Abstract
Purpose
This systematic review intends to give an overview of the current knowledge on how allografts used for the reconstruction of cruciate ligaments and menisci are integrated and specifically perform regarding their biomechanical function.
Methods
Two reviewers reviewed the PubMed and Central Cochrane library with focus on the biomechanical integration of tendon ligament and meniscus allografts. The literature search was conducted in accordance with the PRISMA statement for reporting systematic reviews and meta-analyses.
Results
The analysed literature on tendon allografts shows that they are more vulnerable to overstretching in the phase of degradation compared to autografts as the revascularization process starts later and takes longer. Therefore, to avoid excessive graft loads, allografts for cruciate ligament replacement should be selected that exhibit much higher failure loads than the native ligaments to counteract the detrimental effect of degradation. Further, placement techniques should be considered that result in a minimum of strain differences during knee joint motion, which is best achieved by near-isometric placement. The most important biomechanical parameters for meniscus allograft transplantation are secure fixation and proper graft sizing. Allograft attachment by bone plugs or by a bone block is superior to circumferential suturing and enables the allograft to restore the chondroprotective biomechanical function. Graft sizing is also of major relevance, because too small grafts are not able to compensate the knee joint incongruity and too large grafts may fail due to extrusion. Only adequate sizing and fixation together can lead to a biomechanically functioning allograft. The objective assessment of the biomechanical quality of allografts in a clinical setting is challenging, but would be highly desirable for monitoring the remodelling and incorporation process.
Conclusions
Currently, indicators like ap-stability after ACL reconstruction or meniscal extrusion represent only indirect measures for biomechanical graft integration. These parameters are at best clinical indicators of allograft function, but the overall integration properties comprising e.g. fixation and graft stiffness remain unknown. Therefore, future research should e.g. focus on advanced imaging techniques or other non-invasive methods allowing for in vivo assessment of biomechanical allograft properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In meniscus and cruciate ligament surgery, the use of an allograft is indicated when a regular repair of a structure, autografting or conservative procedure is not possible, for example, in revision cases or because of the severity or complexity of the injury [48, 103]. Clinical concerns against the use of allografts vary between meniscus and tendon allografts for cruciate ligament reconstruction and comprise possible disease transmission, rejection reactions of the donor tissue, delayed incorporation, and graft costs [78]. In turn, allografts can provide the advantage of avoiding donor-site morbidity, reduced implantation time, and selection of stronger grafts [67, 103]. Therefore, using allografts is an accepted method particularly for the reconstruction of the cruciate ligaments and, in fact, is the only current option for a total meniscus replacement [85]. It is of utmost importance for the success of allograft transplantation that it integrates both biologically and biomechanically. While biological integration creates a viable connection to the host tissue and in the long-term leads to cell activity that re-establishes a matrix composition typical of the tissue to be replaced, only biomechanical integration leads to a good functional outcome. That is, the graft must experience loads and strains similar in character and level typical of those of the replaced tissue. By contrast, an allograft, which is not biomechanically integrated, represents a mere space holder that might integrate biologically but will be unable to restore the biomechanical function of the native tissue. However, the boundary between a biomechanically integrated and non-integrated graft is not clearly defined. Depending on the material properties and the remodelling process of an allograft, it is possible that it can only partially fulfil its biomechanical function. Nevertheless, despite incomplete restoration of the biomechanical function, a patient may still benefit from an allograft to a certain extent. For example, an allograft of the cruciate ligament, with its complex fan-like structure of the fibre bundles, cannot be completely restored by a single- or double-bundle tendon graft, but it may provide improved knee-joint translational and rotational stability. Furthermore, it is difficult to evaluate the biomechanical functionality of an allograft in vivo. While it is possible to assess the mechanical properties of allografts post-mortem in animal models, it is far more difficult to assess it in patients in a clinical setting. The related literature comprises many studies on allograft sourcing, preservation and sterilisation methods, operation techniques, in vitro analysis of biomechanical and biological properties, animal models and clinical outcome studies. This includes a variety of review papers on different allograft properties and surgical techniques. However, no review to date has been published specifically focussing on the biomechanical integration of allografts for the reconstruction of joint-related tissues. Therefore, this review intends to provide an overview of the current knowledge on how allografts used for the reconstruction of ligaments and the meniscus are integrated and perform specifically regarding their biomechanical function.
Materials and methods
Quality of methodology
This study was conducted in accordance with the PRISMA statement for reporting systematic reviews and meta-analyses [59, 68].
Eligibility criteria
Study type
-
Any clinical study (randomised controlled trial, non-randomised comparative or case series) or controlled laboratory study written in the English language. Studies that do not contain new data, simulation or computational studies, case reports and operative techniques were excluded.
Participants
-
Any human of any age
-
Any animal species of any age
Intervention
-
Ligament allograft transplantation using any allograft-preservation method and any grafting technique.
-
Tendon allograft transplantation using any allograft-preservation method and any grafting technique.
-
Meniscal allograft transplantation using any allograft-preservation method and any grafting technique.
Comparator
-
If a comparator group exists, it should be a reasonable treatment compared to the tendon or meniscus allograft-transplantation procedure or represent a different tendon or meniscus allograft-transplantation methodology, for example, a different allograft fixation or sterilisation technique.
Outcome measure
-
The primary outcome measure of this systematic review was the biomechanical integration of an allograft at a minimum of 2 weeks post-intervention. This integration was evaluated either directly by mechanical testing or indirectly by medical imaging or clinical scoring. Regarding the latter, the Lysholm, International Knee Documentation Committee (IKDS) and Tegner activity index were considered.
Search strategy
The search strategy applied maximum sensitivity to reduce the risk of failing to identify eligible studies. The presented search strategy was developed using a combination of keywords and medical subject heading, which were selectively explored to maximise the inclusion of potentially relevant studies. The search strategy for PubMed (MEDLINE, Table 1—see Online Appendix) was adapted for the Cochrane library (CENTRAL, Table 2—see Online Appendix). To obtain all relevant studies, the results of the database search were combined with an additional literature search in End-Note using the keywords “allograft*” AND “biomechanic*” AND “knee” for the MEDLINE library.
Selection and appraisal mode
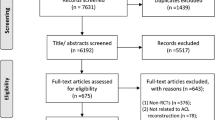
Results of database searches were transferred into End-Note, where duplicates were removed and references were updated when necessary. Our pre-defined criteria were applied to assess the eligibility of the remaining studies. Two independent reviewers evaluated the remaining abstracts for eligibility, and any discrepancies were resolved by discussion. Lastly, the reference lists of the selected papers were reviewed to include relevant studies that were not found during the described selection process. The detailed selection process with the related numbers of studies are presented in Fig. 1. The full papers of the remaining studies were then reviewed. Data from eligible studies were extracted. Potentially redundant publications, that is, when the same patient cohort or participants were used in more than a single study, were only included when different outcomes were reported [42]. In case of similarity, only the most recently published study was analysed.
Results
Allografts for ligament reconstruction
The main biomechanical function of ligaments is the passive stabilisation of joints. Ligaments are predominantly exposed to tensile loads occurring when joints are moved or external loads are acting on the joint. Whereas many ligamentous lesions can heal without surgical intervention because of sufficient vascularisation, tears of the anterior (ACL) and posterior cruciate ligaments (PCL) most frequently require surgical reconstruction using auto- or allografts. The most frequently used source for ACL and PCL reconstructions are tendons harvested from the hamstring muscles or the patella extensor complex. To avoid donor-site morbidity or in the case of revision surgery, allograft tendons harvested from human donors is the method of choice. Allograft choices comprise quadriceps, patellar, Achilles, hamstring, anterior and posterior tibialis tendons, fascia lata and hybrid grafts combining autograft and allograft [6, 21, 28, 29, 33, 38, 62, 67, 76, 77, 79, 84, 96, 100, 101, 103,104,105]. However, allografts bear the risk of disease transmission, infection and immunogenic issues. Therefore, allografts are normally processed to overcome these issues.
A number of different methods exist to preserve, sterilise or decellularise tendon grafts. Freezing and cryopreservation [16, 39, 72, 74, 86, 95], irradiation [20, 22, 37, 40, 41, 87,88,89, 91], gas-sterilisation [8], lyophilising [72] and other methods [17, 44, 80] have been described and tested for potential effects on material properties. These studies revealed that care must be taken to choose processing methods that do not or minimally compromise their biomechanical properties, which would be, of course, highly undesirable. For example, DiBartola et al. and Wang et al. showed in their recent meta-analyses of a large number of publications that Gamma irradiation might negatively affect tendon allograft strength, particularly when the irradiation dose exceeds 2.5 Mrad [22, 102]. Other processing regimens, like gas sterilisation, may preserve strength but decrease stiffness [8], which might be tolerable, provided the allograft properties are sufficiently comparable to the ACL or PCL. Lyophilisation appears to be a good option to preserve a tendon allograft, because on the one hand it did not display any significant changes in biomechanical properties compared to cryopreserved tendons, and, on the other hand, it does not require maintenance of an expensive biobank for storage [72]. The problem of graft selection and processing methods is even more complex, considering that the influence of the sterilisation method on mechanical properties also depends on the type of tendon used for allografting [37]. In conclusion, surgeons are advised to carefully study the current literature to select the processing method of choice for their specific cases.
Three phases of graft integration can be distinguished: inflammatory response with degeneration, tissue maturation with revascularisation and finally graft healing with tissue remodelling. West et al. described these phases in a review paper citing a number of studies that histologically and biomechanically investigated the graft incorporation process in animal models [103]. During the first phase of inflammation, the graft degenerates accompanied by cell death in autografts, while viable cells are normally lacking in the allografts. This process of degeneration is associated with a loss of strength and stiffness [14, 65, 103]. This weakening continues during the second phase when blood vessels start to revascularise the tissue. An early study by Butler et al. in primates demonstrated that after 7 weeks, the strength of an autograft was diminished to only 16% of that of an intact ACL and the stiffness to 24% of the ACL [15]. A few studies have compared the autograft and allograft. A clinical study using magnetic resonance imaging (MRI) showed that allograft revascularisation is slower and initiates later than in autografts [70], which suggests that healing and remodelling might be delayed in allografts. This leads to a slower recovery of biomechanical properties, which was demonstrated by Jackson et al. in a goat model. They reported an increase of failure load of autografts from 265 N at 6 weeks to 1337 N after 6 months. The corresponding increase observed in the allograft group was from 241 N to 578 N during the same time period [43]. The difference in stiffness increase between both groups was even more pronounced. This agrees with a clinical study of Li et al., who found greater antero-posterior (ap) laxity for allografts than for autografts [58] and with an experimental study in sheep showing higher failure load, stiffness and ap-drawer after 1 year for autograft compared to allografts [24]. However, 6 months is a relatively early time point and the graft remodelling process continues after this for a considerable time. Therefore, the third phase of graft integration starts when the vascularisation process has progressed to an extent that new cells invade and deposit a collagenous matrix that enhances the biomechanical properties. However, this last phase appears to persist for at least 1 year, as Bosch et al. demonstrated in a 2-year study on sheep with bone-patellar tendon-bone autografts for PCL reconstruction [14]. Both elastic modulus and maximum stress achieved the final level after 1 year and did not change substantially until 2 years post-op. Given that allografts heal slower because of the later onset of revascularisation, tissue remodelling probably takes longer than a year to reach the final state for allografts. This should be taken into consideration for adequate rehabilitation procedures. Furthermore, it should be noted that the biomechanical properties, regardless whether autograft or allograft, never regain the level of that at the time of implantation [9]. This might also be influenced by the collagenous structure of ligaments and tendons, which differ significantly [10]. Therefore, selecting grafts that are stronger than the tissue to be replaced might to some extent counteract the detrimental effect of graft weakening. This appears to be already accomplished when comparing native ACL failure load (2160 N) with those of allograft options like quadrupled hamstring (4090 N), doubled Tibialis Anterior (4122 N) and Achilles tendon (4617 N) [7]. Therefore, assuming the minimum failure load in the degradation phase in the worst case is only 10% of the initial value, the indicated allografts can still bear loads of approximately 400 N. Such loads do not occur during normal activities and should be avoided during the most vulnerable phase of graft incorporation. Therefore, care should be taken in rehabilitation programmes and a prolonged return to sports should be considered [69]. With respect to the study of Muramatso et al., who found the peak of allograft revascularisation delayed by 12–18 months compared to autografts, a return to sports should be postponed accordingly [70].
Other factors with an impact on the biomechanical integration of an allograft are the type of graft placement [47, 63, 71], initial graft fixation [26, 50, 53, 94] and the technique of graft tensioning [36, 64]. Each of these parameters affect the extent of graft load, loading characteristic during joint motion and joint forces [12, 13, 92]. Excessive loads acting on the graft after implantation can cause tissue overstretching, resulting in increased knee laxity. Specifically, during the second phase of allograft incorporation, when degradation has significantly weakened the graft and the fixation in the bone tunnels is still in the healing phase, high loads can cause irreversible stretching and/or loosening of the fixation. Therefore, it is of utmost importance to select an implantation technique that avoids too high a static or dynamic load. Several authors have tested a variety of fixation techniques and implants for initial fixation strength and stiffness. The failure loads ranged over 471–1323 N and the stiffness over 61–223 N/mm, depending on the graft type and fixation technique [43, 51, 52, 103]. Therefore, any of the tested fixation techniques are secure provided the graft force does not exceed approximately 400 N. To avoid high graft forces, it is important to which preload in which flexion position the graft is tensioned. For example, when choosing a non-isometric placement with a posteriorly located femoral insertion, preloading in 30° flexion could result in increased forces in extension. In this case, a preloading in the extension position would be recommended, thereby decreasing tension while flexing the knee joint. However, any graft placement too far from an isometric position would result in a slack graft with increasing flexion [82]. Again, considering that allografts are even more vulnerable to undesired overstretching than autografts, surgeons should consider favouring a near isometric rather than an anatomic placement, which would avoid excessive graft loads. Another factor compromising graft ingrowth is a possible mismatch between the sizes of the graft and bone tunnel, particularly occurring when the graft diameter varies from distal to proximal. While the larger diameter might fit efficiently into the bone tunnel, a graft portion of a smaller diameter lacks bone contact, with the consequence of impeded graft ingrowth. Lord et al. suggested in an in vitro study to compress autografts or allografts prior to implantation, which allows the use of bone tunnels of smaller diameters. This would lead to a better fit of the graft along the entire tunnel and simultaneously to the preservation of the bone stock without compromising graft mechanical properties [60].
Regarding the donor age of tendon allografts, typically, younger donors are preferred because of supposedly better tissue biomechanical properties. However, there is evidence that the mechanical properties of tendon allografts are independent of age, with either no or only a weak correlation between age and modulus or ultimate tensile load at least for donors younger than 65 years [11, 27, 35, 54]. This is supported by Gagliano et al., who recently reported that the ageing process does not alter tendon structure or tenocyte biological activity [32]. These studies suggest that allografts can also be harvested from donors aged up to 65 years with little risk of inferior material properties.
Taken together, the existing literature illustrates the advantages and disadvantages of autografts and allografts used for the reconstruction of the cruciate ligaments. From a biomechanical point of view, it should be noted that, when deciding upon using an allograft, it is most important to consider the greater vulnerability during the phase of maturation and revascularisation, because this process starts later and takes longer than in autografts. Choosing a graft placement and tensioning technique that avoids the incurrence of excessive loads is essential for an autograft while being even more important for allografts.
Meniscus allografts
The main biomechanical function of the semilunar-shaped menisci is the homogeneous distribution of the knee-joint load on the articular cartilage to protect it from premature degeneration. In the case of an injury, any further treatment depends on the lesion severity and pattern. Additionally, the condition of the adjacent knee cartilage plays an important role. Possible surgical treatment options include meniscal resection and repair, but also its replacement with scaffolds and auto- or allograft transplantation [48, 56, 57, 93]. Meniscus replacement options are clinically indicated for young patients with pain in the affected knee, ACL-deficient patients who had previous meniscectomy and patients that may benefit from the prevention of early cartilage degeneration, for example, young athletic patients who underwent total meniscectomy [34, 93]. Contraindications are advanced chondral degeneration (IRCS < III), osteophytes, synovial disease and inflammatory arthritis, but also obesity and skeletal immaturity [61]. Meniscal transplantation has the same general risks associated with meniscal repair, and additional risks specific to the transplant itself, including disease transmission and injury to the patellar tendon because of an anterior approach [57]. Particularly in patients with mature osteoarthritis, the outcomes are worse, leading to an increased failure rate or revision surgery of the knee [45]. Meniscal allografts are available cryopreserved, fresh frozen, and lyophilized. These types of allograft differ in terms of viable graft cells, immunogenicity and sterilisation options [66, 83]. A recently published study compared cryopreserved and fresh-frozen allografts, indicating superior biomechanical performance of the cryopreserved menisci [3]. However, it should be noted that a meniscal allograft procedure is a bridging operation and not a permanent solution.
Biomechanical studies have demonstrated the ability of meniscal allografts to improve the contact mechanics [5, 23, 49, 73, 75]. Although the native mean pressure on the tibial plateau was not completely restored, the maximum contact pressure was reduced by up to 75% compared with the knee after meniscectomy. The initial and long-term mechanical integration also depends on the graft fixation method [56]. This can be achieved with or without the attached bone. Normally, bone plugs or bony bridges are applied where the meniscal attachments are directly inserted. In an arthroscopic intervention, the bony fixation of the attachments is combined with a capsular suture of the graft through transosseous tunnels. Paletta et al. investigated the meniscal horn fixation, concluding that an insufficient anchorage would lead to biomechanical results comparable to meniscectomy [75]. Alhalki et al. underlined these findings in their study while investigating the impact on meniscotibial contact mechanics during three different fixation methods [4]. However, not only the fixation method plays an important role during meniscal transplantation, but also the placement of the fixation. Sekaran et al. used human cadaver knees and varied the placement of the meniscal insertions [90], finding a significant increase in the contact pressure when the insertions of the meniscal attachments were non-physiological. In a biomechanical study, the partial replacement of the meniscus using an allograft was investigated in a porcine knee model [73]. The authors demonstrated that the tibiofemoral contact pressure could be restored after partial meniscectomy with four horizontal sutures securing the meniscal allograft. Clinical data with a follow-up of 23 months when fixing the allograft exclusively with sutures revealed an extrusion of the allograft under reduced body weight [97]. The authors concluded that this supported the bony block anchorage method. In addition to the fixation of the graft, its sizing plays an additional key role for the success of the biomechanical integration [23, 81]. Generally, too small grafts are unable to restore the joint congruency, whereas too large grafts may fail because of extrusion [23]. Consequently, a stable fixation method including ligamentous and bony attachments in combination with the correct sizing are crucial for restoring the physiological load distribution.
An animal study investigating the biomechanical integration of meniscal allografts suggested an inferior outcome of grafted knees versus meniscectomised knees regarding the tensile modulus of the femoral cartilage compared to non-operated controls [25]. In turn, some authors suggested that a meniscal allograft procedure improved the weight-bearing function of the knee [5, 16, 19, 46, 75, 98]. Aagaard et al. investigated the effect of primary versus delayed, secondary meniscus allograft transplantation in two separate studies [1, 2]. In their 6-month follow-up studies using a sheep model, they found similar results in image analysis and histologic scoring, indicating a better chondroprotective effect when the menisci are primarily implanted.
From a clinical perspective, the evaluation of the biomechanical integration is challenging. Because of the anatomical and biomechanical differences, Yoon et al. investigated the differences between a lateral and a medial allograft procedure [106]. They did not observe any difference in clinical scores in the long-term of up to 125 months. In addition to the known knee scoring systems, clinical imaging using either MRI or sonography could be applied to evaluate the success of a grafting procedure. Overall, most of the studies discussed here reported good results in the short and medium terms with a follow-up of ≤ 2 years [18]. Additionally, in a 10-year follow-up, approximately 75–90% of the patients experienced good results [56]. MRI investigations revealed problems, including graft shrinkage, cartilage and meniscus degeneration, and extrusion [2]. A recently published review concluded that meniscus allograft interventions do not prevent cartilage damage, but protect it at least from further accelerated degeneration [85]. Furthermore, there is clinical evidence clearly showing reduced pain after allograft treatment [34, 55]. This is particularly the case for total meniscectomised knee joints, where significant pain relief and functional improvement are observed in the majority of the patients [56]. Long-term follow-up studies reported success rates of allograft transplantation of approximately 70% [56, 99].
Taken together, clinical studies support the use of meniscal allografts to provide symptomatic pain relief and functional recovery. This indicates that meniscus allografts may at least in part restore the contact mechanics. To achieve this, a combination of proper graft fixation and correct sizing is essential. However, it is still not possible to assess the biomechanical properties of meniscal allograft integration in vivo.
Conclusion
Biomechanical considerations are important for the successful integration of both tendon and meniscal allografts. In ACL replacement by tendon allografts, maturation takes considerably longer than in autografts. A good functional integration of meniscus allografts strongly depends on biomechanical parameters, including fixation and sizing. Although the objective assessment of the biomechanical quality of allografts in a clinical setting is challenging, it would be highly desirable to monitor the remodelling and incorporation process more accurately. Currently, indicators like ap-stability after ACL reconstruction or meniscal extrusion represent only indirect measures for biomechanical graft integration. These parameters are at best clinical indicators of allograft function, but the overall integration properties comprising, for example, fixation and graft stiffness, remain unknown. Therefore, future research should focus, for example, on advanced imaging techniques [30, 31] or other non-invasive methods allowing for in vivo assessment of biomechanical allograft properties.
References
Aagaard H, Jorgensen U, Bojsen-Moller F (2003) Immediate versus delayed meniscal allograft transplantation in sheep. Clin Orthop Relat Res 406:218–227
Aagaard H, Jorgensen U, Bojsen-Moller F (1999) Reduced degenerative articular cartilage changes after meniscal allograft transplantation in sheep. Knee Surg Sports Traumatol Arthrosc 7:184–191
Ahmad S, Singh VA, Hussein SI (2017) Cryopreservation versus fresh frozen meniscal allograft: a biomechanical comparative analysis. J Orthop Surg (Hong Kong) 25(3):1–7
Alhalki MM, Howell SM, Hull ML (1999) How three methods for fixing a medial meniscal autograft affect tibial contact mechanics. Am J Sports Med 27:320–328
Alhalki MM, Hull ML, Howell SM (2000) Contact mechanics of the medial tibial plateau after implantation of a medial meniscal allograft. A human cadaveric study. Am J Sports Med 28:370–376
Almqvist KF, Jan H, Vercruysse C, Verbeeck R, Verdonk R (2007) The tibialis tendon as a valuable anterior cruciate ligament allograft substitute: biomechanical properties. Knee Surg Sports Traumatol Arthrosc 15:1326–1330
Baer GS, Harner CD (2007) Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med 26:661–681
Baldini T, Caperton K, Hawkins M, McCarty E (2016) Effect of a novel sterilization method on biomechanical properties of soft tissue allografts. Knee Surg Sports Traumatol Arthrosc 24:3971–3975
Beynnon BD, Johnson RJ (1996) Anterior cruciate ligament injury rehabilitation in athletes. Biomechanical considerations. Sports Med 22:54–64
Birch HL, Thorpe CT, Rumian AP (2013) Specialisation of extracellular matrix for function in tendons and ligaments. Muscles Ligaments Tendons J 3:12–22
Blevins FT, Hecker AT, Bigler GT, Boland AL, Hayes WC (1994) The effects of donor age and strain rate on the biomechanical properties of bone-patellar tendon-bone allografts. Am J Sports Med 22:328–333
Boguszewski DV, Wagner CT, Butler DL, Shearn JT (2015) Effect of ACL graft material on anterior knee force during simulated in vivo ovine motion applied to the porcine knee: an in vitro examination of force during 2000 cycles. J Orthop Res 33:1789–1795
Boguszewski DV, Wagner CT, Butler DL, Shearn JT (2014) Effect of ACL graft material on joint forces during a simulated in vivo motion in the porcine knee: examining force during the initial cycles. J Orthop Res 32:1458–1463
Bosch U, Decker B, Kasperczyk W, Nerlich A, Oestern HJ, Tscherne H (1992) The relationship of mechanical properties to morphology in patellar tendon autografts after posterior cruciate ligament replacement in sheep. J Biomech 25:821–830
Butler DL, Grood ES, Noyes FR, Olmstead ML, Hohn RB, Arnoczky SP et al (1989) Mechanical properties of primate vascularized vs. nonvascularized patellar tendon grafts; changes over time. J Orthop Res 7:68–79
Chen L, Wu Y, Yu J, Jiao Z, Ao Y, Yu C et al (2011) Effect of repeated freezing-thawing on the Achilles tendon of rabbits. Knee Surg Sports Traumatol Arthrosc 19:1028–1034
Colaco HB, Lord BR, Back DL, Davies AJ, Amis AA, Ajuied A (2017) Biomechanical properties of bovine tendon xenografts treated with a modern processing method. J Biomech 53:144–147
Cole BJ, Dennis MG, Lee SJ, Nho SJ, Kalsi RS, Hayden JK et al (2006) Prospective evaluation of allograft meniscus transplantation: a minimum 2-year follow-up. Am J Sports Med 34:919–927
Cummins JF, Mansour JN, Howe Z, Allan DG (1997) Meniscal transplantation and degenerative articular change: an experimental study in the rabbit. Arthroscopy 13:485–491
Curran AR, Adams DJ, Gill JL, Steiner ME, Scheller AD (2004) The biomechanical effects of low-dose irradiation on bone-patellar tendon-bone allografts. Am J Sports Med 32:1131–1135
Delcroix GJ, Kaimrajh DN, Baria D, Cooper S, Reiner T, Latta L et al (2013) Histologic, biomechanical, and biological evaluation of fan-folded iliotibial band allografts for anterior cruciate ligament reconstruction. Arthroscopy 29:756–765
DiBartola AC, Everhart JS, Kaeding CC, Magnussen RA, Flanigan DC (2016) Maximum load to failure of high dose versus low dose gamma irradiation of anterior cruciate ligament allografts: a meta-analysis. Knee 23:755–762
Dienst M, Greis PE, Ellis BJ, Bachus KN, Burks RT (2007) Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med 35:34–42
Dustmann M, Schmidt T, Gangey I, Unterhauser FN, Weiler A, Scheffler SU (2008) The extracellular remodeling of free-soft-tissue autografts and allografts for reconstruction of the anterior cruciate ligament: a comparison study in a sheep model. Knee Surg Sports Traumatol Arthrosc 16:360–369
Elliott DM, Jones R 3rd, Setton LA, Scully SP, Vail TP, Guilak F (2002) Joint degeneration following meniscal allograft transplantation in a canine model: mechanical properties and semiquantitative histology of articular cartilage. Knee Surg Sports Traumatol Arthrosc 10:109–118
Farmer JM, Lee CA, Curl WW, Martin DF, Kortesis B, Poehling GG (2006) Initial biomechanical properties of staple-anchor Achilles tendon allograft and interference screw bone-patellar tendon-bone autograft fixation for anterior cruciate ligament reconstruction in a cadaveric model. Arthroscopy 22:1040–1045
Flahiff CM, Brooks AT, Hollis JM, Vander Schilden JL, Nicholas RW (1995) Biomechanical analysis of patellar tendon allografts as a function of donor age. Am J Sports Med 23:354–358
Fleming BC, Spindler KP, Palmer MP, Magarian EM, Murray MM (2009) Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med 37:1554–1563
Forsythe B, Haro MS, Bogunovic L, Collins MJ, Arns TA, Trella KJ et al (2016) Biomechanical evaluation of posterior cruciate ligament reconstruction with quadriceps versus Achilles tendon bone block allograft. Orthop J Sports Med 4(8):1–8
Freutel M, Galbusera F, Ignatius A, Durselen L (2015) Material properties of individual menisci and their attachments obtained through inverse FE-analysis. J Biomech 48:1343–1349
Freutel M, Seitz AM, Galbusera F, Bornstedt A, Rasche V, Knothe Tate ML et al (2014) Medial meniscal displacement and strain in three dimensions under compressive loads: MR assessment. J Magn Reson Imaging 40:1181–1188
Gagliano N, Menon A, Cabitza F, Compagnoni R, Randelli P (2018) Morphological and molecular characterization of human hamstrings shows that tendon features are not influenced by donor age. Knee Surg Sports Traumatol Arthrosc 26:343–352
Gill TJ, DeFrate LE, Wang C, Carey CT, Zayontz S, Zarins B et al (2004) The effect of posterior cruciate ligament reconstruction on patellofemoral contact pressures in the knee joint under simulated muscle loads. Am J Sports Med 32:109–115
Giuliani JR, Burns TC, Svoboda SJ, Cameron KL, Owens BD (2011) Treatment of meniscal injuries in young athletes. J Knee Surg 24:93–100
Greaves LL, Hecker AT, Brown CH Jr (2008) The effect of donor age and low-dose gamma irradiation on the initial biomechanical properties of human tibialis tendon allografts. Am J Sports Med 36:1358–1366
Hame SL, Markolf KL, Gabayan AJ, Hunter DM, Davis B, Shapiro MS (2002) The effect of anterior cruciate ligament graft rotation on knee laxity and graft tension: an in vitro biomechanical analysis. Arthroscopy 18:55–60
Hangody G, Szebenyi G, Abonyi B, Kiss R, Hangody L, Pap K (2017) Does a different dose of gamma irradiation have the same effect on five different types of tendon allografts?—a biomechanical study. Int Orthop 41:357–365
Haut Donahue TL, Howell SM, Hull ML, Gregersen C (2002) A biomechanical evaluation of anterior and posterior tibialis tendons as suitable single-loop anterior cruciate ligament grafts. Arthroscopy 18:589–597
Henson J, Nyland J, Chang HC, Caborn DN (2009) Effect of cryoprotectant incubation time on handling properties of allogeneic tendons prepared for knee ligament reconstruction. J Biomater Appl 24:343–352
Hoburg A, Keshlaf S, Schmidt T, Smith M, Gohs U, Perka C et al (2011) Fractionation of high-dose electron beam irradiation of BPTB grafts provides significantly improved viscoelastic and structural properties compared to standard gamma irradiation. Knee Surg Sports Traumatol Arthrosc 19:1955–1961
Hoburg AT, Keshlaf S, Schmidt T, Smith M, Gohs U, Perka C et al (2010) Effect of electron beam irradiation on biomechanical properties of patellar tendon allografts in anterior cruciate ligament reconstruction. Am J Sports Med 38:1134–1140
Huston P, Moher D (1996) Redundancy, disaggregation, and the integrity of medical research. Lancet 347:1024–1026
Jackson DW, Grood ES, Goldstein JD, Rosen MA, Kurzweil PR, Cummings JF et al (1993) A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med 21:176–185
Jones DB, Huddleston PM, Zobitz ME, Stuart MJ (2007) Mechanical properties of patellar tendon allografts subjected to chemical sterilization. Arthroscopy 23:400–404
Kang RW, Lattermann C, Cole BJ (2006) Allograft meniscus transplantation: background, indications, techniques, and outcomes. J Knee Surg 19:220–230
Kelly BT, Potter HG, Deng XH, Pearle AD, Turner AS, Warren RF et al (2006) Meniscal allograft transplantation in the sheep knee: evaluation of chondroprotective effects. Am J Sports Med 34:1464–1477
Kennedy NI, LaPrade RF, Goldsmith MT, Faucett SC, Rasmussen MT, Coatney GA et al (2014) Posterior cruciate ligament graft fixation angles, part 2: biomechanical evaluation for anatomic double-bundle reconstruction. Am J Sports Med 42:2346–2355
Khetia EA, McKeon BP (2007) Meniscal allografts: biomechanics and techniques. Sports Med Arthrosc Rev 15:114–120
Kim JG, Lee YS, Bae TS, Ha JK, Lee DH, Kim YJ et al (2013) Tibiofemoral contact mechanics following posterior root of medial meniscus tear, repair, meniscectomy, and allograft transplantation. Knee Surg Sports Traumatol Arthrosc 21:2121–2125
Kocabey Y, Klein S, Nyland J, Caborn D (2004) Tibial fixation comparison of semitendinosus-bone composite allografts fixed with bioabsorbable screws and bone-patella tendon-bone grafts fixed with titanium screws. Knee Surg Sports Traumatol Arthrosc 12:88–93
Kousa P, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M (2003) The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part I: femoral site. Am J Sports Med 31:174–181
Kousa P, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M (2003) The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part II: tibial site. Am J Sports Med 31:182–188
Krupp R, Nyland J, Smith C, Nawab A, Burden R, Caborn DN (2007) Biomechanical comparison between CentraLoc and Intrafix fixation of quadrupled semitendinosus-gracilis allografts in cadaveric tibiae with low bone mineral density. Knee 14:306–313
Lansdown DA, Riff AJ, Meadows M, Yanke AB, Bach BR Jr (2017) What factors influence the biomechanical properties of allograft tissue for ACL reconstruction? A systematic review. Clin Orthop Relat Res 475:2412–2426
LaPrade RF, Wills NJ, Spiridonov SI, Perkinson S (2010) A prospective outcomes study of meniscal allograft transplantation. Am J Sports Med 38:1804–1812
Lee BS, Kim JM, Sohn DW, Bin SI (2013) Review of meniscal allograft transplantation focusing on long-term results and evaluation methods. Knee Surg Relat Res 25:1–6
Lee SR, Kim JG, Nam SW (2012) The tips and pitfalls of meniscus allograft transplantation. Knee Surg Relat Res 24:137–145
Li J, Kong F, Gao X, Shen Y, Gao S (2016) Prospective randomized comparison of knee stability and proprioception for posterior cruciate ligament reconstruction with autograft, hybrid graft, and gamma-irradiated allograft. Arthroscopy 32:2548–2555
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100
Lord BR, Colaco HB, Gupte CM, Wilson AJ, Amis AA (2018) ACL graft compression: a method to allow reduced tunnel sizes in ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 26:2430–2437
Lubowitz JH, Verdonk PC, Reid JB 3rd Verdonk R (2007) Meniscus allograft transplantation: a current concepts review. Knee Surg Sports Traumatol Arthrosc 15:476–492
Mabe I, Hunter S (2014) Quadriceps tendon allografts as an alternative to Achilles tendon allografts: a biomechanical comparison. Cell Tissue Bank 15:523–529
Markolf KL, McAllister DR, Young CR, McWilliams J, Oakes DA (2003) Biomechanical effects of medial–lateral tibial tunnel placement in posterior cruciate ligament reconstruction. J Orthop Res 21:177–182
Markolf KL, O’Neill G, Jackson SR, McAllister DR (2003) Reconstruction of knees with combined cruciate deficiencies: a biomechanical study. J Bone Jt Surg Am 85-A:1768–1774
Mayr HO, Stoehr A, Dietrich M, von Eisenhart-Rothe R, Hube R, Senger S et al (2012) Graft-dependent differences in the ligamentization process of anterior cruciate ligament grafts in a sheep trial. Knee Surg Sports Traumatol Arthrosc 20:947–956
Mickiewicz P, Binkowski M, Bursig H, Wrobel Z (2014) Preservation and sterilization methods of the meniscal allografts: literature review. Cell Tissue Bank 15:307–317
Miller SL, Gladstone JN (2002) Graft selection in anterior cruciate ligament reconstruction. Orthop Clin N Am 33:675–683
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Mook WR, Civitarese D, Turnbull TL, Kennedy NI, O’Brien L, Schoeberl JB et al (2017) Double-bundle posterior cruciate ligament reconstruction: a biomechanical analysis of simulated early motion and partial and full weightbearing on common reconstruction grafts. Knee Surg Sports Traumatol Arthrosc 25:2536–2544
Muramatsu K, Hachiya Y, Izawa H (2008) Serial evaluation of human anterior cruciate ligament grafts by contrast-enhanced magnetic resonance imaging: comparison of allografts and autografts. Arthroscopy 24:1038–1044
Narvy SJ, Hatch GF 3rd, Ihn HE, Heckmann ND, McGarry MH, Tibone JE et al (2017) Evaluating the femoral-side critical corner in posterior cruciate ligament reconstruction: the effect of outside-in versus inside-out creation of femoral tunnels on graft contact pressure in a synthetic knee model. Arthroscopy 33:1370–1374
Negrin R, Duboy J, Olavarria F, Wainer M, Jimenez H, Las Heras F et al (2016) Biomechanical and histological comparison between the cryopreserved and the lyophilized gracilis tendon allograft for MPFL reconstruction, a cadaveric experimental study. J Exp Orthop 3:20
Nyland J, Campbell K, Kalloub A, Strauss EJ, Kuban K, Caborn DNM (2018) Medial meniscus grafting restores normal tibiofemoral contact pressures. Arch Orthop Trauma Surg 138:361–367
Nyland J, Larsen N, Burden R, Chang H, Caborn DN (2009) Biomechanical and tissue handling property comparison of decellularized and cryopreserved tibialis anterior tendons following extreme incubation and rehydration. Knee Surg Sports Traumatol Arthrosc 17:83–91
Paletta GA Jr, Manning T, Snell E, Parker R, Bergfeld J (1997) The effect of allograft meniscal replacement on intraarticular contact area and pressures in the human knee. A biomechanical study. Am J Sports Med 25:692–698
Palmer JE, Russell JP, Grieshober J, Iacangelo A, Ellison BA, Lease TD et al (2017) A biomechanical comparison of allograft tendons for ligament reconstruction. Am J Sports Med 45:701–707
Pearsall AWT, Hollis JM, Russell GV Jr, Scheer Z (2003) A biomechanical comparison of three lower extremity tendons for ligamentous reconstruction about the knee. Arthroscopy 19:1091–1096
Prokopis PM, Schepsis AA (1999) Allograft use in ACL reconstruction. Knee 6:75–85
Qu J, Thoreson AR, An KN, Amadio PC, Zhao C (2015) What is the best candidate allograft for ACL reconstruction? An in vitro mechanical and histologic study in a canine model. J Biomech 48:1811–1816
Qu J, van Alphen NA, Thoreson AR, Chen Q, An KN, Amadio PC et al (2015) Effects of trypsinization and mineralization on intrasynovial tendon allograft healing to bone. J Orthop Res 33:468–474
Rankin M, Noyes FR, Barber-Westin SD, Hushek SG, Seow A (2006) Human meniscus allografts’ in vivo size and motion characteristics: magnetic resonance imaging assessment under weightbearing conditions. Am J Sports Med 34:98–107
Rayan F, Nanjayan SK, Quah C, Ramoutar D, Konan S, Haddad FS (2015) Review of evolution of tunnel position in anterior cruciate ligament reconstruction. World J Orthop 6:252–262
Rijk PC (2004) Meniscal allograft transplantation—part I: background, results, graft selection and preservation, and surgical considerations. Arthroscopy 20:728–743
Robertson A, Nutton RW, Keating JF (2006) Current trends in the use of tendon allografts in orthopaedic surgery. J Bone Jt Surg Br 88:988–992
Rongen JJ, Hannink G, van Tienen TG, van Luijk J, Hooijmans CR (2015) The protective effect of meniscus allograft transplantation on articular cartilage: a systematic review of animal studies. Osteoarthr Cartil 23:1242–1253
Scheffler SU, Schmidt T, Gangey I, Dustmann M, Unterhauser F, Weiler A (2008) Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy 24:448–458
Schmidt T, Grabau D, Grotewohl JH, Gohs U, Pruss A, Smith M et al (2017) Does sterilization with fractionated electron beam irradiation prevent ACL tendon allograft from tissue damage? Knee Surg Sports Traumatol Arthrosc 25:584–594
Schmidt T, Hoburg A, Broziat C, Smith MD, Gohs U, Pruss A et al (2012) Sterilization with electron beam irradiation influences the biomechanical properties and the early remodeling of tendon allografts for reconstruction of the anterior cruciate ligament (ACL). Cell Tissue Bank 13:387–400
Schwartz HE, Matava MJ, Proch FS, Butler CA, Ratcliffe A, Levy M et al (2006) The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med 34:1747–1755
Sekaran SV, Hull ML, Howell SM (2002) Nonanatomic location of the posterior horn of a medial meniscal autograft implanted in a cadaveric knee adversely affects the pressure distribution on the tibial plateau. Am J Sports Med 30:74–82
Seto AU, Gatt CJ Jr, Dunn MG (2013) Sterilization of tendon allografts: a method to improve strength and stability after exposure to 50 kGy gamma radiation. Cell Tissue Bank 14:349–357
Smith CK, Hull ML, Howell SM (2006) Lengthening of a single-loop tibialis tendon graft construct after cyclic loading: a study using Roentgen stereophotogrammetric analysis. J Biomech Eng 128:437–442
Smith NA, Costa ML, Spalding T (2015) Meniscal allograft transplantation: rationale for treatment. Bone Jt J 97-B:590–594
Smith PA, DeBerardino TM (2015) Tibial fixation properties of a continuous-loop ACL hamstring graft construct with suspensory fixation in porcine bone. J Knee Surg 28:506–512
Suhodolcan L, Brojan M, Kosel F, Drobnic M, Alibegovic A, Brecelj J (2013) Cryopreservation with glycerol improves the in vitro biomechanical characteristics of human patellar tendon allografts. Knee Surg Sports Traumatol Arthrosc 21:1218–1225
Thornton GM, Shao X, Kuchison ME, Marchuk LL, Shrive NG, Frank CB (2009) Healing ligament mechanical properties are improved by repair with interpositional allografts but not by concomitant treatment with hyaluronic acid. J Orthop Res 27:400–407
Verdonk P, Depaepe Y, Desmyter S, De Muynck M, Almqvist KF, Verstraete K et al (2004) Normal and transplanted lateral knee menisci: evaluation of extrusion using magnetic resonance imaging and ultrasound. Knee Surg Sports Traumatol Arthrosc 12:411–419
von Lewinski G, Hurschler C, Allmann C, Wirth CJ (2006) The influence of pre-tensioning of meniscal transplants on the tibiofemoral contact area. Knee Surg Sports Traumatol Arthrosc 14:425–436
von Lewinski G, Milachowski KA, Weismeier K, Kohn D, Wirth CJ (2007) Twenty-year results of combined meniscal allograft transplantation, anterior cruciate ligament reconstruction and advancement of the medial collateral ligament. Knee Surg Sports Traumatol Arthrosc 15:1072–1082
Walz B, Nyland J, Fisher B, Krupp R, Nawab A (2012) Supplemental bio-tenodesis improves tibialis anterior allograft yield load in extremely low density tibiae. Arch Orthop Trauma Surg 132:343–347
Wang HD, Gao SJ, Zhang YZ (2018) Comparison of clinical outcomes after anterior cruciate ligament reconstruction using a hybrid graft versus a hamstring autograft. Arthroscopy 34:1508–1516
Wang HD, Zhu YB, Wang TR, Zhang WF, Zhang YZ (2018) Irradiated allograft versus autograft for anterior cruciate ligament reconstruction: a meta-analysis and systematic review of prospective studies. Int J Surg 49:45–55
West RV, Harner CD (2005) Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg 13:197–207
Yanke A, Bell R, Lee A, Shewman EF, Wang V, Bach BR Jr (2015) Regional mechanical properties of human patellar tendon allografts. Knee Surg Sports Traumatol Arthrosc 23:961–967
Yanke AB, Bell R, Lee AS, Shewman E, Wang VM, Bach BR Jr (2013) Central-third bone-patellar tendon-bone allografts demonstrate superior biomechanical failure characteristics compared with hemi-patellar tendon grafts. Am J Sports Med 41:2521–2526
Yoon KH, Lee SH, Park SY, Kim HJ, Chung KY (2014) Meniscus allograft transplantation: a comparison of medial and lateral procedures. Am J Sports Med 42:200–207
Funding
No funding was required for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors declare that they have any conflict of interest related to this work.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Seitz, A.M., Dürselen, L. Biomechanical considerations are crucial for the success of tendon and meniscus allograft integration—a systematic review. Knee Surg Sports Traumatol Arthrosc 27, 1708–1716 (2019). https://doi.org/10.1007/s00167-018-5185-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-018-5185-y