Abstract
Purpose
Irradiation >30 kGy is required to achieve sterility against bacterial and viral pathogens in ACL allograft sterilization. However, doses >20 kGy substantially reduce the structural properties of soft-tissue grafts. Fractionation of irradiation doses is a standard procedure in oncology to reduce tissue damage but has not been applied in tissue graft sterilization.
Methods
Forty-four human 10-mm wide bone-patellar-tendon-bone grafts were randomized into four groups of sterilization with (1) 34 kGy of ebeam (2) 34 kGy gamma (3) 34 kGy fractionated ebeam, and (4) non sterilized controls. Graft´s biomechanical properties were evaluated at time zero. Biomechanical properties were analyzed during cyclic and load-to-failure testing.
Results
Fractionation of ebeam irradiation resulted in significantly higher failure loads (1,327 ± 305) than with one-time ebeam irradiation (1,024 ± 204; P = 0.008). Compared to gamma irradiation, significantly lower strain (2.9 ± 1.5 vs. 4.6 ± 2.0; P = 0.008) and smaller cyclic elongation response (0.3 ± 0.2 vs. 0.6 ± 0.4; P = 0.05), as well as higher failure loads (1,327 ± 305 vs. 827 ± 209; P = 0.001), were found. Compared to non-irradiated BPTB grafts, no significant differences were found for any of the biomechanical parameters. Non-irradiated controls had significantly lower cyclic elongation response and higher failure loads than ebeam and gamma irradiation.
Conclusions
In this study, it was found that fractionation of high-dose electron beam irradiation facilitated a significant improvement of viscoelastic and structural properties of BPTB grafts compared to ebeam and gamma irradiation alone, while maintaining levels of non-irradiated controls. Therefore, this technique might pose an important alternative to common methods for sterilization of soft-tissue allografts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior cruciate ligament (ACL) reconstruction is the gold standard for restoring knee stability and preserving patient’s activity level. Different graft options exist with both auto- and allografts showing satisfactory clinical outcomes [5, 9, 19, 20, 27, 36]. However, especially in Europe, allograft use has been limited, mainly due to legal regulations, insufficient availability, and the potential risk of disease transmission [4, 26].
Different preservation and sterilization methods have been evaluated to address these legal requirements and eliminate the risk of disease transmission [7, 22, 32]. Currently, ionizing radiation is the most accepted procedure for terminal sterilization of soft-tissue grafts using gamma rays or electron emitting beams (Ebeam), which provide good bactericidal and virucidal properties [37, 40, 41]. It is known that high-dose irradiation beyond 20 kGy results in the deterioration of structural and biomechanical properties of soft-tissue grafts [8, 11, 23, 30, 31]. Low-dose irradiation (<20 kGy) is sufficient to eliminate bacterial pathogens, while dosages >30 kGy are required to provide tissue sterility [29], especially against certain viral pathogens.
Electron beam irradiation has been shown to be an efficient alternative to gamma irradiation, allowing for improved control of dose application and shorter irradiation times [1]. It is successfully used in the radiation therapy of malignant tumors, since it allows direct delivery of the radiation dose to the tumor, while limiting the exposure to tumor-free tissue [10, 17]. For damage reduction of the irradiated tissue, fractionation of the overall irradiation dosage has become a common procedure in radiotherapy [21, 28]. Further, it has been shown that fractionation did not affect the efficiency of malignant cell elimination compared to single-dose therapy [10]. Rationale of fractionation is a possible reduction of free radical formation, which has been suspected to play an important role in the impairment of mechanical properties of irradiated tissue [1, 15].
However, the concept of fractionating overall radiation dosages in the sterilization of soft-tissue grafts has not been examined yet. It was therefore the purpose of this study to evaluate the biomechanical properties of bone-patellar tendon-bone (BPTB) grafts after fractionation of high-dose (>30 kGy) ebeam sterilization and compare these to identical grafts following single-time standard gamma and Ebeam sterilization, as it is commonly carried out with sterilization of allografts for ACL reconstruction. It was hypothesized that fractionation of high-dose Ebeam irradiation would not significantly deteriorate the biomechanical properties, while single-time high-dose ebeam and gamma irradiation would result in significantly reduced biomechanical properties compared to non-irradiated soft-tissue grafts.
Materials and methods
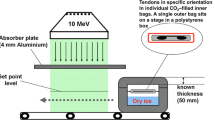
Forty-four 10-mm-wide human bone-patellar tendon-bone grafts were used in this study (Fig. 1). Donor age was between 43 and 73 years (mean 62.5 years; Fig. 1). They were randomized into four groups (n = 11 per group): (1) non-irradiated grafts, (2) 34-kGy ebeam irradiated grafts, (3) 34-kGy fractionated ebeam irradiated grafts, and (4) 34-kGy gamma irradiated grafts. All specimens were individually packed in gas-impermeable sterilization bags at an accredited authorized sterilization facility (DIZG, German Institute of Cell and Tissue Replacement, Berlin, Germany) and deep-frozen at −70°C. Ebeam and gamma sterilization were performed at a center for industrial application of ionizing radiation (Gamma Service Produktbestrahlung GmbH, Radeberg, Germany). Grafts were placed on a stage in a dry ice-filled polystyrene box, maintaining −70°C during the irradiation procedure (Fig. 2). Sterilization was either performed with 34 kGy of gamma or Ebeam radiation. Ebeam fractionation was carried out with repeated irradiation of 3.4 kGy for 10 times. Average time duration of the sterilization procedure was 30 s. The fractionation of Ebeam sterilization extended the procedure to 11.8 min. Average duration of gamma irradiation was 17.5 h. During the sterilization procedure, the applied dosage was very tightly controlled with a maximum dose deviation of +2.5 kGy for fractionated Ebeam, +2.6 kGy for Ebeam, and +5.4 kGy for Gamma irradiation. After sterilization, all grafts were stored in polystyrene boxes at −70°C until the day of mechanical testing. Maximum time of storage was 10 days.
For biomechanical testing, grafts were thawed at room temperature and both patella and tibia were potted in polymethyl methacrylate and mounted on aluminum clamps for fixation on a material testing machine (model 1455; Zwick GmbH, Ulm, Germany; Fig. 3) with the loading direction aligned parallel to the longitudinal graft axis. The grafts were kept moist throughout the entire testing. The biomechanical testing procedure included: preconditioning (10 cycles, 0–20 N); cyclic loading (200 cycles, 20–200 N); and a load-to-failure (LTF) test. The strain rate was 150 mm/min.
Graft motion during cyclic loading was tracked with an infrared motion analysis system (MacReflex, Qualisys Inc.). For this purpose, four circular reflective tape markers were glued to the tendon surface: two in the midsubstance and two at the bony insertions sites of the grafts. An additional marker was attached to each clamp (Fig. 3). The infrared motion analysis system consisted of two high-speed cameras that measured motion along all three axes. Our preliminary tests showed that out-of-plane motion was negligible; therefore, we only recorded motion along the longitudinal axis of the testing machine between the reflective tape markers attached to the clamps. Prior to each test, a special calibration frame was used to calibrate the system, which provided a precision of ≤0.1 mm for longitudinal graft motion. Customized software (MacReflex, Qualisys Inc.) was used to record and transfer these data to Excel (Microsoft, Redmond, WA, USA) software for final data processing and quantification of overall graft motion during the cyclic loading test. Overall strain and the cyclic elongation response of the grafts were also calculated from the motion analysis system during cyclic loading testing. Data are reported as strain difference, which was defined as the difference in length at 200 N between the 200th and 1st cycle divided by the initial length of the graft in percent (Strain difference: (L200−L1)/L0) × 100). Overall strain was measured at the last cycle as follows: (L200−L0)/L0) × 100. L0 was calculated from the distance between both markers attached to the respective clamps of the patellar tendon at the last cycle of preconditioning at 20 N. The cyclic elongation response was calculated from the difference in elongation at 200 N between the 200th and first cycle (Cyclic elongation response: Elong200−Elong1). Structural properties such as failure load and displacement at failure were recorded, and stiffness was derived from these values at the linear region of the load-elongation curve. Failure loads were measured at the peak of the linear load-elongation curve, which always corresponded to intratendinous rupture or tendon peal-off at the tibial insertion site, which was considered graft failure. A more detailed description of the biomechanical testing setup has been previously published [16, 33].
Statistical analysis
A power analysis was performed prior to the conduction of this study, which indicated that a sample size of 11 specimen would provide statistical power of at least 80% to detect mean differences in outcome parameters of one standard deviation between the groups (beta = 0.2, alpha = 0.05). A Mann–Whitney U test was used to test for significant differences between the respective groups regarding their biomechanical properties after determination of non-parametic data distribution. The level of significance was set at P < 0.05.
Results
Structural properties are presented in Table 1. Failure loads in the gamma irradiation group were significantly lower than in the single-time ebeam irradiation group (P = 0.046). Failure loads after gamma and single-time ebeam irradiation were significantly lower than after fractionation of ebeam irradiation (P = 0.001; P = 0.008) and in the non-irradiated controls (P = 0.000; P = 0.002). BPTB grafts after fractionation of 34 kGy ebeam irradiation showed no significant differences from non-irradiated controls (P = 0.270). Stiffness values revealed no significant differences between either of the groups.
All grafts failed by either intratendinous rupture (n = 24) or peal off from the tibial insertion site (n = 20) in all groups.
The analysis of the cyclic elongation response showed similar results (Table 1) with significant differences between gamma compared to fractionated ebeam irradiated grafts (P = 0.05). No statistically significant differences in cyclic elongation were found between single-time and fractionated ebeam irradiation (P = 0.076). Non-irradiated controls had a significantly smaller cyclic elongation response than gamma (P = 0.002) and single-time ebeam irradiation (P = 0.004). Again, no significant differences were found between Ebeam fractionation and non-irradiated controls (P = 0.332).
Strain values (Table 1) were significantly lower in fractionated ebeam irradiated and non-irradiated grafts compared to the gamma group (P = 0.008; P = 0.002), while no significant differences were found between gamma and single-time ebeam (P = 0.890) as well as ebeam fractionation and non-irradiated controls (P = 0.401).
Discussion
The most important finding of the present study was that fractionation of high-dose irradiation did not reduce the biomechanical properties of bone-patellar tendon-bone grafts compared to non-irradiated grafts. This was in contrast to the significant reduction in biomechanical properties after single-time high-dose irradiation.
As reconstruction of the ACL with allograft tissue has become an accepted and increasingly popular procedure not only in revision surgeries but also in primary reconstruction of the ACL, different preservation methods and terminal sterilization procedures of allografts have been introduced to prevent disease transmission and to comply with legal requirements of local authorities [2]. Most European countries require suppliers of soft-tissue grafts to reliably prove tissue sterility, necessitating graft sterilization. Since current sterilization techniques have inherent disadvantages with respect to mechanical and/or biomechanical properties, allograft supplies have been almost non-existent in Europe.
Deep-freezing and freeze-drying of grafts contribute to allograft safety by reduction of antigeniticity and the deleterious effect of free radicals, as they are less active at these temperatures [6, 7]. However, these sterilization techniques cannot achieve complete protection from bacterial and viral pathogens [24, 39]. The current standard sterilization procedures use ionizing radiation for terminal sterilization of soft-tissue allografts. Ionizing radiation allows for high penetration depth is effective in eliminating bacterial and viral pathogens at high dosages and can eliminate the risk of secondary contamination, as it has been observed during final packaging [40, 41].
Gamma irradiation is currently the most commonly used type of ionizing radiation due to its sterilization properties, good availability, low costs, and extensive experiences following its widespread use over many years. However, due to the well-documented impairment of structural properties at high dosages above 20 kGy [23, 30, 31], continuing attempts have been made to compensate for the adverse affects of high-dose irradiation while maintaining optimal sterilization efficacy [2, 22, 34, 35].
Different studies have evaluated the effect of different radical scavengers. Akkus et al. evaluated the effect of Thiourea as radical scavenger on Gamma irradiated (36.4 kGy) bone allografts and found an improvement in post yield and fracture energies with this treatment. Free radicals are created by hydrolysis of water during the irradiation procedure [25]. It is believed that these radicals play an important role in the reduction of biomechanical properties of soft-tissue grafts, as they are responsible for scissoring covalent bonds and therefore altering the tertiary structure of the collagenous material of ligaments and tendons [2, 15]. But still, a significant decrease of its biomechanical properties was shown compared to non-irradiated controls [1].
Similar results have been shown by Seto et al. who evaluated the effect of one of three radical scavenging regimens (mannitol, ascorbate, or riboflavin) and cross-linking (1-ethyl-3-[3-dimethyl aminopropyl] carbodiimide [EDC]) procedures on irradiated Achilles tendons. They found a protective effect on strength and elastic modulus, especially in combination of treatments, but also observed an inherent reduction in toughness and strength of the treated graft tissues compared to non-irradiated grafts [34, 35].
Electron beam is an ionizing radiation similar to gamma radiation. High energy electrons cause chemical changes similar to gamma irradiation. As Ebeam offers the aforementioned advantages to gamma irradiation, it might be economically more feasible while achieving identical sterility. Its decreased penetrability compared to gamma irradiation only becomes evident at high tissue densities and tissues thicker than 50 mm. This disadvantage is therefore negligible for soft-tissue graft typically used in ACL reconstruction [41]. Even though better biomechanical properties are maintained with Ebeam compared to gamma irradiation, a significant decrease compared to non-irradiated tissue has been observed at high dosages (>30 kGy), too [18]. Kaminsky et al. evaluated BPTB grafts sterilized with 25–100 kGy Ebeam irradiation and found a decrease in structural properties around 20% compared to non-irradiated controls [18].
Additional reduction of tissue deterioration might be achieved by fractionation of radiation dosages, as it has become a standard procedure in the treatment of malignant tumors [3, 13]. Different fractionation protocols as hypo- or hyperfractionation are being used in radiation therapy depending on the ability of the adjacent tissue to regenerate [14]. In hypofractionation, the dose is split in large fractions once a day, as in hyperfractionation small fractions are applied several times a day. Dosage and intervals of fractionation are hereby adjusted to the size and type of the tumor and its adjacent tissue. Concerns about the feasibility of multiple smaller dosages to obtain identical sterility levels can be discarded, as it is the overall dose that determines the extent of cellular elimination [38]. This has been shown by the efficacy of irradiation procedures, using multiple small doses, to destroy malignant tumors compared to single-time dose applications [3, 13, 14]. Additionally as results in biologic healing of irradiated and non-irradiated grafts at dosages below 30 kGy have been shown to be identical despite a slight hypervascularistion, the possibility of greater deterioration due to dose fractionation should be negligible [12].
This study showed that fractionation of irradiation dosages had a protective effect on the biomechanical properties of a bone-patellar tendon-bone graft as it is typically used in ACL reconstruction. Therefore, a similar effect was observed as in tumor irradiation. High-dose irradiation of 34 kGy, which had been shown to have detrimental effects on the biomechanical properties of soft-tissue grafts when applied as a single-dose [30], did not produce impaired structural and viscoelastic properties compared to non-irradiated grafts. The biomechanical properties of fractionated Ebeam sterilized BPTB grafts were significantly higher than in single-time Ebeam and Gamma irradiated grafts.
The significant decrease of the biomechanical properties in the high-dose gamma irradiation group was consistent with the findings of previous studies [2, 18, 23, 30, 31, 35]. Also, an advantage of standard Ebeam irradiation compared to gamma treatment at high dosages has been previously published [18, 35].
The positive effect of dose fractionation in this study cannot be explained by improved tissue regeneration as in tumor irradiation, as no vital tissue was sterilized. It is most likely that the new dose distribution throughout the cycles is responsible for this phenomenon. Smaller energies produce smaller radiochemical reactions at a certain time, such as free radical release, which occurs for fractions of a second after the immediate dose application. Therefore, the alteration of tissue structure might be much more limited with the application of multiple small instead of one large dose. Future studies will have to focus on the actual quantification of free radical release and visualization of its immediate effects on the collagenous parts of soft-tissue grafts.
This study has certain limitations, as it was only evaluated the grafts behavior at time zero and postimplantation behavior of such treated grafts cannot be predicted. Also different fractionation protocols were not compared, and its efficiency of sterilization will have to be evaluated. Future studies evaluating the in vivo behavior of such treated grafts, as well as sterilization efficiency of this procedure are warranted.
Conclusion
This study showed that the fractionation of Ebeam irradiation preserves biomechanical properties of BPTB grafts in vitro at dosages that would provide reliable sterility against bacterial and viral pathogens. Therefore, this technique can be used as an important alternative to current sterilization methods of soft-tissue allografts. This might have important clinical implications for soft-tissue allograft availability in countries, where terminal sterilization is a requirement prior to allograft implantation.
References
Akkus O, Belaney RM, Das P (2005) Free radical scavenging alleviates the biomechanical impairment of gamma radiation sterilized bone tissue. J Orthop Res 23:838–845
Azar FM (2009) Tissue processing: role of secondary sterilization techniques. Clin Sports Med 28:191–201
Bernier J, Hall EJ, Giaccia A (2004) Radiation oncology: a century of achievements. Nat Rev Cancer 4:737–747
Buck BE, Malinin TI, Brown MD (1989) Bone transplantation and human immunodeficiency virus. An estimate of risk of acquired immunodeficiency syndrome (AIDS). Clin Orthop Relat Res 240:129–136
Carey JL, Dunn WR, Dahm DL, Zeger SL, Spindler KP (2009) A systematic review of anterior cruciate ligament reconstruction with autograft compared with allograft. J Bone Joint Surg Am 91:2242–2250
Dzhafarov AI, Kol’s OR (1976) Change in the antiradical activity of cell organoid lipids during deep freezing. Biofizika 21:653–655
Dziedzic-Goclawska A, Kaminski A, Uhrynowska-Tyszkiewicz I, Stachowicz W (2005) Irradiation as a safety procedure in tissue banking. Cell Tissue Bank 6:201–219
Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T (1995) Gamma irradiation: effects on biomechanical properties of human bone-patellar tendon-bone allografts. Am J Sports Med 23:643–646
Foster TE, Wolfe BL, Ryan S, Silvestri L, Kaye EK (2010) Does the graft source really matter in the outcome of patients undergoing anterior cruciate ligament reconstruction? An evaluation of autograft versus allograft reconstruction results: a systematic review. Am J Sports Med 38:189–199
Gerbi BJ, Antolak JA, Deibel FC, Followill DS, Herman MG, Higgins PD, Huq MS, Mihailidis DN, Yorke ED, Hogstrom KR, Khan FM (2009) Recommendations for clinical electron beam dosimetry: supplement to the recommendations of task group 25. Med Phys 36:3239–3279
Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR (1991) Effects of gamma irradiation on the initial mechanical and material properties of goat bone-patellar tendon-bone allografts. J Orthop Res 9:209–218
Goertzen MJ, Clahsen H, Burrig KF, Schulitz KP (1995) Sterilisation of canine anterior cruciate allografts by gamma irradiation in argon. Mechanical and neurohistological properties retained 1 year after transplantation. J Bone Joint Surg Br 77:205–212
Hall EJ (1985) Radiation biology. Cancer 55:2051–2057
Hall EJ (1991) Weiss lecture. The dose-rate factor in radiation biology. Int J Radiat Biol 59:595–610
Hawkins CL, Davies MJ (1997) Oxidative damage to collagen and related substrates by metal ion/hydrogen peroxide systems: random attack or site-specific damage? Biochim Biophys Acta 1360:84–96
Hoburg AT, Keshlaf S, Schmidt T, Smith M, Gohs U, Perka C, Pruss A, Scheffler S (2010) Effect of electron beam irradiation on biomechanical properties of patellar tendon allografts in anterior cruciate ligament reconstruction. Am J Sports Med 38:1134–1140
Hogstrom KR, Almond PR (2006) Review of electron beam therapy physics. Phys Med Biol 51:R455–R489
Kaminski A, Gut G, Marowska J, Lada-Kozlowska M, Biwejnis W, Zasacka M (2009) Mechanical properties of radiation-sterilised human bone-tendon-bone grafts preserved by different methods. Cell Tissue Bank 10:215–219
Kustos T, Balint L, Than P, Bardos T (2004) Comparative study of autograft or allograft in primary anterior cruciate ligament reconstruction. Int Orthop 28:290–293
Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH (2010) Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy 26:41–49
Ma CM, Pawlicki T, Lee MC, Jiang SB, Li JS, Deng J, Yi B, Mok E, Boyer AL (2000) Energy- and intensity-modulated electron beams for radiotherapy. Phys Med Biol 45:2293–2311
McAllister DR, Joyce MJ, Mann BJ, Vangsness CT Jr (2007) Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med 35:2148–2158
McGilvray KC, Santoni BG, Turner AS, Bogdansky S, Wheeler DL, Puttlitz CM (2010) Effects of (60)Co gamma radiation dose on initial structural biomechanical properties of ovine bone-patellar tendon-bone allografts. Cell Tissue Bank. doi:10.1007/s10561-010-9170-z
Minami A, Ishii S, Ogino T, Oikawa T, Kobayashi H (1982) Effect of the immunological antigenicity of the allogeneic tendons on tendon grafting. Hand 14:111–119
Monboisse JC, Gardes-Albert M, Randoux A, Borel JP, Ferradini C (1988) Collagen degradation by superoxide anion in pulse and gamma radiolysis. Biochim Biophys Acta 965:29–35
Nemzek JA, Arnoczky SP, Swenson CL (1994) Retroviral transmission by the transplantation of connective-tissue allografts. An experimental study. J Bone Joint Surg Am 76:1036–1041
Poehling GG, Curl WW, Lee CA, Ginn TA, Rushing JT, Naughton MJ, Holden MB, Martin DF, Smith BP (2005) Analysis of outcomes of anterior cruciate ligament repair with 5-years follow-up: allograft versus autograft. Arthroscopy 21:774–785
Polaczek-Grelik K, Orlef A, Dybek M, Konefal A, Zipper W (2010) Linear accelerator therapeutic dose-induced radioactivity dependence. Appl Radiat Isot 68:763–766
Pruss A, Kao M, Gohs U, Koscielny J, von Versen R, Pauli G (2002) Effect of gamma irradiation on human cortical bone transplants contaminated with enveloped and non-enveloped viruses. Biologicals 30:125–133
Rasmussen TJ, Feder SM, Butler DL, Noyes FR (1994) The effects of 4 Mrad of gamma irradiation on the initial mechanical properties of bone-patellar tendon-bone grafts. Arthroscopy 10:188–197
Salehpour A, Butler DL, Proch FS, Schwartz HE, Feder SM, Doxey CM, Ratcliffe A (1995) Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone-patellar tendon-bone allografts. J Orthop Res 13:898–906
Scheffler SU, Gonnermann J, Kamp J, Przybilla D, Pruss A (2008) Remodeling of ACL allografts is inhibited by peracetic acid sterilization. Clin Orthop Relat Res 466:1810–1818
Scheffler SU, Schmidt T, Gangey I, Dustmann M, Unterhauser F, Weiler A (2008) Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy 24:448–458
Seto A, Gatt CJ Jr, Dunn MG (2008) Radioprotection of tendon tissue via crosslinking and free radical scavenging. Clin Orthop Relat Res 466:1788–1795
Seto A, Gatt CJ Jr, Dunn MG (2009) Improved tendon radioprotection by combined cross-linking and free radical scavenging. Clin Orthop Relat Res 467:2994–3001
Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB (2009) Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy 25:750–759
Tomford WW, Doppelt SH, Mankin HJ, Friedlaender GE (1983) bone bank procedures. Clin Orthop Relat Res 174:15–21
Withers HR (1999) Radiation biology and treatment options in radiation oncology. Cancer Res 59:1676s–1684s
Woo SL, Orlando CA, Camp JF, Akeson WH (1986) Effects of postmortem storage by freezing on ligament tensile behavior. J Biomech 19:399–404
Yusof N (2000) Irradiation for sterilising tissue grafts for viral inactivation. Malays J Nucl Sci 18:23–35
Yusof N (2006) Radiation in tissue banking—basic science and clinical applications of irradiated tissue allografts. World Scientific Publishing Co. Ptc. Ltd., Singapore
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoburg, A., Keshlaf, S., Schmidt, T. et al. Fractionation of high-dose electron beam irradiation of BPTB grafts provides significantly improved viscoelastic and structural properties compared to standard gamma irradiation. Knee Surg Sports Traumatol Arthrosc 19, 1955–1961 (2011). https://doi.org/10.1007/s00167-011-1518-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-011-1518-9