Abstract
Purpose of Review
Over the past several decades, the number of hepatobiliary surgeries performed has increased, with laparoscopic cholecystectomy now the most frequently performed abdominal surgery in the United States. Due to the increase in surgeries performed, medicolegal concerns, and the availability of different types of imaging, radiologists are increasingly requested to evaluate for complications following hepatobiliary surgery.
Recent Findings
Early radiologic identification and assessment of the extent of biliary injuries allows prompt diagnosis and management of postoperative biliary complications. A wide variety of imaging modalities can be utilized, including computed tomography, nuclear medicine, and magnetic resonance imaging.
Summary
This article provides a review of the broad spectrum of biliary injuries following hepatobiliary surgery, with an emphasis on postcholecystectomy imaging findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The number of cholecystectomies performed has drastically increased with the introduction of laparoscopic technique. It is estimated that over 750,000 cholecystectomies are performed each year [1•, 2] and the majority are performed laparoscopically. Laparoscopic technique is associated with less postoperative pain, better cosmetic results, and earlier return to normal activities. The advantage of a laparoscopic technique has been demonstrated for both symptomatic cholelithiasis and acute cholecystitis, with reduction of postoperative morbidity, mortality, and hospital stay using a laparoscopic technique [3].
Despite these advantages, laparoscopic cholecystectomy is not without risks and can be associated with morbidity as well as occasional mortality. In particular, multiple studies have demonstrated an increased risk of biliary injury following laparoscopic cholecystectomy compared to open cholecystectomy, with bile duct injury rates after elective laparoscopic cholecystectomy ranging from 0.3% to 0.5%, compared to 0.1 to 0.2% after open cholecystectomy [4,5,6•, 7,8,9]. While more recent series demonstrate lower rates of biliary injury, as low as 0.2% [10], another study following patients after single-incision laparoscopic cholecystectomy noted an injury rate of 0.7% [11]. In addition to morbidity and mortality, biliary complications have been noted to be a common cause of medicolegal claims and the most likely to be associated with a successful claim [12].
In addition to cholecystectomy, biliary injuries can be seen after a number of other surgeries or procedures, including liver biopsy, hepatic resection, liver transplantation, percutaneous cholecystostomy, transcatheter arterial embolization, gastric bypass, and pancreatic resection. In the setting of hepatic transplants, biliary injuries are a dreaded complication associated with significant morbidity and mortality. Biliary injury occurs in 10–34% of graft recipients and is the second leading cause of graft dysfunction after rejection [13, 14]. Radiologists play a critical role in the early diagnosis of biliary injury, allowing for prompt management to prevent graft failure.
In order to reduce morbidity and mortality, as well as minimize litigation, early recognition of bile duct injury is critical. As clinical symptoms of biliary injuries are often nonspecific, radiologists will increasingly encounter imaging studies performed to evaluate for postoperative complications. An understanding of expected postsurgical appearance and imaging findings of biliary complications across a wide array of modalities is critical. Imaging options are numerous, including ultrasound (US), computed tomography (CT), magnetic resonance cholangiopancreatography (MRCP), hepatobiliary scintigraphy, fluoroscopy with contrast injected through an existing drainage catheter, and percutaneous transhepatic cholangiography (PTC).
Complications of biliary surgery can be divided into early and late complications. Early complications of cholecystectomy generally present within one month and include biliary leak and obstruction. Late complications include papillary stenosis, choledocholithiasis, biliary stricture, remnant gallbladder, and dropped gallstones. Prompt recognition of these injuries and complications allows for assessment of injury extent, procedural planning, and decreased morbidity and mortality.
Expected Postsurgical Appearance
Within the first several postoperative days after cholecystectomy, residual intra-abdominal air can be expected, especially after open surgery. If the patient’s surgery was limited to laparoscopy, the amount of intraperitoneal air should be minimal as insufflated carbon dioxide is rapidly absorbed. Surgical drains are uncommon after laparoscopic cholecystectomy but can be seen after open technique. Metallic clips in the gallbladder fossa should be easily identified after cholecystectomy. A variable length of cystic duct is left in place following cholecystectomy, usually 1–2 cm but occasionally up to 6 cm [15].
The normal postoperative hepatic transplant appearance varies depending on the type of anastomosis performed. Choledochocholedochostomy (end-to-end anastomosis between donor and recipient ducts), hepaticocholedochostomy, or a biliary-enteric anastomosis such as choledochojejunostomy all may be performed during a transplant, depending on the donor and recipient anatomy. A duct-to-duct anastomosis is often the preferred surgical technique as it is technically less challenging compared to a biliary-enteric anastomosis. This technique also preserves sphincter of Oddi function, thereby reducing risk of biliary infections. Biliary-enteric anastomoses are usually reserved for cases when the recipient ducts are abnormal, such as patients with primary sclerosing cholangitis. A knowledge of the utilized anastomotic technique is important to ascertain if the imaging appearance is expected or abnormal.
A biliary tube (straight tube or T tube) placed across the anastomosis may be left in place following hepatic transplant for up to 6 months. A size discrepancy between the donor bile duct and the recipient bile duct in a transplant patient may be seen, usually with the recipient bile duct being larger in caliber than the donor’s duct. There should be gradual tapering of the bile duct lumen at the anastomosis rather than abrupt caliber change which aids in distinguishing between a normal postoperative appearance and a biliary stricture.
Biliary-enteric anastomoses such as choledochojejunostomy or Roux-en-Y anastomosis may also be utilized for other surgical procedures, including a Whipple procedure, repair of biliary injury, hepatectomy, or hepatic transplant. This type of anastomosis is performed between a normal bile duct segment and a portion of the gastrointestinal tract, often a Roux-en-Y loop of jejunum.
After any biliary surgery, a small amount of fluid is often seen in the surgical bed, usually with mixed air and fluid content. These collections may contain blood, resulting in an increased attenuation collection on CT. Hemostatic agents such as Surgicel™ (oxidized regenerated cellulose) can be used intraoperatively to control hemorrhage and may mimic the appearance of hematoma, abscess, or tumor [16]. These agents most commonly appear as complex collections with intermixed gaseous foci and an attenuation of 40–50 HU on CT [17] (Fig. 1). The radiologist must recognize the imaging appearance of hemostatic agents to avoid unnecessary surgery or treatment.
Early Complications of Biliary Surgery
Biliary Leaks and Bilomas
Bile duct leak is one of the most serious biliary complications that may occur after open and laparoscopic cholecystectomy, hepatic resection, transplantation, or liver biopsy. Following cholecystectomy, biliary leak most commonly occurs from the cystic duct stump when clips become dislodged or do not entirely occlude the duct. Less commonly, bile leaks can arise from the accessory ducts of Luschka, common bile duct, common hepatic duct, or liver bed. Several factors predispose to bile leaks, including variant biliary anatomy, chronic cholecystitis, common bile duct stone, and dysfunction of the sphincter of Oddi [18].
Symptoms of biliary leak are nonspecific and often overlap with expected postsurgical symptoms, making the diagnosis difficult. Patients commonly present within a week following surgery, though others may not experience symptoms for 30 days. Persistent abdominal pain, generalized malaise, and anorexia are the most common presenting symptoms. Laboratory findings may include elevated liver enzymes, bilirubin, or leukocytosis. Unrecognized bile leaks can result in development of biliary peritonitis, intra-abdominal abscess, or cholangitis, with the patient presenting with more acute symptoms [19].
Iatrogenic bile duct injuries are often classified using the Bismuth or Strasberg classification. The Bismuth classification was developed in the 1980s prior to the advent of laparoscopy, with types I–IV designating injuries at progressively higher levels in the biliary tree [20]. The Strasberg classification [5] is more comprehensive, including a broader spectrum of extrahepatic bile duct injuries (Table 1). A Strasberg type A injury is the most common biliary injury following cholecystectomy, with bile leakage from the cystic duct or bile ducts of Luschka. These classification systems are useful for surgical planning, as the length of intact duct distal to the biliary confluence determines whether a choledochojejunostomy or hepaticojejunostomy is preferred. Therefore, it is important for the interpreting radiologist to have some familiarity with these classifications when communicating with the ordering physician.
Patients with suspected biliary leak often undergo CT or ultrasound as part of their initial diagnostic work-up. CT studies should be performed with intravenous contrast if possible. A bile leak can either be free flowing within the peritoneum or localized within a collection called a biloma. Bilomas tend to occur when the bile leak rate of flow is low. The most common location for bile accumulation is the subhepatic region, though the right paracolic gutter and suprahepatic regions are also common. More unusual locations can also be seen, with fluid accumulating in the greater and lesser sac, pouch of Douglas, or left paracolic gutter. Unfortunately, bile-containing collections cannot be accurately distinguished from other postoperative collections, including blood, pus, or serous fluid. While CT provides higher spatial resolution and better demonstrates the location of the fluid collections compared to US, neither can establish the precise location of the leak nor determine whether there is an active leak.
Hepatobiliary scintigraphy, also known as hepatobiliary iminodiacetic acid (HIDA) scan, is an effective noninvasive imaging tool for evaluating complications of biliary surgeries, providing functional information with accurate demonstration of the leak. In the past, the primary limitation of scintigraphic imaging was inherently poor spatial resolution of planar imaging. This can largely be overcome by the use of SPECT/CT, allowing accurate localization of radiotracer accumulation. The addition of SPECT/CT has been found to provide additional information from planar images alone in 76% of cases [21]. A study by Sharma et al. [22] demonstrated significantly improved diagnostic accuracy for SPECT/CT versus planar HIDA at 96.8% versus 65.6% with no false-positive results when using SPECT/CT. If SPECT/CT cannot be performed, other anatomic imaging modalities such as CT, MR, ultrasound, or ERCP can be used in conjunction with HIDA imaging.
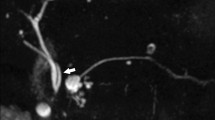
HIDA imaging is performed after intravenous administration of a Tc99m-labeled hepatobiliary agent (mebrofenin or disofenin). Sequential planar images are acquired demonstrating homogenous uptake within the liver after several minutes followed by visualization of the common hepatic duct, common bile duct, duodenum, and jejunum [23••]. A review of dynamic images is often helpful to confirm this appropriate pattern of filling. When radiotracer is seen outside of these locations, a bile leak is confirmed (Fig. 2). It is also necessary to understand the patient’s biliary anatomy including history of any prior biliary-enteric anastomoses.
Bile leak after hepatic transplantation. a Radionuclide hepatobiliary image obtained 40 min after radiotracer administration demonstrates large volume bile leak extending into the peritoneal cavity (arrowhead). b SPECT/CT image localizes the site of bile leak to the region of the choledochojejunostomy (white arrow). c ERCP image confirms high-grade bile leak, with nearly all contrast injected entering the leak at the anastomosis (black arrow), with minimal contrast opacifying the transplant biliary system
Another noninvasive imaging modality for detection of biliary leak is contrast-enhanced MR cholangiopancreatography (MRCP) using a hepatobiliary contrast agent, most commonly gadoxetate disodium (Eovist™). Approximately 50% of this hepatocyte specific contrast agent dose is taken up by functional hepatocytes and excreted into the biliary system, allowing morphologic and functional assessment of the biliary system [24•, 25]. MR cholangiography accurately localizes the anatomic site of bile leakage as well as the type of bile duct injury. Bile leak can be seen with hepatobiliary contrast excretion from the biliary defect into the intra-abdominal space (Fig. 3). Advantages of this study include accurate localization of bile leak and delineation of fluid collection of biliary and nonbiliary origin. In fact, studies have verified that MRCP with hepatocyte specific contrast agent has near 100% accuracy for detection of bile leak, with the exact location of the leak determined in 79–85% of studies [26]. One limitation of MR cholangiography is impaired excretion of contrast in the setting of obstructive jaundice and hepatic dysfunction [25]. This may require repeat delayed imaging for adequate opacification of the biliary tree. In the setting of high-grade obstruction, no excretion may occur.
Bile leak after cholecystectomy. a Axial T2 HASTE MRI image demonstrates a large fluid collection in the cholecystectomy bed (arrowhead). A drain was subsequently placed in the collection, with return of bilious fluid. b Axial MRI T1 fat saturation delayed post contrast image after administration of hepatobiliary contrast agent demonstrates contrast excretion from the cystic duct stump into the fluid gallbladder fossa collection (white arrow). Additional images demonstrate contrast filling the intra-abdominal drain (not shown)
Endoscopic retrograde cholangiopancreatography (ERCP) is a highly sensitive method for determining the presence and location of a biliary leak. In addition, the leak can often be treated during the procedure with approximately 89% success rate [27]. The limitations of ERCP are detection and treatment of leaks at the periphery of the biliary system as well as in patients with altered biliary anatomy such as post Roux-en-Y. In addition, an ERCP Is invasive, with a complication rate of 15.9% [28] including cholangitis (5%), pancreatitis (3.8%), perforation (1.1%), and hemorrhage (0.9%).
Biliary Obstruction
Biliary obstruction can occur after approximately 1% of laparoscopic cholecystectomies [17]. Like biliary leaks, this complication is more common using a laparoscopic approach and is usually secondary to inadvertent transection or ligation of the extrahepatic common bile duct instead of the cystic duct. Thermal injury to the biliary tract or excision of an aberrant bile duct can also result in obstruction. Anatomic biliary variations that increase the risk of inadvertent bile duct excision or ligation include low and medialized insertion of the cystic duct, a parallel course of the cystic duct with the common hepatic duct, or aberrant right hepatic duct [15].
Patients with acute postoperative biliary obstruction often present with abdominal pain and jaundice as well as elevated liver function tests and bilirubin. Biliary obstruction places the patient at risk for cholangitis and development of intrahepatic abscesses, with the patient presenting with signs of infection including fever and leukocytosis.
Biliary obstruction is best assessed by MRI with MRCP, demonstrating diffuse or segmental duct dilation with abrupt tapering at the site of obstruction (Fig. 4). It is important for the radiologist to note the level of the biliary injury and the length of the obstruction, allowing for surgical planning. Though less sensitive for biliary dilation, CT may help identify the inadvertent placement of metallic surgical clips.
Iatrogenic ligation of the hepatic duct during laparoscopic cholecystectomy. a MRCP MIP image demonstrates diffuse dilation of the intrahepatic bile ducts (arrowhead) with abrupt narrowing of the proximal hepatic duct (white arrow). b Coronal T2W image demonstrates linear susceptibility artifact (black arrow) at the level of ductal caliber change compatible with a surgical clip. The distal common bile duct is normal in caliber
MR cholangiography with hepatobiliary contrast agent may offer additional information beyond standard MRI with MRCP. These agents can allow classification of the severity of bile duct obstruction based on the degree of contrast agent filling distal to the stricture. This is optimally evaluated at least 30 min after intravenous injection of the contrast agent, with a complete obstruction demonstrating absence of contrast agent distal to the stricture and a partial obstruction allowing limited passage of contrast agent beyond the apparent stricture [24•].
Late Complications of Cholecystectomy
Late complications of cholecystectomy include choledocholithiasis, biliary stricture, remnant gallbladder, papillary stenosis, and dropped gallstones. Patients may present several weeks up to many years after biliary surgery. Imaging plays an important role in diagnosing each of these conditions, especially as clinical suspicion for iatrogenic bile injury may be low if the patient is not recently postoperative.
Choledocholithiasis
Choledocholithiasis can be an early or delayed complication and is common following cholecystectomy, with retained or recurrent stones occurring in 1.2–14% of patients [29]. A stone is considered retained if identified within two years of surgery and recurrent if noted more than two years after surgery. Retained stones may have been present in the common bile duct prior to surgery or migrate from the cystic duct into the common bile duct intraoperatively. A laparoscopic cholecystectomy does not permit common bile duct exploration, and residual lithiasis is more common than when the procedure is performed using an open technique. The risk of retained lithiasis is increased if MRCP is not performed prior to cholecystectomy.
ERCP is highly sensitive for the detection of choledocholithiasis and also allows stone removal. However, MRCP is preferred as a first-line investigation due to the invasive nature and associated morbidity and mortality of ERCP. MRCP is an accurate test for the detection of stones, with sensitivity of 99% for stones larger than 6 mm and 89% for smaller stones [30]. Choledocholithiasis is identified on MRI as filling defects within the dependent portion of the common bile duct that are usually most accurately identified on T2-weighted imaging, especially the MRCP portion of the examination due to high spatial resolution (Fig. 5). Small calculi may be obscured on MIP images and are best seen on the thin section source images. Ideally, the stone should be identified on two images in different planes to maximize specificity. Pneumobilia can mimic common bile duct stones but is more commonly in a nondependent location. CT and US may also be performed for detection of choledocholithiasis but are less sensitive and specific than MRI.
Less commonly, residual or recurrent stones can occur in the cystic duct remnant following cholecystectomy. These patients may present with obstructive symptoms due to extrinsic compression by the cystic duct stone on the common hepatic duct, otherwise known as Mirizzi syndrome. Imaging will demonstrate one or more gallstones at the junction of the cystic duct and common hepatic duct with intrahepatic biliary ductal dilation (Fig. 6). Longstanding or recurrent stone impaction may eventually lead to a cholecystobiliary fistula, biliary stricture, or cholangitis [31, 32].
Mirizzi syndrome. a Coronal T2-weighted image demonstrates a large stone in the cystic duct remnant (arrowhead) obstructing the common bile duct. b Coronal MRCP MIP demonstrates biliary ductal dilation with abrupt narrowing at the level of the stone (white arrow), with normal caliber distal common bile duct (curved arrow)
Sphincter of Oddi Dysfunction
Sphincter of Oddi dysfunction (SOD), also known as postcholecystectomy syndrome, papillary stenosis, or biliary dyskinesia, is a clinical syndrome of obstruction and thought to be due to functional abnormality of the sphincter of Oddi. Patients present with recurrent biliary pain following surgery, occurring in 1% to 1.5% of postcholecystectomy patients. Imaging studies are most commonly performed on these patients to exclude other causes of recurrent abdominal pain. There are no specific findings on routine imaging studies, with CT and MR demonstrating variable biliary dilatation to the level of the ampulla of Vater without underlying structural abnormality.
The diagnostic gold standard for diagnosis of papillary stenosis is sphincter of Oddi manometry. However, the study is time-consuming, not widely available, and associated with complications such as pancreatitis [33]. Recently, less invasive approaches have been introduced for the diagnosis of sphincter of Oddi dysfunction. Nuclear medicine hepatobiliary imaging with morphine provocation has been demonstrated to identify patients who may respond to endoscopic sphincterotomy [34]. Morphine is known to cause spasm of the sphincter of Oddi and it is hypothesized that administration of morphine accentuates functional abnormalities in patients with SOD. Therefore, patients with SOD are more likely to have delayed biliary excretion with higher residual radiotracer activity in the biliary system at 60 min.
MR cholangiography can also be performed to evaluate bile kinetics. SOD is suggested with delayed or absent passage of contrast material beyond the ampulla of Vater on images obtained 30 min to 1 h after administration of contrast [24•]. SOD can be excluded if there is normal passage of contrast material into the bile ducts on images obtained 20–30 min after contrast injection. Recently, the use of secretin-stimulated MRCP has been explored [35] which may provide additional functional information, but has not been widely adapted into clinical practice to date.
Remnant Gallbladder
Incomplete resection of the gallbladder is surprisingly common, occurring in up to 13.3% of laparoscopic cholecystectomies [29]. Risk factors for incomplete gallbladder resection include poor visualization of the gallbladder fossa during surgery, adhesions, excessive bleeding, or variant gallbladder or biliary morphology. This is rarely symptomatic but may be associated with pain from residual or recurrent gallstones in the residual gallbladder. Imaging features of remnant gallbladder are a fluid filled structure in the gallbladder fossa with or without stones (Fig. 7). It may be difficult to distinguish between a postoperative fluid collection and a remnant gallbladder in the early postoperative setting.
Remnant gallbladder in a patient with prior cholecystectomy presenting with gallstone pancreatitis. a Axial CECT demonstrates a fluid collection in the gallbladder fossa (arrowhead) with small hyperdense focus posteriorly (white arrow). The patient is also noted to have necrotizing pancreatitis with multiple acute necrotic collections (curved arrow). Subsequent ERCP (b) later confirms the presence of a remnant gallbladder (black arrow)
Biliary Stricture
Biliary stricture after cholecystectomy is often related to duct fibrosis or scarring, and most commonly occurs in the common bile duct near the insertion of the cystic duct or at the hepatic confluence due to inadvertent ligation, thermal injury, or chronic irritation from surgical clips. Biliary strictures are rare, occurring after 0.4 to 0.6% of laparoscopic cholecystectomies [36]. Patients with biliary strictures may present several months to several years following cholecystectomy and demonstrate symptoms of biliary obstruction, with elevated bilirubin and alkaline phosphatase.
Both CT and MRI may demonstrate varying levels of biliary dilation with abrupt transition at the point of stricture. MRCP provides a noninvasive depiction of the biliary system above and below the level of the stricture and is more sensitive than CT for identification of biliary dilatation and stricture. The length of structure is often overestimated by MR as the bile duct immediately distal to the stricture is often collapsed. This can often be overcome by evaluating the source MRCP images. ERCP has therapeutic advantages over MRCP, allowing stent placement at the site of the stricture. However, there may be inadequate evaluation of the bile ducts upstream to a severe stricture.
Benign biliary strictures are smooth with tapered regular margins and symmetric narrowing. These must be distinguished from malignant strictures, which are often longer and more irregular with shouldered margins [37]. Malignant strictures are also often associated with thickening and hyperenhancement of the bile duct wall.
Biliary strictures are the most frequent late complication in patients that have undergone hepatic transplantation, occurring in 15–18% of liver transplant recipients [14]. These strictures most frequently occur at the level of the biliary anastomosis due to scar formation or iatrogenic trauma. Anastomotic strictures appear as short-segment narrowing at the anastomosis with upstream dilatation. Nonanastomotic strictures are less common and usually caused by ischemia, most commonly due to hepatic artery thrombosis or stenosis [38]. Rejection and cholangitis are less common causes of nonanastomotic strictures. Nonanastomotic strictures are typically multifocal long segment strictures, usually involving the biliary confluence and extending into the right and left hepatic ducts [15, 39••] (Fig. 8). MRCP has been found to be 96% sensitive and 94% specific in the depiction of biliary obstruction in hepatic transplants [40]. Initial treatment of biliary stricture usually involves balloon dilation with or without stent placement, though surgical reconstruction may be required [41]. Treatment of nonanastomotic strictures focuses on revascularization of the hepatic artery with intra-arterial heparin therapy or angioplasty, though the strictures are usually irreversible.
Ischemic cholangiopathy in a hepatic transplant. a MRCP MIP demonstrates diffuse irregularity of the intrahepatic bile ducts with multiple long segment stenoses (white arrows). The common bile duct below the anastomosis appears normal (arrowhead). b Contrast-enhanced CT 3D reconstruction demonstrates the hepatic artery graft taking a sharp turn with focal stenosis (curved arrow)
Dropped Gallstones
Perforation of the gallbladder with bile spillage occurs not infrequently during laparoscopic cholecystectomy at a rate of 10–40% [42]. The rate of gallstone spillage is less frequent, estimated at 4–6% [17, 43]. Initially, dropped gallstones were thought to be of no clinical significance. Over the past several decades, however, their complications have been well recognized, with rates ranging from 0.08% to 12% [43, 44]. While the primary complication is abscess formation, chronic inflammation, and fistula formation are other symptomatic complications. Patients may be asymptomatic or present with nonspecific symptoms such as abdominal pain and fever. This presentation can be a variable time after cholecystectomy, ranging from days to years.
CT imaging findings are straightforward if the dropped gallstone is calcified, with CT demonstrating calcified densities with or without associated fluid collections (Fig. 9). These are often more easily identified on noncontrast CT images. Dropped stones are most commonly located near the liver in the subhepatic space, specifically Morrison’s pouch. Unusual locations for dropped gallstones have been described, including the right hemithorax, subphrenic space, the paracolic gutters, as well as abdominal wall at trocar sites [45]. Small or noncalcified dropped stones may not be visible by CT. In these instances, the presence of a fluid collection or abscess in Morrison’s pouch in a postcholecystectomy patient should raise the suspicion of dropped stones. US can be useful with the dropped gallstones appearing as hyperechoic foci with posterior acoustic shadowing (Fig. 9). Stones causing abscess vary in size, ranging from several millimeters to 2.5 cm. [46].
Dropped gallstone with abscess. a Axial CECT image demonstrates cholecystectomy clips in the gallbladder fossa and a rim-enhancing fluid collection in the right subhepatic space (curved arrow). Within the abscess, a focus of calcification is noted consistent with a gallstone (arrowhead). a Ultrasound of a different patient demonstrates multiple hyperechoic internal foci (white arrows) with posterior acoustic shadowing consistent with dropped gallstones in the right subhepatic space
On MRI, pigmented dropped gallstones may appear hyperintense on T1-weighted images, while other stones are typically hypointense on T1 .++and T2-weighted images [47]. The stones themselves should not enhance on CT or MRI. However, adjacent enhancement will likely be present in the setting of abscess or inflammatory fibrous tissue.
Dropped gallstones and their associated complications have overlapping imaging features with other conditions, most commonly peritoneal metastases. Soft tissue attenuation perihepatic nodules may often be confused with metastatic implants. Though the presence of internal calcification suggests dropped stones, some mucin producing tumors can contain calcium. Dropped gallstones should be considered when a perihepatic soft tissue nodule or fluid collection is noted to avoid unnecessary diagnostic work up for metastatic disease.
The recognition of dropped gallstone with associated abscess is crucial as the definitive treatment requires removal of the retained gallstone whereas other small intra-abdominal abscesses can often be treated with antibiotics and/or percutaneous drainage. If an abscess associated with a dropped gallstone is treated conservatively, the abscess is likely to recur until the patient undergoes definitive surgical treatment. Although less common, fistula formation can be seen with dropped gallstones. Gallstones can migrate through bowel, diaphragm, and the abdominal wall.
Clip Migration
Dropped surgical clips are commonly noted following multiple different types of abdominal surgeries. Patients are rarely symptomatic and are considered low risk for abscess formation [46]. However, clip migration into the common bile duct can be problematic. While uncommon, migration of a surgical clip into the bile duct can act as a nidus for stone formation and infection, resulting in choledocholithiasis and cholangitis. A review of case reports evaluating postcholecystectomy clip migration noted that the median time from surgery to presentation was 2 years, and patients generally presented with jaundice, cholangitis, biliary colic, or pancreatitis [48]. Imaging findings are often straightforward, with a radiopaque clip demonstrated in the biliary system with a variable degree of upstream biliary dilatation (Fig. 10). As the clips act as a nidus for stone formation, choledocholithiasis and findings of cholangitis may also be present. The majority of clip migration can be treated with ERCP, with surgery reserved for refractory cases.
Conclusion
Iatrogenic biliary injuries can present within days or years after the initial surgery. Therefore, a high clinical suspicion and constant vigilance for pathology are imperative when evaluating the imaging studies of a patient who has undergone hepatobiliary surgery. Radiologists should be aware of the expected postoperative appearance following biliary surgery, identify abnormal findings, recommend additional imaging studies that may further characterize the pathology, and provide optimal diagnostic information to aid in treatment planning. Prompt recognition and diagnosis of injury can minimize unnecessary additional work--up of the patient and improve outcomes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Stewart L. Iatrogenic biliary injuries: identification, classification, and management. Surg Clin North Am. 2014;94(2):297–310. This review article provides a surgical perspective of biliary injuries and classification.
Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. 2012;6(2):172–87.
Agabiti N, Stafoggia M, Davoli M, Fusco D, Barone AP, Perucci CA. Thirty-day complications after laparoscopic or open cholecystectomy: a population-based cohort study in Italy. BMJ Open. 2013;3(2):e001943.
Deziel DJ. Complications of cholecystectomy. Incidence, clinical manifestations, and diagnosis. Surg Clin North Am. 1994;74(4):809–23.
Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180(1):101–25.
• Vecchio R, MacFadyen BV, Latteri S. Laparoscopic cholecystectomy: an analysis on 114,005 cases of United States series. Int Surg. 1998;83(3):215–9. One of the largest retrospective studies evaluating outcomes of laparoscopic cholecystectomies in the United States.
Nuzzo G, Giuliante F, Giovannini I, Ardito F, D'Acapito F, Vellone M, et al. Bile duct injury during laparoscopic cholecystectomy: results of an Italian national survey on 56 591 cholecystectomies. Arch Surg. 2005;140(10):986–92.
Waage A. Iatrogenic bile duct injury: a population-based study of 152,776 cholecystectomies in the Swedish Inpatient Registry. Arch Surg. 2006;141:1207–13.
Massoumi H, Kiyici N, Hertan H. Bile leak after laparoscopic cholecystectomy. J Clin Gastroenterol. 2007;41(3):301–5.
Pekolj J, Alvarez FA, Palavecino M, Sánchez Clariá R, Mazza O, de Santibañes E. Intraoperative management and repair of bile duct injuries sustained during 10,123 laparoscopic cholecystectomies in a high-volume referral center. J Am Coll Surg. 2013;216(5):894–901.
Joseph M, Phillips MR, Farrell TM, Rupp CC. Single incision laparoscopic cholecystectomy is associated with a higher bile duct injury rate: a review and a word of caution. Ann Surg. 2012;256(1):1–6.
Alkhaffaf B, Decadt B. 15 years of litigation following laparoscopic cholecystectomy in England. Ann Surg. 2010;251(4):682–5.
Girometti R, Cereser L, Bazzocchi M, Zuiani C. Magnetic resonance cholangiography in the assessment and management of biliary complications after OLT. World J Radiol. 2014;6(7):424–36.
Gunawansa N, McCall JL, Holden A, Plank L, Munn SR. Biliary complications following orthotopic liver transplantation: a 10-year audit. HPB (Oxford). 2011;13(6):391–9.
Hoeffel C, Azizi L, Lewin M, Laurent V, Aubé C, Arrivé L, et al. Normal and pathologic features of the postoperative biliary tract at 3D MR cholangiopancreatography and MR imaging. Radiographics. 2006;26(6):1603–20.
Thurley PD, Dhingsa R. Laparoscopic cholecystectomy: postoperative imaging. AJR Am J Roentgenol. 2008;191(3):794–801.
Tonolini M, Ierardi AM, Patella F, Carrafiello G. Early cross-sectional imaging following open and laparoscopic cholecystectomy: a primer for radiologists. Insights Imaging. 2018;9(6):925–41.
De Palma GD, Galloro G, Iuliano G, Puzziello A, Persico F, Masone S, et al. Leaks from laparoscopic cholecystectomy. Hepatogastroenterology. 2002;49(46):924–5.
LeBedis CA, Bates DDB, Soto JA. Iatrogenic, blunt, and penetrating trauma to the biliary tract. Abdom Radiol (NY). 2017;42(1):28–45.
Bismuth H, Majno PE. Biliary strictures: classification based on the principles of surgical treatment. World J Surg. 2001;25(10):1241–4.
Arun S, Santhosh S, Sood A, Bhattacharya A, Mittal BR. Added value of SPECT/CT over planar Tc-99m mebrofenin hepatobiliary scintigraphy in the evaluation of bile leaks. Nucl Med Commun. 2013;34(5):459–66.
Sharma P, Kumar R, Das KJ, Singh H, Pal S, Parshad R, et al. Detection and localization of post-operative and post-traumatic bile leak: hybrid SPECT-CT with 99mTc-Mebrofenin. Abdom Imaging. 2012;37(5):803–11.
•• Matesan M, Bermo M, Cruite I, Shih CH, Elojeimy S, Behnia F, et al. Biliary leak in the postsurgical abdomen: a primer to HIDA scan interpretation. Semin Nucl Med. 2017;47(6):618–29. This article provides an excellent review of normal and abnormal nuclear medicine imaging findings following hepatobiliary surgery.
• Lee NK, Kim S, Lee JH, Lee SH, Kang DH, Kim DH, et al. Biliary MR imaging with Gd-EOB-DTPA and its clinical applications. Radiographics 2009;29:1707–24. This article outlines the use of hepatobiliary contrast agents and their role in imaging of the biliary system.
Seale MK, Catalano OA, Saini S, Hahn PF, Sahani DV. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics. 2009;29(6):1725–48.
Aduna M, Larena JA, Martín D, Martínez-Guereñu B, Aguirre I, Astigarraga E. Bile duct leaks after laparoscopic cholecystectomy: value of contrast-enhanced MRCP. Abdom Imaging. 2005;30(4):480–7.
Tewani SK, Turner BG, Chuttani R, Pleskow DK, Sawhney MS. Location of bile leak predicts the success of ERCP performed for postoperative bile leaks. Gastrointest Endosc. 2013;77(4):601–8.
Christensen M, Matzen P, Schulze S, Rosenberg J. Complications of ERCP: a prospective study. Gastrointest Endosc. 2004;60(5):721–31.
Greenfield NP, Azziz AS, Jung AJ, Yeh BM, Aslam R, Coakley FV. Imaging late complications of cholecystectomy. Clin Imaging. 2012;36(6):763–7.
Guarise A, Baltieri S, Mainardi P, Faccioli N. Diagnostic accuracy of MRCP in choledocholithiasis. Radiol Med. 2005;109(3):239–51.
Borz-Baba C, Levy DA, Cohen ME. Post-cholecystectomy Mirizzi syndrome: a case report and review of the literature. Am J Case Rep. 2019;20:1290–8.
Gandhi D, Ojili V, Nepal P, Nagar A, Hernandez-Delima FJ, Bajaj D, et al. A pictorial review of gall stones and its associated complications. Clin Imaging. 2020;60(2):228–36.
Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335(13):909–18.
Thomas PD, Turner JG, Dobbs BR, Burt MJ, Chapman BA. Use of (99m)Tc-DISIDA biliary scanning with morphine provocation for the detection of elevated sphincter of Oddi basal pressure. Gut. 2000;46(6):838–41.
Pereira SP, Gillams A, Sgouros SN, Webster GJ, Hatfield AR. Prospective comparison of secretin-stimulated magnetic resonance cholangiopancreatography with manometry in the diagnosis of sphincter of Oddi dysfunction types II and III. Gut. 2007;56(6):809–13.
Sahajpal AK, Chow SC, Dixon E, Greig PD, Gallinger S, Wei AC. Bile duct injuries associated with laparoscopic cholecystectomy: timing of repair and long-term outcomes. Arch Surg. 2010;145(8):757–63.
Choi SH, Han JK, Lee JM, Lee KH, Kim SH, Lee JY, et al. Differentiating malignant from benign common bile duct stricture with multiphasic helical CT. Radiology. 2005;236(1):178–83.
Buis CI, Verdonk RC, Van der Jagt EJ, van der Hilst CS, Slooff MJ, Haagsma EB, et al. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007;13(5):708–18.
•• Camacho JC, Coursey-Moreno C, Telleria JC, Aguirre DA, Torres WE, Mittal PK. Nonvascular post-liver transplantation complications: from US screening to cross-sectional and interventional imaging. Radiographics. 2015;35(1):87–104. Excellent review article describing imaging findings of complications after liver transplantation.
Jorgensen JE, Waljee AK, Volk ML, Sonnenday CJ, Elta GH, Al-Hawary MM, et al. Is MRCP equivalent to ERCP for diagnosing biliary obstruction in orthotopic liver transplant recipients? A meta-analysis. Gastrointest Endosc. 2011;73(5):955–62.
Ryu CH, Lee SK. Biliary strictures after liver transplantation. Gut Liver. 2011;5(2):133–42.
Sathesh-Kumar T, Saklani AP, Vinayagam R, Blackett RL. Spilled gall stones during laparoscopic cholecystectomy: a review of the literature. Postgrad Med J. 2004;80(940):77–9.
Schäfer M, Suter C, Klaiber C, Wehrli H, Frei E, Krähenbühl L. Spilled gallstones after laparoscopic cholecystectomy. A relevant problem? A retrospective analysis of 10,174 laparoscopic cholecystectomies. Surg Endosc. 1998;12(4):305–9.
Tumer AR, Yüksek YN, Yasti AC, Gözalan U, Kama NA. Dropped gallstones during laparoscopic cholecystectomy: the consequences. World J Surg. 2005;29(4):437–40.
Morrin MM, Kruskal JB, Hochman MG, Saldinger PF, Kane RA. Radiologic features of complications arising from dropped gallstones in laparoscopic cholecystectomy patients. AJR Am J Roentgenol. 2000;174(5):1441–5.
Singh AK, Levenson RB, Gervais DA, Hahn PF, Kandarpa K, Mueller PR. Dropped gallstones and surgical clips after cholecystectomy: CT assessment. J Comput Assist Tomogr. 2007;31(5):758–62.
Nayak L, Menias CO, Gayer G. Dropped gallstones: spectrum of imaging findings, complications and diagnostic pitfalls. Br J Radiol. 2013;86(1028):20120588.
Chong VH, Chong CF. Biliary complications secondary to post-cholecystectomy clip migration: a review of 69 cases. J Gastrointest Surg. 2010;14(4):688–96.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Laura Linstroth MD: None. Akram Shaaban MD: Receives royalties from Elsevier. Sherry S. Wang MBBS: Receives royalties from Elsevier.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Gastrointestinal Imaging.
Rights and permissions
About this article
Cite this article
Linstroth, L., Shaaban, A. & Wang, S.S. Imaging of Postoperative Biliary Complications. Curr Radiol Rep 8, 23 (2020). https://doi.org/10.1007/s40134-020-00368-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s40134-020-00368-w