Abstract
Introduction
Improved soft tissue tendon graft mechanical properties have led to their increased use for anterior cruciate ligament (ACL) reconstruction. Because they do not have an osseous component; however, there are greater concerns regarding tibial graft slippage during early postoperative rehabilitation and activities of daily living, particularly in patients with poor bone mineral density (BMD), such as older patients, women, smokers, and patients undergoing revision ACL reconstruction surgery.
Methods
This in vitro biomechanical study attempted to determine the effectiveness of supplemental ACL graft fixation in low BMD tibiae. Eight paired knees (16 specimens) were harvested from female cadavers (mean age = 76, range = 60–88 years). Tibiae were assigned to either a combination bioabsorbable interference screw, bio-tenodesis screw group (Group 1, n = 8, apparent BMD = 0.44 ± 0.13 g/cm2) or a bioabsorbable interference screw group (Group 2, n = 8, apparent BMD = 0.44 ± 0.14 g/cm2). Double-strand (single loop) tibialis anterior tendon allografts were fixed in matched diameter tibial tunnels. Using a custom 6° of freedom jig, potted constructs were mounted on to a servo hydraulic device with the axial loading force aligned directly with the tibial tunnel. Constructs underwent progressive cyclic tensile loading from 10 to 150 N with a 25 N load increase every 20 cycles. This was followed by yield load to failure testing (20 mm/min).
Results
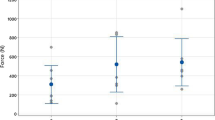
Groups did not display displacement differences during progressive cyclic loading. Group 1 (312.7 ± 67.5 N) displayed 25% greater yield load at failure than Group 2 (235.0 ± 47.6 N), P = 0.045. Both groups displayed fixation levels well below the previously reported minimal safe threshold estimate for early unrestricted weight bearing, accelerated rehabilitation and activities of daily living.
Conclusion
Supplemental bio-tenodesis fixation may improve early tibial-soft tissue tendon graft fixation in patients that have poor tibial BMD, but study results suggest that both methods may require weightbearing, rehabilitation, and activity of daily living restrictions during the early postoperative period to prevent graft slippage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The advantageous biomechanical properties of soft tissue tendon grafts have lead to their increased use for anterior cruciate ligament (ACL) reconstruction. With soft tissue tendon graft use however greater concerns exist regarding tibial graft slippage during the early postoperative period as compared to grafts that possess an osseous component. Because of its metaphyseal trabecular cancellous bone volume and the bone density reducing effects of ACL injury and tunnel drilling [1–7], tibial fixation is particularly prone to ACL graft slippage [8, 9]. Because of these concerns, some knee surgeons use supplemental tibial-soft tissue tendon graft fixation making use of stronger adjacent cortical bone [6, 10]. Because a bio-tenodesis screw is countersunk within a separate bone socket through the cortical tibial surface, it would be less likely to produce skin irritation and adjacent tissue morbidity than permanent extra-cortical supplemental fixation methods, such as metal staples, mini-plates, or synthetic buttons [9]. The intra-tunnel fixation provided by a bioabsorbable interference screw and the supplemental fixation provided by a bio-tenodesis screw combines the positive attributes of extra-tunnel cortical bone fixation with the graft-tunnel compression provided by intra-tunnel cancellous bone fixation.
As a greater number of older patients desire to continue participating in sports and recreational activities that require sudden pivoting or running direction changes more are undergoing ACL reconstruction [10–17]. This raises concerns regarding the greater likelihood that some of these older patients will possess poor tibial bone mineral density (BMD) [1–9]. In addition, patients that have a history of chronic lower extremity injuries, women, smokers, or those that require revision ACL reconstruction may have additional concerns related to poor tibial BMD and its negative influence on ACL graft fixation [2, 6, 8, 9].
Although they possess excellent biomechanical strength and stiffness when used in a looped configuration, soft tissue tendon grafts, such as the tibialis anterior [18] undergo a longer and more variable postoperative time period for successful graft-tunnel osteointegration than grafts that possess an osseous component [6, 19]. Studies using animal models that have reported successful soft tissue tendon graft-tunnel osteointegration by as early at 9–12 weeks may represent a best-case scenario [20–22]. These findings may not translate directly to the in vivo human condition, particularly when a soft tissue tendon allograft is used [22]. When soft tissue tendon grafts are used for ACL reconstruction in older tibiae with lower BMD, concerns increase regarding potentially decreased trabecular cancellous bone volume in the tunnel region of interest. Therefore, soft tissue tendon graft tibial tunnel slippage during the early postoperative period is a concern [2, 20, 23]. The purpose of this in vitro biomechanical study was to determine the effectiveness of supplemental bio-tenodesis screw fixation to bioabsorbable interference screw fixation of tibialis anterior allografts for ACL reconstruction in low BMD cadaveric tibiae.
Methods
Eight paired knees (16 specimens) were harvested from female cadavers (mean age = 76, range = 60–88 years). All specimens had been “lightly embalmed” using 7.6 L of a formaldehyde-based arterial conditioner (#120014 Metasyn, Cambridge, MA) to better maintain normal tissue moisture content and mechanical properties [24]. Short-term embalming using low formalin concentrations reportedly does not adversely affect bone mechanical properties under compression [25] and tensile [26] loading conditions. Proximal tibiae were harvested and all soft tissues were removed. Apparent BMD was determined using a dual energy X-ray absorptiometry scanner (Hologic QDR-1000 Whole Body X-Ray Bone Densitometer, Hologic Inc., Waltham, MA). Using a fiberglass reinforced filler compound (#265, Bondo Corporation, Atlanta, GA) with the longitudinal axis aligned vertically, specimens were embedded in 3 in. diameter polyvinylchloride tubes and were assigned to one of two groups of eight specimens with similar apparent BMD (Group 1 = 0.44 ± 0.13 g/cm2, Group 2 = 0.44 ± 0.14 g/cm2).
Fresh frozen human tibialis anterior tendons (CryoLife, Marietta, GA) were re-hydrated in room temperature sterile saline (0.9%) solution for 60 min. Doubled (single loop) tibialis anterior tendon allografts were prepared using 2-0 braided polyblend suture (AR-7200, FiberWire, Arthrex, Naples, FL) and running, interlocking whipstitches over each strand end for 3–4 cm [27]. Allograft diameter was measured to the nearest 0.5 mm using a standard sizing block and 8 allografts of comparable diameter were assigned to each group.
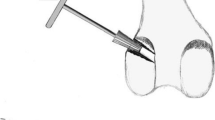
Following guidewire placement, extraction drilled tunnels were prepared that matched allograft diameter at a 50º sagittal plane angle from the tibial axis [28], placing the proximal tunnel within the posterior half of the native ACL insertion footprint [29]. The looped end of the tibialis anterior tendon allograft was then passed through the tunnel with a suture. Following this a stainless steel bar was positioned within the proximal allograft loop to maintain a consistent counterforce during intra-tunnel fixation and to ensure approximately 25 mm of looped graft for biomechanical testing, thereby simulating the length of the intra-articular portion of the ACL allograft. Both groups received a 35-mm long bio-interference screw (Arthrex, Naples, FL) with a diameter 1 mm > tibial tunnel diameter. Following tibial tunnel allograft fixation, a bone socket of equal diameter to allograft diameter was created in Group 1 tibiae approximately 2.5 cm distal to the center of the primary tunnel (Fig. 1). One of the whip-stitched allograft suture limbs was then passed through a cannulated Bio-Tenodesis Screwdriver (Arthrex, Naples, FL) and the allograft was inserted into the tibial socket. A 9-mm diameter × 23-mm-long Bio-Tenodesis screw (Arthrex) was advanced into the tibial socket over the retracting driver tip that maintained allograft tension. The ends of the allograft delivery sutures were then secured around the bio-tenodesis screw rim with a Mulberry knot thereby completing the secondary fixation. Maximum screw insertion torque was measured using a digital torque wrench (Mark-10, Hicksville, NY).
Constructs were mounted in a custom-made jig with 6º of freedom to enable the servo hydraulic device (Model #858, MTS, Eden Prairie, MN) axial tensile force vector to be aligned directly with the tibial tunnel (worst-case scenario) (Fig. 2). A steel cross pin passed through the looped end of the allograft served as the force application point. Constructs were preloaded to 10 N at a 1 N/s rate and were maintained at this load for 30 s. Constructs then underwent progressive cyclic loading at a 0.5 Hz rate from 10 to 150 N with a 25 N load increase every 20 cycles. This was followed by yield load to failure testing (20 mm/min).
Statistical analysis
Non-parametric Mann–Whitney U tests were used to compare group differences for displacement during progressive cyclic loading and yield load at failure. An alpha level of P < 0.05 was selected to indicate statistical significance. All statistical analysis was performed using SPSS version 11.0 software (SPSS Inc., Chicago, IL).
Results
Specimen preparation characteristics are reported in Table 1. Statistically significant displacement differences were not evident during progressive cyclic loading with comparable specimen survival (Table 2). Yield load to failure testing revealed a 25% greater yield load for Group 1 = 312.7 ± 67.5 N, Group 2 = 235.0 ± 47.6 N, P = 0.045. All specimens in both groups failed by graft slippage.
Discussion
Tibial trabecular cancellous bone density and architectural variability has a strong influence on ACL graft fixation [6, 8, 9]. Patients that have experienced chronic lower extremity injuries, women, smokers, and those undergoing revision ACL reconstruction may have tibial BMD that is less suitable for soft tissue tendon graft fixation [1, 2, 4, 6, 7]. A previous report demonstrated that supplemental soft tissue tendon allograft-tibial fixation using a bio-tenodesis screw increased construct ultimate load at failure and stiffness compared to bioabsorbable interference screw fixation alone in tibiae with BMD values that were in the lower range of normal [9]. The extremely low BMD of the cadaveric tibia used in this current study was approximately 40% of that reported for non-impaired 21-year-old females [7], representing a worst-case scenario. In the previous study using cadaveric tibiae with mean 0.9 ± 0.16 g/cm2 apparent BMD, the group that received bioabsorbable interference screw fixation and supplementary bio-tenodesis fixation displayed a mean 52% greater ultimate load at failure value (467 ± 184 vs. 223 ± 66 N) as compared to the group that received bioabsorbable interference screw fixation alone [9]. In addition, the failure mode for the group that received bioabsorbable interference screw fixation alone was allograft slippage, while the group that received supplemental bio-tenodesis fixation displayed a combination of graft slippage and graft–suture interface elongation [9]. In this study, all specimens in both groups failed by graft slippage. Poor tibial BMD and low screw insertion torques likely were the primary factors for the low yield loads at failure that we observed [23].
The yield load at failure that we observed for both groups was considerably lower than the recommended minimal time zero ACL graft fixation requirement estimate needed to enable safe participation in full weightbearing, aggressive or accelerated rehabilitation and unrestricted activities of daily living during the early postoperative period [30–32]. This suggests that an early period of restricted weightbearing, activities of daily living, and avoidance of aggressive or accelerated therapeutic exercises may be indicated for this population during the early postoperative period to prevent graft slippage irregardless if bioabsorbable interference screw fixation alone or combined bioabsorbable interference screw and supplemental bio-tenodesis screw fixation are used. Although the validity of a single safe, time zero threshold value such as 445–450 N has been called into question [33], having sufficient graft fixation over the early weeks post-surgery is essential. In addition, soft tissue tendon graft-tunnel healing studies that have reported sufficient healing by as early as 6–9 weeks post-surgery have been largely based on canine and ovine study models. The precise timetable for clinical soft tissue tendon graft-tunnel integration is unknown, likely longer, and more variable. The healing timetable is potentially even more delayed when soft tissue tendon allografts are used [22]. Although our study supported the hypothesis that supplemental tibial soft tissue tendon graft fixation using a bio-tenodesis screw provided superior time zero yield load at failure results, the relatively low yield loads that we observed suggest that both groups would require an early time period of restricted range of motion, weightbearing, and low-intensity rehabilitation exercises to prevent early graft-tunnel slippage.
This study is limited in that only eight paired cadaveric tibiae specimens with extremely low BMD were used. While “light” embalming with a formalin solution may have slightly affected bone mechanical properties, all paired specimens were similarly prepared allowing for direct group comparisons. Also, tensile loads applied directly inline with the tibial tunnel represent a worse case scenario unlikely to occur in vivo. Although the combination of extremely low BMD tibiae and a tensile loading vector aligned directly with the tibial tunnel are unlikely to occur in vivo, this in vitro model was useful to determine the specific contribution from secondary bio-tenodesis fixation. Another study limitation is that we did not perform a direct comparison with other hybrid fixation methods, such as extra-cortical buttons. The focus of our study was on bioabsorbable tibial ACL graft fixation methods and the paired study design did not allow for other comparisons. Future research of other hybrid tibial ACL graft fixation methods is indicated.
Because of their increasing desire to continue participation in stressful athletic activities that challenge both ligamentous and dynamic knee stability, more patients of >50 years of age are considering ACL reconstruction [11, 12, 14, 16]. This raises concerns about the efficacy of soft tissue tendon graft use among this population given the increased likelihood that they have less than ideal, highly variable trabecular cancellous BMD and volume in the tibial tunnel region of interest for ACL reconstruction, and the known negative BMD influences of gender, previous knee injury history, ACL reconstruction, revision ACL reconstruction, behaviors such as smoking, and chronic lower extremity conditions. Although the findings we report were observed with use of a single looped tibialis anterior tendon allograft, since poor tibial BMD was the primary fixation limiting factor we would expect similar results with supplemental bio-tenodesis fixation of semitendinosus-gracilis autografts compared with solely intra-tunnel bioabsorbable interference screw fixation.
References
Sievanen H, Kannus P, Heinonen A, Oja P, Vuori I (1994) Bone mineral density and muscle strength of lower extremities after long-term strength training, subsequent knee ligament injury and rehabilitation: a unique 2-year follow-up of a 26-year-old female student. Bone 15:85–90
Nyland J, Fisher B, Brand E, Krupp R, Caborn DN (2010) Osseous deficits after anterior cruciate ligament injury and reconstruction: a systematic review with suggestions to improve osseous homeostasis. Arthroscopy 26:1248–1257
Andersson SM, Nilsson BE (1979) Changes in bone mineral content following ligamentous knee injuries. Med Sci Sports 11:351–353
Leppala J, Kannus P, Natri A, Pasanen M, Sievanen H, Vuori I, Jarvinen M (1999) Effect of anterior cruciate ligament injury of the knee on bone mineral density of the spine and affected lower extremity: a prospective one-year follow-up study. Calcif Tissue Int 64:357–363
Kannus P, Sievanen H, Jarvinen M, Heinonen A, Oja P, Vuori I (1992) A cruciate ligament injury produces considerable permanent osteoporosis in the affected knee. J Bone Miner Res 7:1429–1434
Klein SA, Nyland J, Caborn DN, Kocabey Y, Nawab A (2005) Comparison of volumetric bone mineral density in the tibial region of interest for ACL reconstruction. Surg Radiol Anat 27:372–376
Vuori I, Heinonen A, Sievanen H, Kannus M, Pasanen M, Oja P (1994) Effects of unilateral strength training and detraining on bone mineral density and content in young women: a study of mechanical loading and deloading on human bones. Calcif Tissue Int 55:59–67
Bailey SB, Grover DM, Howell SM, Hull ML (2004) Foam-reinforced elderly human tibia approximates young human tibia better than porcine tibia. Am J Sports Med 32:755–764
Klein SA, Nyland J, Kocabey Y, Wozniak T, Nawab A, Caborn DN (2004) Tendon graft fixation in ACL reconstruction: in vitro evaluation of bioabsorbable tenodesis screw. Acta Orthop Scand 75:84–88
Kuechle DK, Pearson SE, Beach WR, Freeman EL, Pawlowski DF, Whipple TL, Caspari Dagger RB, Meyers JF (2002) Allograft anterior cruciate ligament reconstruction in patients over 40 years of age. Arthroscopy 18:845–853
Dahm DL, Wulf CA, Dajani KA, Dobbs RE, Levy BA, Stuart MA (2008) Reconstruction of the anterior cruciate ligament in patients over 50 years. J Bone Joint Surg Br 90:1446–1450
Arbuthnot JE, Brink RB (2010) The role of anterior cruciate ligament reconstruction in the older patients, 55 years or above. Knee Surg Sports Traumatol Arthrosc 18:73–78
Marquass B, Hepp P, Engel T, Dusing T, Lill H, Josten C (2007) The use of hamstrings in anterior cruciate ligament reconstruction in patients over 40 years. Arch Orthop Trauma Surg 127:835–843
Osti L, Papalia R, Del Buono A, Leonardi F, Denaro V, Maffulli N (2011) Surgery for ACL deficiency in patients over 50. Knee Surg Sports Traumatol Arthrosc 19:412–417
Plancher KD, Steadman JR, Briggs KK, Hutton KS (1998) Reconstruction of the anterior cruciate ligament in patients who are at least forty years old. A long-term follow-up and outcome study. J Bone Joint Surg Am 80:184–197
Trojani C, Sane J-C, Coste J-S, Boileau P (2009) Four-strand hamstring tendon autograft for ACL reconstruction in patients aged 50 years or older. Orthop Traumatol Surg Res 95:22–27
Viola R, Vianello R (1999) Intra-articular ACL reconstruction in the over-40-year-old patient. Knee Surg Sports Traumatol Arthrosc 7:25–28
Haut Donahue TL, Howell SM, Hull ML, Gregersen C (2002) A biomechanical evaluation of anterior and posterior tibialis tendons as suitable single-loop anterior cruciate ligament grafts. Arthroscopy 18:589–597
Lu Y, Markel MD, Nemke B, Wynn S, Graf B (2009) Comparison of single- versus double-tunnel tendon-to-bone healing in an ovine model: a biomechanical and histological analysis. Am J Sports Med 37:512–517
Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF (1993) Tendon-healing in a bone tunnel: a biomechanical and histological study in the dog. J Bone Joint Surg Am 75:1795–1803
Scranton PE, Lanzer WL, Ferguson MS, Kirkman TR, Pflaster DS (1998) Mechanisms of anterior cruciate ligament neovascularization and ligamentization. Arthroscopy 14:702–716
Scheffler SU, Schmidt T, Gangey I, Dustmann M, Unterhauser F, Weiler A (2008) Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy 24:448–458
Brand JC Jr, Pienkowski D, Steenlage E, Hamilton D, Johnson DL, Caborn DN (2000) Interference screw fixation strength of a quadrupled hamstring tendon graft is directly related to bone mineral density and insertion torque. Am J Sports Med 28:705–710
Anderson SD (2006) Practical light embalming technique for use in the surgical fresh tissue dissection laboratory. Clin Anat 19:8–11
Ohman C, Dall’Ara E, Baleani M, Van Sint Jan S, Viceconti M (2008) The effects of embalming using 4% formalin solution on the compressive mechanical properties of human cortical bone. Clin Biomech 23:1294–1298
Sedlin ED, Hirsch C (1966) Factors affecting the determination of the physical properties of femoral cortical bone. Acta Orthop Scand 37:29–48
Charlick DA, Caborn DN (2000) Technical note: alternative soft-tissue graft preparation technique for cruciate ligament reconstruction. Arthroscopy 16:E20
Goble EM, Downey DJ, Wilcox TR (1995) Positioning of the tibial tunnel for anterior cruciate ligament reconstruction. Arthroscopy 11:688–695
Howell SM, Wallace MP, Hull ML, Deutsch ML (1999) Evaluation of the single-incision arthroscopic technique for anterior cruciate ligament replacement. A study of tibial tunnel placement, intraoperative graft tension, and stability. Am J Sports Med 27:284–293
Morrison JB (1969) Function of the knee joint in various activities. Biomed Eng 4:573–580
Noyes FR, Butler DL, Grood ES, Zernicke RF, Hefzy MS (1984) Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Joint Surg Am 66:344–352
Noyes FR, Butler DL, Paulos LE, Grood ES (1983) Intra-articular cruciate reconstruction. I: Perspectives on graft strength, vascularization, and immediate motion after replacement. Clin Orthop Rel Res 172:71–77
Jarvinen TLN, Alami GB, Karlsson J (2010) Anterior cruciate ligament graft fixation—a myth busted? Arthroscopy 26:681–684
Acknowledgments
Equipment support was provided by Arthrex, Inc. Thanks to Jack Martin and HillCo. Medical, Louisville, KY.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walz, B., Nyland, J., Fisher, B. et al. Supplemental bio-tenodesis improves tibialis anterior allograft yield load in extremely low density tibiae. Arch Orthop Trauma Surg 132, 343–347 (2012). https://doi.org/10.1007/s00402-011-1374-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-011-1374-6