in diagnosis of an active bile leak [58, 61]. Through direct visualization of biliary flow, CE-MRC can also depict other surgical complications such as biliary strictures [10].
MR imaging protocol
MR imaging of the biliary tree is generally performed using a 1.5 T or 3 T magnet and a phased-array surface coil. For precontrast imaging, axial T1-weighted in-phase/opposed-phase and fat-suppressed T1 gradient echo sequences are obtained in addition to axial and coronal T2-weighted breath-hold half-Fourier acquisition single-shot turbo spin echo imaging (HASTE), T2-weighted rapid acquisition with relaxation enhancement (RARE), and three-dimensional MRC sequences. Heavily T2-weighted sequences are used to depict the fluid-contained biliary tree and pancreatic duct [62]. Since hepatobiliary-specific agents cause T2 shortening, T2-weighted MRC should always be performed prior to excretion of contrast into the biliary tree [63]. Suggested MRI protocol is summarized in Table 1.
Mangafodipir trisodium (Teslascan, GE Healthcare) was one of the first approved hepatobiliary contrast agents. Although it allows for hepatobiliary imaging, it precludes dynamic imaging and assessment of vascular structures since it is solely a hepatobiliary-specific agent. This agent was discontinued in the United States due to concerns of toxicity [61, 64]. Gadobenate dimeglumine (Gd-BOPTA; Multihance, Bracco Imaging) and gadoxetate disodium (Gd-EOB-DTPA, Bayer Healthcare, Primovist in Europe, Eovist in the United States) can be used for both early dynamic and delayed hepatobiliary phase imaging as they share properties of extracellular gadolinium-based agents. Only 3–5% of Gd-BOPTA is excreted in the bile allowing for biliary imaging at about 45 min after injection. Gd-EOB-DTPA is more often used to assess biliary injury as 50% of it is excreted in bile, allowing for improved and earlier biliary imaging at 20 min [58]. For both agents the remainder of contrast is excreted through the kidneys [65]. Gd-EOB-DTPA (Eovist) is the only agent currently approved in the United States, as an off-label use, for biliary imaging [66].
The suggested dose of Gd-EOB-DTPA by the FDA is 0.1 mL/kg (0.025 mmol/kg), which is the minimum effective dose. Higher doses have been recommended to improve enhancement as there is overall a small volume injected with hepatobiliary agents and they are associated with transient respiratory motion in the arterial phase [67, 68]. In order to improve acquisition timing and truncation artifacts, a timing bolus or fluoroscopic trigger is recommended [60, 67, 69]. In our institution’s protocol, we administer 0.1 mL/kg of Gd-EOB-DTPA at a rate of 1 mL/s through a fluoroscopic trigger and obtain dynamic images including arterial, portal venous, transitional, and hepatobiliary phases.
Increasing the flip angle to 35°–45° in delayed phase imaging using Gd-EOB-DTPA can lead to improvements in the signal-to-noise ratio and contrast-to-noise ratio [64, 65]. A higher flip angle is possible due to the high concentration of Gd-EOB-DTPA excreted in the bile ducts, which helps to decrease background noise from the liver (also retains hepatobiliary contrast agents) [68]. The duration of delayed imaging can also improve the sensitivity of biliary leakage. Cieszanowski et al. found in a retrospective study that the overall sensitivity of CE-MRC was improved in combined 20–25 min and 60–90 min delayed images, and even further improved with combined 20–25 min, 60–90 min, and 150–180 min delayed images (92.9% and 96.4%, respectively, as compared to 42.9% for 20–25 min delay only) [60, 67, 70, 71]. For delayed imaging, we use a 30° flip angle in coronal and axial fat-suppressed T1 gradient echo sequences acquired 20 min post-injection, with instruction to obtain further delayed images if there is inadequate biliary filling or a high suspicion for biliary leak. When waiting to acquire delayed images (e.g., 180 min), other patients can be imaged so that MR throughput is not compromised. If the diagnosis is a bile leak, an additional thin-sliced coronal fat-suppressed T1 gradient echo sequence is performed to look for filling of hepatic ducts and the CBD.
Liver dysfunction can contribute to inadequate biliary excretion of contrast as Gd-EOB-DTPA is actively transported through hepatocytes. Tschirch et al. found that patients with serum total bilirubin greater than 30 μmoles/L or MELD scores greater than 11 had insufficient visualization of the biliary tree 20 min after administration of Gd-EOB-DTPA [69]. However, in a study conducted by Kul et al., there was no statistically significant difference between bilirubin levels in patients who required more delayed phase imaging and patients who did not. Nevertheless, if there is a high clinical suspicion for a bile leak even with normal lab values more delayed imaging should be pursued [67, 71].
MR imaging of post-cholecystectomy complications
Biloma
Bilomas, usually the first consideration of bile duct injury on imaging, are defined as well-demarcated collections of bile outside the biliary tree. They are commonly located in the subhepatic and perihepatic space. Less common locations are intrahepatic and retroperitoneal space [72]. It is difficult to distinguish between post-operative seromas, lymphoceles, hematomas, and abscesses solely based on CT. In addition, hemostatic agents such as Surgicel (oxidized regenerated cellulose, Johnson and Johnson Ethicon) can mimic the appearance of an abscess or hematoma [73]. MRI can often differentiate between abscess and Surgicel since the latter demonstrates low signal on T2-weighted images while an abscess has high T2 signal intensity [9].
On conventional MRI, bilomas appear as fluid collections with a thin wall that are hypointense on T1-weighted MRI and hyperintense on T2-weighted MRI. Occasionally layering high T1 and low T2 signal can be seen within the biloma which represents concentrated bile (Fig. 3) [74]. A thick enhancing rim raises suspicion for superinfection and abscess [57]. Lymphatic and serous post-operative collections can mimic biloma on MRI. On CE-MRC, a biloma may show delayed contrast agent filling if there is an active leak [57]. A walled-off biloma will not demonstrate contrast accumulation and potentially exclude an active leak as there will be no communication between the injury and biloma [66].
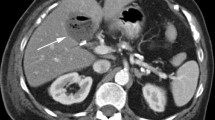
45-year-old female patient with post-cholecystectomy bilomas. a and b Axial T2-weighted MRI (a) and axial post-contrast T1-weighted MRI (b) show mildly-rim-enhancing fluid collections in the subhepatic space and hepatorenal recess (arrows). The dominant collection was aspirated percutaneously showing high bilirubin level consistent with biloma
Bile duct injury
CE-MRC can also be advantageous in identifying the precise anatomic location of leakage (Fig. 4). There are numerous classifications used to describe bile duct injuries. The Stewart–Way classification is based on mechanism of injury and includes associated vascular injuries (Table 2). The Hannover classification, proposed based on study of 72 consecutive cases of iatrogenic bile injury after laparoscopic cholecystectomies, is shown to have high association with the surgical treatment chosen (Table 3) [3, 75]. The Bismuth–Strasberg classification, which is based on location of injury in the biliary tract, is currently the most widely used classification and is discussed here (Table 4; Fig. 5).
38-year-old female patient with history of cholecystectomy which was complicated by bile duct injury. a Axial T1-weighted MR precontrast image of bile duct injury. b Axial T1-weighted MRI (20 min after administration of Gd-EOB-DTPA) shows excreted contrast within the intrahepatic ducts and with hyperintensity in the non-dependent portion of the perihepatic fluid (arrow). c More delayed images, obtained with 35-min delay, demonstrate expanding extraluminal contrast (arrow) within the perihepatic fluid confirming active bile leak. Patients were found to have Strasberg-type E1 injury (injury to extrahepatic bile duct > 2 cm distal to confluence) following attempted cholecystectomy
Type A bile duct injuries are leaks from the cystic duct or ducts of Luschka [12, 58]. Leaks from the cystic duct commonly occur when clips on the cystic duct become dislodged or do not encompass the entire duct [62]. Lacerations to the cystic duct, ductal necrosis due to cholecystitis, or distal obstruction by a stone shearing the cystic duct remnant are other potential etiologies. Leaks from the smaller ducts of Luschka occur mainly with the presence of an anatomic abnormality such as an intrahepatic position of gallbladder or an adherent gallbladder from chronic cholecystitis. MRC will demonstrate a T2-hyperintense fluid collection within the gallbladder fossa. A small connection between the fluid collection and cystic duct may be seen [5]. CE-MRC will show extravasation of contrast agent from the cystic duct remnant or ducts of Luschka [46]. These leaks are usually self-resolving, unless they become secondarily infected [76].
Type B and C injuries are due to occlusion or transection of the aberrant RHD, respectively. They usually stem from misidentification of the aberrant RHD as the cystic duct, resulting in the RHD being mistakenly clipped [62]. If the duct is occluded, patients are often asymptomatic and the injury can be missed until a late presentation of cholangitis occurs. MRC will show an obstructed segment lacking continuity with the distal biliary tree. CE-MRC will reveal focal duct dilation and lack of excretion of the contrast agent from the bile duct at the site of occlusion. This may be accompanied by segmental hepatic atrophy which shows up on CE-MRC as volume loss with poor contrast uptake in the corresponding segment [63]. On conventional MRC, complete lack of visualization of the duct is usually indicative of a transection or excision, but it can occasionally be difficult to distinguish from a stricture. In type C injury CE-MRC will show discontinuation of the contrast at the aberrant RHD, with pooling of contrast nearby due to a resultant biloma [47]. Type B and C injuries are often not detected on ERCP since the injured duct is not in continuity with the intrahepatic and common bile ducts.
Type D bile duct injuries result in a partial lateral wall injury to the CHD or CBD. They are due to misidentification of any of the major bile ducts as the cystic duct. They present on CE-MRC as narrowing in CHD or CBD (with an adjacent subhepatic biloma accumulating contrast in the hepatobiliary phase) and an otherwise intact biliary tree [8].
Type E injuries are injuries to the main hepatic duct and are organized from E1 to E5 based on distance from the bile duct bifurcation, the involvement of the bile duct bifurcation, and involvement of the aberrant RHD (Figs. 6, 7, 8, 9) [77]. They are usually due to anatomical variants such as a low medial insertion of the cystic duct, a cystic duct parallel to the CHD, and an aberrant RHD. CE-MRC allows for the detection of maintenance of the biliary confluence and length of the cystic duct. Mechanisms of injury include transection, ligation, thermal injury, or occlusion. If injury is specifically from a ligation, a ductal segment on CE-MRC may show narrowing or discontinuation [78].
46-year-old female patient with history of chronic cholecystitis and underwent laparoscopic cholecystectomy which was complicated by a Strasberg-type E2 injury (injury to common hepatic duct < 2 cm distal to confluence). a Axial T1-weighted MRI (20 min after administration of Gd-EOB-DTPA) shows excreted contrast within the common bile duct (arrow) and renal collecting system. b Axial image obtained at the level of common hepatic duct shows marked narrowing of common hepatic duct at this level. Note the cholecystectomy clips (seen as signal void) adjacent to the area of stricture. No bile leak was seen. c and d Maximum-intensity projection MRCP (c) and subsequent ERCP (d) confirm marked stricture of common hepatic duct. Note a right percutaneous transhepatic biliary stent in place
44-year-old male patient with history of acute cholecystitis and underwent laparoscopic cholecystectomy which was complicated by a Strasberg-type E3 injury (injury at the level of hilum with preserved biliary confluence). a Coronal contrast-enhanced CT shows cholecystectomy clips adjacent to the biliary confluence with associated intrahepatic biliary ductal dilation and perihepatic fluid collection. b and c Coronal maximum-intensity projection MRCP (b) and subsequent ERCP (c) show stricture just below the level of biliary confluence (arrow)
52-year-old male patient with history of laparoscopic cholecystectomy which was complicated by a Strasberg-type E4 injury (injury at the level of biliary confluence). a Axial post-contrast T1-weighted MRI shows bilobar intrahepatic biliary ductal dilation without discernible biliary confluence. Note the cholecystectomy clip (arrow) in hilum. b Coronal T2-weighted MRI also shows separation of the dilated right and left hepatic ducts
60-year-old male patient with history of right upper quadrant pain and underwent laparoscopic cholecystectomy which was complicated by a Strasberg-type E5 injury (injury to both aberrant right posterior duct and common hepatic duct). a Axial T1-weighted MRI (20 min after administration of Gd-EOB-DTPA) shows extraluminal contrast within the gallbladder fossa (arrow) consistent with active biliary leak. b Subsequent cholangiogram also showed narrowing in common hepatic duct (arrow). Patient was found to have injury to an aberrant right posterior hepatic duct
Retained Stone/Mirizzi syndrome
Although often asymptomatic, gallstones may remain within the cystic duct remnant, intrahepatic ducts, or extrahepatic ducts following a cholecystectomy [62]. It is difficult to determine the exact incidence of retained gallstones, but it is reported to range between 2 and 12% [79]. They are a major contributor to post-cholecystectomy syndrome [19]. In a prospective study of 272 patients who presented with post-cholecystectomy syndrome, 17.6% of cases were attributed to retained gallstones or cystic duct remnant (Fig. 10) [80]. Diagnosis using MRC is often helpful as other etiologies of post-cholecystectomy syndrome such as strictures or pancreatitis can be ruled out. Retained stones on MRC will appear as smoothly marginated filling defects within the common bile duct or cystic duct remnant, usually in the dependent position, surrounded by a thin border of hyperintense bile (Fig. 11) [19]. Thin slice HASTE images may show a filling defect in the center of the duct related to flow artifact which is not seen on balanced steady state-free precession techniques (Fig. 12).
39-year-old male with remote history of orthotopic liver transplantation with duct-to-duct biliary anastomosis for hepatic failure caused by bupropion. a and b Coronal maximum-intensity projection MRCP (a) and longitudinal duplex ultrasound (b) through the hilum show a fluid collection (arrow) near in location of cystic remnant suggestive of mucocele of cystic duct remnant, which has been slowly growing over the years
47-year-old male presenting with right upper quadrant pain 1 week post-cholecystectomy. a Axial contrast-enhanced CT shows a fluid collection versus large remnant in the gallbladder fossa (arrow). b Coronal maximum-intensity projection MRCP demonstrates a collection in continuity with the cystic duct, suggestive of gallbladder remnant, with suggestion of filling defect (arrow) in cystic duct. c Coronal contrast-enhanced MRC shows non-filling of gallbladder remnant suggestive of cystic duct obstruction which was confirmed on subsequent ERCP (d). Note the meniscus (arrow) in cystic duct consistent with obstructing stone. Patient underwent endoscopic therapy with direct cannulation of cystic duct and removal of cystic duct stone
58-year-old male patient with history of abnormal liver function tests to rule out primary sclerosing cholangitis. a and b Axial thin slice single-shot (a) and T1-weighted (b) MR images show large gallstone in the fundus of the gallbladder (small arrow) and non-dilated common bile duct with a small low signal in the distal CBD on the T2-weighted sequence (a, arrow) most likely represent flow artifact and is not appreciated on balanced steady state-free precession techniques image (b, arrow)
Mirizzi syndrome is defined as inflammation from a cystic duct or gallbladder infundibulum stone resulting in extrinsic compression of the CHD and can either present preoperatively or post-operatively due to remnant cystic duct stones [81]. This inflammation can make laparoscopic cholecystectomy challenging due to distortion of normal anatomy, which can increase the risk of bile duct injury [82]. A systematic review found that a preoperative diagnosis can decrease the conversion rate of laparoscopy to laparotomy [83]. The accuracy of Mirizzi syndrome on MRC is 50–76% [84, 85] and can be improved to 94% when used in conjunction with CT [86]. MRC can delineate characteristic findings of Mirizzi syndrome such as a stone in the CHD, extrinsic compression of the CHD, and dilation of the CHD with a normal-sized CBD [80].
MRC is the preferred imaging modality for post-cholecystectomy Mirizzi syndrome with a sensitivity ranging from 89 to 92% for diagnosis of post-cholecystectomy syndrome [29]. MRC findings of post-operative Mirizzi syndrome include a stone in the cystic duct remnant with extrinsic compression of the CHD and disproportionate dilation of the upstream biliary tree. Inflammation can be visualized in the biliary ducts and MRC may be useful in distinguishing Mirizzi syndrome from other biliary conditions such as cancer. ERCP is additionally useful for evaluation of possible accompanying cholecystobiliary fistula, which is important in determining the subtype of Mirizzi syndrome. It also offers therapeutic interventions through stenting or papillotomy [29, 85].
Vascular injury
Iatrogenic vascular injuries (Fig. 13) are another complication of laparoscopic cholecystectomy and have been observed in 0.25% of cases [87]. Frequent causes of vascular injury include inadequate ligation of the cystic artery, right hepatic artery injury, and pseudoaneurysm formation. The right hepatic artery is the most commonly injured due to anatomical variations leading to confusion with the cystic artery [88]. This injury often occurs concomitantly with a bile duct injury and thus patients can present with hemobilia, right hepatic lobe ischemia, or an associated hepatic abscess [41]. Although US with Doppler or CTA is usually the initial modality used to diagnose arterial injury, MRI can also assist with diagnosis as vascular structures can be examined in the hepatic arterial and portal venous phases through dynamic imaging [58, 63, 89].
59-year-old male patient presenting with pain and fever 2 months following cholecystectomy. Patient underwent MRI showing fluid collection (arrow) in the gallbladder fossa demonstrating high signal on precontrast T1-weighted (a) and intermediate signal on T2-weighted MRI (b), respectively, suggestive of hematoma
Right hepatic artery pseudoaneurysm is a rare complication of laparoscopic cholecystectomy. Precipitating factors include direct arterial injury, bile leak/infection, and erosion via clip displacement. Pseudoaneurysms present as pulsatile masses that can rupture into the peritoneal cavity, biliary tree, or duodenum and lead to life-threatening hemorrhage. Pseudoaneurysms may manifest as rounded fluid-filled structures and should be considered in the differential diagnoses of a fluid collection in the gallbladder fossa following cholecystectomy [90]. On MRC, a pseudoaneurysm may appear as heterogeneous mixed signal intensity on T1- and T2-weighted sequences with flow voids in the patent portion. CE-MRC will show brisk enhancement during the arterial phase [91].
Ischemic cholangiopathy is a rare entity, characterized by focal or diffuse injury of the bile duct because of impaired blood supply. Damage to the arterial supply of the CBD, such as the right hepatic artery, can result in biliary strictures and other ischemic complications [92]. US, although unable to directly identify biliary ischemia, is a useful modality for evaluation of consequences of biliary ischemia such as biliary ductal dilation and intrahepatic biloma formation. On CECT, cases of severe ischemic cholangiopathy with biliary necrosis manifest with water density necrotic tissue and debris in peribiliary space and porta hepatis abutting the central bile ducts as well as intrahepatic bilomas. In the affected segments, the peripheral bile ducts become dilated secondary to downstream intrinsic strictures or extrinsic compression by the peribiliary necrotic tissue. MRC is excellent for detecting bile duct morphological changes and complications of biliary ischemia (Fig. 14). Biliary dilation, biliary strictures, and sludge are well depicted on MRC except in cases of severe biliary ischemia as the bile leakage into the portal tracts show high signal intensity, limiting the assessment of the central ducts. On diffusion-weighted imaging (DWI), ischemic bile ducts may show high signal intensity [93].
56-year-old male with remote history of cholecystectomy with reported bile duct injury at the time of surgery (details of injury not known). a and b Coronal thick-slab MRCP (a) and axial post-contrast T1-weighted MRI (b) show focal narrowing (stricture) (arrow) and associated segmental dilation of bile ducts in segment VI, presumably due to ischemic cholangiopathy. No mass or malignancy on ERCP and brushing
Dropped gallstones
Another post-operative complication of laparoscopic cholecystectomy is spillage of gallstones within the abdominal cavity. Dropped gallstones occur in 25–30% of cholecystectomies and become symptomatic in the form of an abscess in 0.3% of patients [94]. The abscesses typically present months after cholecystectomy, although late presentations several years after the surgery have also been reported [95]. Fistulas created by the migration of dropped gallstones to the gastrointestinal tract, diaphragm, or abdominal wall are other less frequent complications. MRI is useful in identifying non-calcified gallstones that are not easily visible on CT. Stones appear as well-defined signal voids on T2-weighted sequences. Their signal intensity on T1-weighted MRI is variable. Surrounding inflammation appears hyperintense on T2-weighted MRI with variable degrees of enhancement following injection of gadolinium (Fig. 15). An abnormality in the right posterior subhepatic space often prompts investigation for a history of cholecystectomy due to its characteristic location for dropped gallstones. Mimics of dropped gallstones include peritoneal loose bodies arising from appendices epiploica, dropped appendicoliths, colonic diverticuli, and peritoneal metastases [96]. Clinical history and a multimodality approach to imaging can help narrow the diagnosis.
45-year-old male presenting with intermittent right upper quadrant pain. Patient had cholecystectomy 7 years earlier. a Axial contrast-enhanced CT shows a gallbladder remnant (arrow). b and c Subsequent axial T2-weighted MRI (b) and coronal thick-slab MRCP (c) show a stone within the infundibulum of the gallbladder remnant (arrow). The remnant and cystic duct did not fill with radiotracer during scintigraphy (not shown). Patient underwent completion cholecystectomy with resolution of symptoms
Iatrogenic biliary strictures
Ninety-five percent of biliary strictures are secondary to biliary tract surgery (Fig. 16) [47]. Strictures usually arise from thermal injury or irritation from surgical clips leading to fibrosis [12]. Clinical presentation often occurs as obstructive jaundice or liver dysfunction months to years after laparoscopic cholecystectomy. On MRC, they manifest as intra or extrahepatic ductal dilation with gentle tapering, focal ductal narrowing, and non-depiction of part of a duct [12, 62]. A disadvantage of conventional MRC is that there tends to be an overestimation of strictures based on ductal appearance because it offers no functional information about resistance to flow. There can also be overestimation of the length of the stricture on T2-weighted MRC if the duct distal to the stricture has collapsed [59]. CE-MRC offers functional information that can help distinguish non-obstructive dilation vs. obstruction from stricture. The degree of obstruction can be classified based on delay of contrast flow through bile duct obstruction. Complete obstruction is the absence of contrast agent in the proximal portion of the stricture. Near-complete obstruction is delayed contrast filling only in the proximal part of the stricture, while partial obstruction is the passage of contrast agent beyond the stricture [57, 59].
20-year-old female presenting with painless jaundice, 2 weeks after elective cholecystectomy. a and b Coronal maximum-intensity projection MRCP (a) and subsequent ERCP (b) show severe stricture of common hepatic duct (arrow). c Patient underwent endoscopic therapy with several biliary stenting and ballooning with eventual resolution of stricture, as shown on follow up ERCP
Reporting the number and location of strictures is important for patient management. Stenting, dilation, and surgery are the main options for treatment [97]. Short-segment benign strictures are more amenable to dilation compared to long-segment or multifocal strictures [98]. For post-procedural evaluation, absence of ductal dilation on T2-weighted MRC may suggest stent patency, but susceptibility artifacts from the metallic stent and associated pneumobilia make it difficult to see the internal lumen. CE-MRC can visualize stent patency by demonstrating contrast present above and below the stent [57].
Management
The treatment approach to bile duct injuries is based on timing and recognition of injury, location of injury, and extent of injury [99]. Biliary anatomy is always thoroughly investigated prior to repair as it improves success rate. Exploratory surgery should not be performed and is associated with higher morbidity and mortality [100]. Although bile duct injuries only occur in 20–30% of cases, intraoperative recognition of them is associated with superior outcomes. If bile duct injuries are recognized post-operatively, then the goal is to control sepsis first through antibiotics and drainage of bile. Elective reconstruction can then be performed 6–8 weeks later after inflammation is more controlled [101].
Leaks from cystic ducts (Strasberg type A) are usually managed non-operatively either with stenting or sphincterotomy through ERCP [100]. As mentioned previously, RHD injuries (Strasberg types B and C) are often asymptomatic and underrecognized. Thus, imaging with MRC is crucial to diagnosis and management as these are usually repaired through image-guided PTC placement for external drainage. Partial defects such as Strasberg type D injuries can be repaired with primary closure and subhepatic drainage, while complete defects (Strasberg type E) require Roux-en-Y hepaticojejunostomy. The location of injury in complete defects is also important for operative technique. Lowering of the hilar plate, which improves exposure, only needs to be performed in bile duct injuries where the biliary confluence is disrupted [99].
Associated vascular injuries can make biliary reconstruction more difficult due to the increased risk of hemorrhage [3, 100]. These injuries, such as visceral pseudoaneurysms, are increasingly being treated via endovascular approach using coil embolization [102]. Dropped gallstone-associated abscesses can be managed by antibiotics and percutaneous drainage although they often require stone removal via open or laparoscopic surgery for definitive treatment [103].
Conclusion
Knowledge of variations in biliary anatomy and the classification systems that grade bile duct injuries is helpful for proper diagnosis and management of complications following cholecystectomy. MRC can facilitate localization of bile duct injuries as well as identification of other complications such as strictures, vascular injuries, and displaced gallstones. CE-MRC can provide additional information regarding biliary excretion and exclude non-biliary sources of fluid collections. Timely detection and localization of bile duct injuries and other cholecystectomy complications can help optimize surgical approaches and reduce morbidity and mortality.
References
Cohen JT, Charpentier KP, Beard RE (2019) An update on iatrogenic biliary injuries: identification, classification, and management. Surg Clin North Am 99 (2):283-299. https://doi.org/10.1016/j.suc.2018.11.006
Gadacz TR, Talamini MA (1991) Traditional versus laparoscopic cholecystectomy. Am J Surg 161 (3):336-338. https://doi.org/10.1016/0002-9610(91)90591-z
Lau WY, Lai EC (2007) Classification of iatrogenic bile duct injury. Hepatobiliary Pancreat Dis Int 6 (5):459-463
Salvolini L, Urbinati C, Valeri G, Ferrara C, Giovagnoni A (2012) Contrast-enhanced MR cholangiography (MRCP) with GD-EOB-DTPA in evaluating biliary complications after surgery. Radiol Med 117 (3):354-368. https://doi.org/10.1007/s11547-011-0731-4
Mungai F, Berti V, Colagrande S (2013) Bile leak after elective laparoscopic cholecystectomy: role of MR imaging. J Radiol Case Rep 7 (1):25-32. https://doi.org/10.3941/jrcr.v7i1.1261
Pucher PH, Brunt LM, Davies N, Linsk A, Munshi A, Rodriguez HA, Fingerhut A, Fanelli RD, Asbun H, Aggarwal R (2018) Outcome trends and safety measures after 30 years of laparoscopic cholecystectomy: a systematic review and pooled data analysis. Surg Endosc 32 (5):2175-2183. https://doi.org/10.1007/s00464-017-5974-2
Flores-Rangel GA, Chapa-Azuela O, Rosales AJ, Roca-Vasquez C, Bohm-Gonzalez ST (2018) Quality of life in patients with background of iatrogenic bile duct injury. World J Surg 42 (9):2987-2991. https://doi.org/10.1007/s00268-018-4564-3
Mercado MA, Domínguez I (2011) Classification and management of bile duct injuries. World J Gastrointest Surg 3 (4):43-48. https://doi.org/10.4240/wjgs.v3.i4.43
Thurley PD, Dhingsa R (2008) Laparoscopic cholecystectomy: postoperative imaging. AJR Am J Roentgenol 191 (3):794-801. https://doi.org/10.2214/ajr.07.3485
Melamud K, Lebedis CA, Anderson SW, Soto JA (2014) Biliary imaging: Multimodality approach to imaging of biliary injuries and their complications. Radiographics 34 (3):613-623. https://doi.org/10.1148/rg.343130011
Abdalla S, Pierre S, Ellis H (2013) Calot’s triangle. Clin Anat 26 (4):493-501. https://doi.org/10.1002/ca.22170
Desai NS, Khandelwal A, Virmani V, Kwatra NS, Ricci JA, Saboo SS (2014) Imaging in laparoscopic cholecystectomy—What a radiologist needs to know. Eur J Radiol 83 (6):867–879. https://doi.org/10.1016/j.ejrad.2014.02.016
Sarawagi R, Sundar S, Raghuvanshi S, Gupta SK, Jayaraman G (2016) Common and uncommon anatomical variants of intrahepatic bile ducts in magnetic resonance cholangiopancreatography and its clinical implication. Pol J Radiol 81:250–255. https://doi.org/10.12659/PJR.895827
Andall RG, Matusz P, du Plessis M, Ward R, Tubbs RS, Loukas M (2016) The clinical anatomy of cystic artery variations: a review of over 9800 cases. Surg Radiol Anat 38 (5):529-539. https://doi.org/10.1007/s00276-015-1600-y
Shaw MJ, Dorsher PJ, Vennes JA (1993) Cystic duct anatomy: an endoscopic perspective. Am J Gastroenterol 88 (12):2102-2106
Puente SG, Bannura GC (1983) Radiological anatomy of the biliary tract: variations and congenital abnormalities. World J Surg 7 (2):271-276. https://doi.org/10.1007/bf01656159
Turner MA, Fulcher AS (2001) The cystic duct: normal anatomy and disease processes. Radiographics 21 (1):3-22. https://doi.org/10.1148/radiographics.21.1.g01ja093
Duca S, Bãlã O, Al-Hajjar N, Lancu C, Puia IC, Munteanu D, Graur F (2003) Laparoscopic cholecystectomy: incidents and complications. A retrospective analysis of 9542 consecutive laparoscopic operations. HPB (Oxford) 5 (3):152–158. https://doi.org/10.1080/13651820310015293
Girometti R, Brondani G, Cereser L, Como G, Del Pin M, Bazzocchi M, Zuiani C (2010) Post-cholecystectomy syndrome: spectrum of biliary findings at magnetic resonance cholangiopancreatography. Br J Radiol 83 (988):351-361. https://doi.org/10.1259/bjr/99865290
Isherwood J, Oakland K, Khanna A (2019) A systematic review of the aetiology and management of post cholecystectomy syndrome. Surgeon 17 (1):33-42. https://doi.org/10.1016/j.surge.2018.04.001
Chaib E, Kanas AF, Galvão FHF, D’Albuquerque LAC (2014) Bile duct confluence: anatomic variations and its classification. Surgical and Radiologic Anatomy 36 (2):105-109. https://doi.org/10.1007/s00276-013-1157-6
Kullman E, Borch K, Lindstrom E, Svanvik J, Anderberg B (1996) Value of routine intraoperative cholangiography in detecting aberrant bile ducts and bile duct injuries during laparoscopic cholecystectomy. Br J Surg 83 (2):171-175. https://doi.org/10.1046/j.1365-2168.1996.02190.x
Mariolis-Sapsakos T, Kalles V, Papatheodorou K, Goutas N, Papapanagiotou I, Flessas I, Kaklamanos I, Arvanitis DL, Konstantinou E, Sgantzos MN (2012) Anatomic variations of the right hepatic duct: results and surgical implications from a cadaveric study. Anatomy Research International. https://doi.org/10.1155/2012/838179
Khan MH, Howard TJ, Fogel EL, Sherman S, McHenry L, Watkins JL, Canal DF, Lehman GA (2007) Frequency of biliary complications after laparoscopic cholecystectomy detected by ERCP: experience at a large tertiary referral center. Gastrointest Endosc 65 (2):247-252. https://doi.org/10.1016/j.gie.2005.12.037
Babel N, Sakpal SV, Paragi P, Wellen J, Feldman S, Chamberlain RS (2009) Iatrogenic bile duct injury associated with anomalies of the right hepatic sectoral ducts: a misunderstood and underappreciated problem. HPB Surg. https://doi.org/10.1155/2009/153269
Schnelldorfer T, Sarr MG, Adams DB (2012) What is the duct of Luschka?--a systematic review. J Gastrointest Surg 16 (3):656-662. https://doi.org/10.1007/s11605-011-1802-5
Ko K, Kamiya J, Nagino M, Oda K, Yuasa N, Arai T, Nishio H, Nimura Y (2006) A study of the subvesical bile duct (duct of Luschka) in resected liver specimens. World J Surg 30 (7):1316-1320. https://doi.org/10.1007/s00268-005-0469-z
Ramia JM, Muffak K, Mansilla A, Villar J, Garrote D, Ferron JA (2005) Postlaparoscopic cholecystectomy bile leak secondary to an accessory duct of Luschka. Jsls 9 (2):216-217
Phillips MR, Joseph M, Dellon ES, Grimm I, Farrell TM, Rupp CC (2014) Surgical and endoscopic management of remnant cystic duct lithiasis after cholecystectomy--a case series. J Gastrointest Surg 18 (7):1278-1283. https://doi.org/10.1007/s11605-014-2530-4
Yeh BM, Liu PS, Soto JA, Corvera CA, Hussain HK (2009) MR imaging and CT of the biliary tract. Radiographics 29 (6):1669-1688. https://doi.org/10.1148/rg.296095514
Terhaar OA, Abbas S, Thornton FJ, Duke D, O'Kelly P, Abdullah K, Varghese JC, Lee MJ (2005) Imaging patients with “post-cholecystectomy syndrome”: an algorithmic approach. Clin Radiol 60 (1):78-84. https://doi.org/10.1016/j.crad.2004.02.014
McArthur TA, Planz V, Fineberg NS, Berland LL, Lockhart ME (2015) CT evaluation of common duct dilation after cholecystectomy and with advancing age. Abdom Imaging 40 (6):1581-1586. https://doi.org/10.1007/s00261-014-0308-5
Yegiyants S, Collins JC (2008) Operative strategy can reduce the incidence of major bile duct injury in laparoscopic cholecystectomy. Am Surg 74 (10):985-987
Overby DW, Apelgren KN, Richardson W, Fanelli R (2010) SAGES guidelines for the clinical application of laparoscopic biliary tract surgery. Surg Endosc 24 (10):2368-2386. https://doi.org/10.1007/s00464-010-1268-7
Nuzzo G, Giuliante F, Giovannini I, Ardito F, D'Acapito F, Vellone M, Murazio M, Capelli G (2005) Bile duct injury during laparoscopic cholecystectomy: results of an Italian national survey on 56 591 cholecystectomies. Arch Surg 140 (10):986-992. https://doi.org/10.1001/archsurg.140.10.986
Tornqvist B, Waage A, Zheng Z, Ye W, Nilsson M (2016) Severity of acute cholecystitis and risk of iatrogenic bile duct injury during cholecystectomy, a population-based case-control study. World J Surg 40 (5):1060-1067. https://doi.org/10.1007/s00268-015-3365-1
Jabłońska B, Lampe P (2009) Iatrogenic bile duct injuries: Etiology, diagnosis and management. World J Gastroenterol 15 (33):4097. https://doi.org/10.3748/wjg.15.4097
Karanikas M, Bozali F, Vamvakerou V, Markou M, Memet Chasan ZT, Efraimidou E, Papavramidis TS (2016) Biliary tract injuries after lap cholecystectomy—types, surgical intervention and timing. Ann Transl Med 4 (9):163–163. https://doi.org/10.21037/atm.2016.05.07
Kohn JF, Trenk A, Kuchta K, Lapin B, Denham W, Linn JG, Haggerty S, Joehl R, Ujiki MB (2018) Characterization of common bile duct injury after laparoscopic cholecystectomy in a high-volume hospital system. Surg Endosc 32 (3):1184-1191. https://doi.org/10.1007/s00464-017-5790-8
Elshaer M, Gravante G, Thomas K, Sorge R, Al-Hamali S, Ebdewi H (2015) Subtotal cholecystectomy for “difficult gallbladders”: systematic review and meta-analysis. JAMA Surg 150 (2):159-168. https://doi.org/10.1001/jamasurg.2014.1219
Wu YV, Linehan DC (2010) Bile duct injuries in the era of laparoscopic cholecystectomies. Surg Clin North Am 90 (4):787-802. https://doi.org/10.1016/j.suc.2010.04.019
Strasberg SM (2002) Avoidance of biliary injury during laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg 9 (5):543-547. https://doi.org/10.1007/s005340200071
Sicklick JK, Camp MS, Lillemoe KD, Melton GB, Yeo CJ, Campbell KA, Talamini MA, Pitt HA, Coleman J, Sauter PA, Cameron JL (2005) Surgical management of bile duct injuries sustained during laparoscopic cholecystectomy: perioperative results in 200 patients. Ann Surg 241 (5):786–792; discussion 793–785. https://doi.org/10.1097/01.sla.0000161029.27410.71
Jahangir K, Thomas S (2016) Noninvasive imaging of the biliary system relevant to percutaneous interventions. Semin Intervent Radiol 33 (04):277-282. https://doi.org/10.1055/s-0036-1592328
Lee CM, Stewart L, Way LW (2000) Postcholecystectomy abdominal bile collections. Archives of Surgery 135 (5):538-544. https://doi.org/10.1001/archsurg.135.5.538
Ragozzino A, De Ritis R, Mosca A, Iaccarino V, Imbriaco M (2004) Value of MR cholangiography in patients with iatrogenic bile duct injury after cholecystectomy. AJR Am J Roentgenol 183 (6):1567-1572. https://doi.org/10.2214/ajr.183.6.01831567
Thompson CM, Saad NE, Quazi RR, Darcy MD, Picus DD, Menias CO (2013) Management of iatrogenic bile duct injuries: role of the interventional radiologist. Radiographics 33 (1):117-134. https://doi.org/10.1148/rg.331125044
Dinkel HP, Moll R, Gassel HJ, Knupffer J, Timmermann W, Fieger M, Schindler G (1999) Helical CT cholangiography for the detection and localization of bile duct leakage. AJR Am J Roentgenol 173 (3):613-617. https://doi.org/10.2214/ajr.173.3.10470888
Sandoval BA, Goettler CE, Robinson AV, O'Donnell JK, Adler LP, Stellato TA (1997) Cholescintigraphy in the diagnosis of bile leak after laparoscopic cholecystectomy. Am Surg 63 (7):611-616
Kim JS, Moon DH, Lee SG, Lee YJ, Park KM, Hwang S, Lee HK (2002) The usefulness of hepatobiliary scintigraphy in the diagnosis of complications after adult-to-adult living donor liver transplantation. Eur J Nucl Med Mol Imaging 29 (4):473-479. https://doi.org/10.1007/s00259-001-0706-0
Morosi C, Civelli E, Battiston C, Schiavo M, Mazzaferro V, Severini A, Marchianò A (2009) CT cholangiography: assessment of feasibility and diagnostic reliability. Eur J Radiol 72 (1):114-117. https://doi.org/10.1016/j.ejrad.2008.05.011
Marin D, Bova V, Agnello F, Youngblood R, Midiri M, Brancatelli G (2010) Gadoxetate disodium-enhanced magnetic resonance cholangiography for the noninvasive detection of an active bile duct leak after laparoscopic cholecystectomy. J Comput Assist Tomogr 34 (2):213-216. https://doi.org/10.1097/RCT.0b013e3181c1a72c
Fidelman N, Kerlan RK, Jr., Laberge JM, Gordon RL (2011) Accuracy of percutaneous transhepatic cholangiography in predicting the location and nature of major bile duct injuries. J Vasc Interv Radiol 22 (6):884-892. https://doi.org/10.1016/j.jvir.2011.02.007
van de Graaf FW, Zaimi I, Stassen LPS, Lange JF (2018) Safe laparoscopic cholecystectomy: A systematic review of bile duct injury prevention. Int J Surg 60:164-172. https://doi.org/10.1016/j.ijsu.2018.11.006
Ding G-Q, Cai W, Qin M-F (2015) Is intraoperative cholangiography necessary during laparoscopic cholecystectomy for cholelithiasis? World J Gastroenterol 21 (7):2147-2151. https://doi.org/10.3748/wjg.v21.i7.2147
Altieri MS, Yang J, Obeid N, Zhu C, Talamini M, Pryor A (2018) Increasing bile duct injury and decreasing utilization of intraoperative cholangiogram and common bile duct exploration over 14 years: an analysis of outcomes in New York State. Surg Endosc 32 (2):667-674. https://doi.org/10.1007/s00464-017-5719-2
Lee NK, Kim S, Lee JW, Lee SH, Kang DH, Kim GH, Seo HI (2009) Biliary MR imaging with Gd-EOB-DTPA and its clinical applications. Radiographics 29 (6):1707-1724. https://doi.org/10.1148/rg.296095501
Cieszanowski A, Stadnik A, Lezak A, Maj E, Zieniewicz K, Rowinska-Berman K, Grudzinski IP, Krawczyk M, Rowinski O (2013) Detection of active bile leak with Gd-EOB-DTPA enhanced MR cholangiography: comparison of 20–25 min delayed and 60–180 min delayed images. Eur J Radiol 82 (12):2176-2182. https://doi.org/10.1016/j.ejrad.2013.08.021
Boraschi P, Donati F (2013) Biliary-enteric anastomoses: spectrum of findings on Gd-EOB-DTPA-enhanced MR cholangiography. Abdom Imaging 38 (6):1351-1359. https://doi.org/10.1007/s00261-013-0007-7
Kantarcı M, Pirimoglu B, Karabulut N, Bayraktutan U, Ogul H, Ozturk G, Aydinli B, Kizrak Y, Eren S, Yilmaz S (2013) Non-invasive detection of biliary leaks using Gd-EOB-DTPA-enhanced MR cholangiography: comparison with T2-weighted MR cholangiography. Eur Radiol 23 (10):2713-2722. https://doi.org/10.1007/s00330-013-2880-4
Aduna M, Larena JA, Martin D, Martinez-Guerenu B, Aguirre I, Astigarraga E (2005) Bile duct leaks after laparoscopic cholecystectomy: value of contrast-enhanced MRCP. Abdom Imaging 30 (4):480-487. https://doi.org/10.1007/s00261-004-0276-2
Hoeffel C, Azizi L, Lewin M, Laurent V, Aubé C, Arrivé L, Tubiana J-M (2006) Normal and pathologic features of the postoperative biliary tract at 3D MR cholangiopancreatography and MR imaging. Radiographics 26 (6):1603-1620. https://doi.org/10.1148/rg.266055730
Seale MK, Catalano OA, Saini S, Hahn PF, Sahani DV (2009) Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics 29 (6):1725-1748. https://doi.org/10.1148/rg.296095515
Frydrychowicz A, Lubner MG, Brown JJ, Merkle EM, Nagle SK, Rofsky NM, Reeder SB (2012) Hepatobiliary MR imaging with gadolinium-based contrast agents. J Magn Reson Imaging 35 (3):492-511. https://doi.org/10.1002/jmri.22833
Stelter L, Freyhardt P, Grieser C, Walter T, Geisel D, Baur A, Seehofer D, Denecke T (2014) An increased flip angle in late phase Gd-EOB-DTPA MRI shows improved performance in bile duct visualization compared to T2w-MRCP. Eur J Radiol 83 (10):1723-1727. https://doi.org/10.1016/j.ejrad.2014.06.005
Gupta RT, Brady CM, Lotz J, Boll DT, Merkle EM (2010) Dynamic MR imaging of the biliary system using hepatocyte-specific contrast agents. AJR Am J Roentgenol 195 (2):405-413. https://doi.org/10.2214/AJR.09.3641
Van Beers BE, Pastor CM, Hussain HK (2012) Primovist, Eovist: What to expect? J Hepatol 57 (2):421-429. https://doi.org/10.1016/j.jhep.2012.01.031
Welle CL, Miller FH, Yeh BM (2020) Advances in MR Imaging of the Biliary Tract. Magn Reson Imaging Clin N Am 28 (3):341-352. https://doi.org/10.1016/j.mric.2020.03.002
Tschirch FT, Struwe A, Petrowsky H, Kakales I, Marincek B, Weishaupt D (2008) Contrast-enhanced MR cholangiography with Gd-EOB-DTPA in patients with liver cirrhosis: visualization of the biliary ducts in comparison with patients with normal liver parenchyma. Eur Radiol 18 (8):1577-1586. https://doi.org/10.1007/s00330-008-0929-6
Petrillo M, Ierardi AM, Tofanelli L, Maresca D, Angileri A, Patella F, Carrafiello G (2019) Gd-EOB-DTP-enhanced MRC in the preoperative percutaneous management of intra and extrahepatic biliary leakages: does it matter? Gland Surg 8 (2):174–183. https://doi.org/10.21037/gs.2019.03.09
Kul M, Erden A, Düşünceli Atman E (2017) Diagnostic value of Gd-EOB-DTPA-enhanced MR cholangiography in non-invasive detection of postoperative bile leakage. Br J Radiol 90 (1072):20160847. https://doi.org/10.1259/bjr.20160847
Copelan A, Bahoura L, Tardy F, Kirsch M, Sokhandon F, Kapoor B (2015) Etiology, diagnosis, and management of bilomas: A current update. Tech Vasc Interv Radiol 18 (4):236-243. https://doi.org/10.1053/j.tvir.2015.07.007
O’Connor AR, Coakley FV, Meng MV, Eberhardt SC (2003) Imaging of retained surgical sponges in the abdomen and pelvis. AJR Am J Roentgenol 180 (2):481-489. https://doi.org/10.2214/ajr.180.2.1800481
Nikpour AM, Knebel RJ, Cheng D (2016) Diagnosis and management of postoperative biliary leaks. Semin Intervent Radiol 33 (4):307-312. https://doi.org/10.1055/s-0036-1592324
Bektas H, Schrem H, Winny M, Klempnauer J (2007) Surgical treatment and outcome of iatrogenic bile duct lesions after cholecystectomy and the impact of different clinical classification systems. Br J Surg 94 (9):1119-1127. https://doi.org/10.1002/bjs.5752
Saad N, Darcy M (2008) Iatrogenic bile duct injury during laparoscopic cholecystectomy. Tech Vasc Interv Radiol 11 (2):102-110. https://doi.org/10.1053/j.tvir.2008.07.004
Chun K (2014) Recent classifications of the common bile duct injury. Korean J Hepatobiliary Pancreat Surg 18 (3):69. https://doi.org/10.14701/kjhbps.2014.18.3.69
Bharathy KG, Negi SS (2014) Postcholecystectomy bile duct injury and its sequelae: pathogenesis, classification, and management. Indian J Gastroenterol 33 (3):201-215. https://doi.org/10.1007/s12664-013-0359-5
Demirbas BT, Gulluoglu BM, Aktan AO (2015) Retained abdominal gallstones after laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech 25 (2):97-99. https://doi.org/10.1097/sle.0000000000000105
Shirah BH, Shirah HA, Zafar SH, Albeladi KB (2018) Clinical patterns of postcholecystectomy syndrome. Ann Hepatobiliary Pancreat Surg 22 (1):52–57. https://doi.org/10.14701/ahbps.2018.22.1.52
Schäfer M, Schneiter R, Krähenbühl L (2003) Incidence and management of Mirizzi syndrome during laparoscopic cholecystectomy. Surg Endosc 17 (8):1186–1190; discussion 1191–1182. https://doi.org/10.1007/s00464-002-8865-z
Lai EC, Lau WY (2006) Mirizzi syndrome: history, present and future development. ANZ J Surg 76 (4):251-257. https://doi.org/10.1111/j.1445-2197.2006.03690.x
Antoniou SA, Antoniou GA, Makridis C (2010) Laparoscopic treatment of Mirizzi syndrome: a systematic review. Surg Endosc 24 (1):33-39. https://doi.org/10.1007/s00464-009-0520-5
Senra F, Navaratne L, Acosta A, Martínez-Isla A (2020) Laparoscopic management of type II Mirizzi syndrome. Surg Endosc 34 (5):2303-2312. https://doi.org/10.1007/s00464-019-07316-6
Kulkarni SS, Hotta M, Sher L, Selby RR, Parekh D, Buxbaum J, Stapfer M (2017) Complicated gallstone disease: diagnosis and management of Mirizzi syndrome. Surg Endosc 31 (5):2215-2222. https://doi.org/10.1007/s00464-016-5219-9
Yun EJ, Choi CS, Yoon DY, Seo YL, Chang SK, Kim JS, Woo JY (2009) Combination of magnetic resonance cholangiopancreatography and computed tomography for preoperative diagnosis of the Mirizzi syndrome. J Comput Assist Tomogr 33 (4):636-640. https://doi.org/10.1097/RCT.0b013e31817710d5
Deziel DJ, Millikan KW, Economou SG, Doolas A, Ko ST, Airan MC (1993) Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg 165 (1):9-14. https://doi.org/10.1016/s0002-9610(05)80397-6
Bao-Qiang W, Jun H, Wen-Song L, Yong J, Xue-Min C, Dong-Lin S (2019) Laparoscopic repair of transected right hepatic artery during cholecystectomy: A report of two cases. Annals of Hepatology. https://doi.org/10.1016/j.aohep.2019.11.004
Lebedis CA, Bates DDB, Soto JA (2017) Iatrogenic, blunt, and penetrating trauma to the biliary tract. Abdom Radiol (NY) 42 (1):28-45. https://doi.org/10.1007/s00261-016-0856-y
Madanur MA, Battula N, Sethi H, Deshpande R, Heaton N, Rela M (2007) Pseudoaneurysm following laparoscopic cholecystectomy. Hepatobiliary Pancreat Dis Int 6 (3):294-298
Craig EV, Heller MT (2019) Complications of liver transplant. Abdom Radiol (NY). https://doi.org/10.1007/s00261-019-02340-5
Deltenre P, Valla DC (2008) Ischemic cholangiopathy. Semin Liver Dis 28 (3):235-246. https://doi.org/10.1055/s-0028-1085092
Alabdulghani F, Healy GM, Cantwell CP (2020) Radiological findings in ischaemic cholangiopathy. Clin Radiol 75 (3):161-168. https://doi.org/10.1016/j.crad.2019.10.017
Horton M, Florence MG (1998) Unusual abscess patterns following dropped gallstones during laparoscopic cholecystectomy. Am J Surg 175 (5):375-379. https://doi.org/10.1016/s0002-9610(98)00048-8
Arishi AR, Rabie ME, Khan MS, Sumaili H, Shaabi H, Michael NT, Shekhawat BS (2008) Spilled gallstones: the source of an enigma. Jsls 12 (3):321-325
Nayak L, Menias CO, Gayer G (2013) Dropped gallstones: spectrum of imaging findings, complications and diagnostic pitfalls. Br J Radiol 86 (1028):20120588. https://doi.org/10.1259/bjr.20120588
Kapoor S, Nundy S (2012) Bile duct leaks from the intrahepatic biliary tree: a review of its etiology, incidence, and management. HPB Surg. https://doi.org/10.1155/2012/752932
Kapoor BS, Mauri G, Lorenz JM (2018) Management of biliary strictures: state-of-the-art review. Radiology 289 (3):590-603. https://doi.org/10.1148/radiol.2018172424
Lau WY, Lai EC, Lau SH (2010) Management of bile duct injury after laparoscopic cholecystectomy: a review. ANZ J Surg 80 (1-2):75-81. https://doi.org/10.1111/j.1445-2197.2009.05205.x
Pesce A, Palmucci S, La Greca G, Puleo S (2019) Iatrogenic bile duct injury: impact and management challenges. Clin Exp Gastroenterol Volume 12:121-128. https://doi.org/10.2147/ceg.s169492
Feng X, Dong J (2017) Surgical management for bile duct injury. BioScience Trends 11 (4):399-405. https://doi.org/10.5582/bst.2017.01176
Saad NE, Saad WE, Davies MG, Waldman DL, Fultz PJ, Rubens DJ (2005) Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics 25 Suppl 1:S173-189. https://doi.org/10.1148/rg.25si055503
Lentz J, Tobar MA, Canders CP (2017) Perihepatic, pulmonary, and renal abscesses due to spilled gallstones. J Emerg Med 52 (5):e183-e185. https://doi.org/10.1016/j.jemermed.2016.12.016
Funding
No funding or grant support was used in the preparing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reddy, S., Lopes Vendrami, C., Mittal, P. et al. MRI evaluation of bile duct injuries and other post-cholecystectomy complications. Abdom Radiol 46, 3086–3104 (2021). https://doi.org/10.1007/s00261-020-02947-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02947-z