Abstract
Purpose
To evaluate the in vitro biomechanical characteristics of patellar tendon ligaments (BTB) when stored as fresh frozen or as glycerol cryopreserved allografts.
Methods
Seventy patellar tendons were harvested from 35 cadaveric human donors and randomly assigned into seven groups. Grafts in group FRESH were mechanically tested within 2 h of harvesting. FROZ-3, FROZ-6, and FROZ-9 were deep-frozen to −80°C for 3, 6, and 9 months, respectively. Grafts in groups CRYO-3, CRYO-6, and CRYO-9 were initially incubated with 10 % glycerol in a phosphate-buffered saline for 1 h and then stored in glycerol solution (10 % glycerol in PBS) at −80°C for 3, 6, and 9 months, respectively. Grafts were mechanically tested with two cycling modes (50–250°N and 150–500°N) and then loaded to failure.
Results
Cryopreserved grafts demonstrated more consistent results and expressed lower elongation rates after both cycling loading protocols compared to their frozen counterparts at all storage times. During load-to-failure analysis, ultimate stiffness levels were predominantly higher (23.9–61.5 %) in cryopreserved grafts compared with frozen grafts, and ultimate stress levels were 26 % (13.3–47.7 %) higher, regardless of the storage time. Moreover, cryopreserved grafts revealed similar ultimate elongation and uniformly higher ultimate stiffness and ultimate stress levels compared to fresh grafts.
Conclusion
The results of this in vitro study demonstrated superior mechanical properties of cryopreserved grafts compared to frozen grafts within a preservation period of 9 months. Cryopreservation with glycerol solution might be used to further improve the quality of preserved soft-tissue allografts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The anterior cruciate ligament (ACL) is the most commonly injured ligament in the knee, and more than 250,000 reconstructions of this ligament are made annually in Europe and the US [9, 20]. Primary ACL reconstructions are typically performed with autologous tissues with a high success rate [16]. Autologous tissues offer excellent tissue compatibility and avoid both immunogenicity and a risk of disease transmission. However, their usage may induce functional disability of the harvested area, and there are limitations in graft size, shapes, and availability [16]. Individual variation in anatomic structures such as patella baja, thin fasciae, or hypoplastic tendons or previous injuries (for example, tendon ruptures) may cause technical difficulties and inferior results of the autologous ACL reconstruction [6, 12, 30]. The use of allogenic tissues has become increasingly popular in reconstructive surgery of the knee, and it is estimated that 20 % of primary ACL reconstructions in the US are performed by an allograft [9, 22]. The percentage is significantly lower in the EU, where allografts are still predominately reserved for multi-ligament knee injuries and revision surgeries [7]. The main advantages of allografts are the shorter operative time, better cosmetic results, availability of larger grafts, lower incidence of postoperative arthrofibrosis, and no donor site morbidity [22, 25]. On the other hand, they carry a risk of disease transmission, the graft incorporation rate is slower (as there is a potentially subclinical immune response), and they increase operative costs [16, 17, 28, 29].
The storage of allogenic musculoskeletal tissues is most commonly performed by deep-freezing, cryopreservation, or freeze-drying. Deep-frozen and freeze-dried allografts have no viable cells remaining at the time of the transplantation [5]. Park et al. performed histomorphological examination of fresh frozen tendons in the frozen state and observed many spaces in interfibrillar substances. The collagen fibrils were divided and squeezed by these spaces, and the lines of collagen fibrils were distorted. They suggested that these spaces could be ice crystals, because the spaces disappeared after thawing [26]. Cryopreservation, a process of a controlled-rate freezing with extraction of cellular water by cryoprotectant media (glycerol or dimethylsulphoxide—DMSO), was shown to preserve up to 80 % of cell viability due to the alteration of water crystallisation during the freezing process [5, 35]. Cryoprotectants were additionally found to promote angiogenesis and reduce hosts’ intravascular immune response to frozen bone grafts, potentially contributing to an improvement of their biological properties [37]. Even though glycerol has shown positive results as a cryoprotectant for sperm, embryos, meniscal cartilage, skin stem cells, vein, chondrocytes, and bone marrow, evidence of its efficacy for ligament allograft preservation is limited [3, 8, 35, 36].
The purpose of this study was to evaluate the in vitro biomechanical characteristics of patellar tendon ligaments or bone tendon bone (BTB) when stored as fresh frozen or as glycerol cryopreserved allografts. Cryopreservation of BTB allografts was hypothesised to decrease the strain in cyclic loading and increases ultimate stress and ultimate stiffness in load-to-failure (LTF) testing when compared to fresh frozen allografts.
Materials and methods
Graft procurement and preservation
The study protocol was approved by the National Medical Ethics Committee. Seventy patellar tendons were harvested from 35 human cadaveric donors (mean age, 50.5; ±10.0 years; 44 men and 26 women). The donors had no recorded disease or injury history that would have deteriorated the quality of the patellar tendon. All patellar tendons were harvested within 36 h after death under aseptic conditions. Standard 10-mm wide BTB grafts were procured from the central part of patellar tendons with a ligament stripper and a chisel. The bone blocks were trimmed to a width of 10 mm, a thickness of 10 mm, and a length of 20 mm, and all remaining soft tissues were removed. The procured BTB grafts were macroscopically intact. The grafts were randomly assigned into seven groups with ten specimens in each group. Grafts in the fresh group (FRESH) were mechanically tested within 2 h of harvesting. Grafts in each of the three frozen groups (FROZ-3, FROZ-6, and FROZ-9) were immediately stored in plastic cuvettes and deep-frozen to −80°C within 1 h, for 3, 6, and 9 months, respectively. Grafts in the cryopreserved groups (CRYO-3, CRYO-6, and CRYO-9) were initially incubated with 10 % glycerol (Sigma-Aldrich Co, St. Louis, USA) in a phosphate-buffered saline (PBS; pH 7.4, Sigma-Aldrich Co, St. Louis, USA) for 1 h. These grafts were then stored in glycerol solution (10 % glycerol in PBS) at −80°C for 3, 6, and 9 months. Grafts removed from storage were warmed to room temperature for 2 h. Their osseous parts were potted in polyurethane resin (polyol and isocyanate, Axson technologies, France) for 2 min and left for an additional hour at room temperature to harden prior mechanical testing. The tendinous portions of BTB were protected from the polymer contact. Grafts were kept moist with normal saline at all times during preparation and mechanical testing.
Mechanical testing
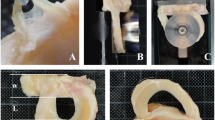
Mechanical tests were performed on the universal testing machine (Zwick/Roel Z050, Ulm, Germany) under stable ambient conditions at a temperature of 22–24°C and a humidity of 50–55 %. The accuracy of measurements was 0.4 % for force and 0.02 % for displacement. The potted osseous part of each BTB graft was securely clamped into the testing machine (Fig. 1a). To minimise imperfections of clamping, a specimen was initially loaded with a 10°N force and then carefully unloaded until 0°N was reached. The initial length of the tendon between the bone blocks was recorded. The graft was then preconditioned for 2 min with a constant load of 50°N. After 2 min, the width and thickness of the tendon were measured across the three points: 10 mm from tibial bone block, at the midpoint, and 10 mm from the femoral bone block with a digital calliper (Mitutoyo, Illinois, USA). The cross-sectional area of the patellar tendon graft was calculated as the average of all three measurements. The next step was cyclic testing of the grafts performed in a load control mode in the interval 50–250°N at a constant rate of 100 mm/min for 200 cycles. After the last cycle, the specimen was carefully unloaded back to 0°N. The graft was subsequently loaded with a 150°N force for 2 min and then subjected to additional 200 loading cycles in the 150–500°N interval at a constant rate of 100 mm/min. After the last cycle, the specimen was carefully unloaded to 0°N. The end stage of the mechanical testing was based on tensile loading-to-failure (LTF). The force–elongation to break curve at a constant rate of 100 mm/min was recorded. The strain was defined as εn = (L n –L 1)/L 1,where L n is the absolute length of the ligament at the upper-limit of force for the n-th cycle and L 1 is the absolute length of the ligament at the upper-limit of force for the first cycle. It should be emphasised that in this definition the initial length of the ligament L 0 does not appear and L 1 is used instead. We chose such a definition because we explicitly focused on the mechanical behaviour of the ligament during cyclical loading. The ultimate stress at load-to-failure test was defined as the ratio of the ultimate force at disruption of the ligament to the average cross-sectional area of ligament after preconditioning. Ultimate stiffness was defined as the ratio of the ultimate force at disruption of the ligament to the relative ligament elongation at the break.
Statistical analysis
A chi-squared test was performed to check the distribution of sex in the groups, and ANOVA was performed to test for differences in age, cross-sectional area, and initial length distribution between groups. We confirmed a normal distribution for the cyclic and LTF testing data using the Shapiro–Wilks W test. Therefore, we used unpaired Student’s t tests for statistical analysis amongst groups. All statistical tests were performed at a probability level of 95 % (α = 0.05). We used SPSS® for Windows® version 17.0 (SPSS, Chicago, Illinois, USA) for the statistical analysis. Results for cyclic testing and LTF tests are presented as the mean and standard deviation.
Results
Sixty-three of the grafts withstood the whole protocol. Four tendons from the frozen groups and three tendons from the cryopreserved groups failed for reasons other than mechanical problems. Of the remaining 63 grafts, all disrupted between the substance during LTF testing (Fig. 1b). In addition, no failures at the bone fixation clamp and no specimen slippage from the fixation device occurred. There were no statistical differences in the distribution of sex, age, mean cross-sectional area, and initial length between the graft subgroups (Table 1).
Typical cyclic loading and LTF diagrams are given in Fig. 2. After settling in of biological fibres, the elongation steps in the first cycling process were within a 0.2-mm interval (Fig. 2a). The response of the ligament was non-linear in the whole domain. A similar difference between the first and last elongation steps was observed when the ligament was subjected to 150–500°N cycling loading (Fig. 2b). In contrast to the first cycling loading regime, the mechanical response of the specimen was close to linear when the load exceeded 200°N. Force–elongation from 0°N to disruption of the ligament is shown in Fig. 2c. A non-linear response of the ligament was recorded in the 0–200°N interval again, whereas a linear response was observed from 200–800°N. The ligament disruption occurred around 1,100°N.
The first cyclic loading (50–250°N; Fig. 3a) revealed higher elongation rates for FROZ compared to FRESH. These rates were not statistically significantly different except between the FROZ-6 and FRESH groups. The elongation rates of cryopreserved grafts showed only minimal non-significant strain increase at 200th cycle compared with FRESH, except between CRYO-6 and FRESH. The second cyclic loading (150–500°N; Fig. 3b) induced strain increase at the 200th cycle as a function of preservation time in frozen grafts. Strain increments compared with FRESH were measured as following: 5.2 % for FROZ-3, 115.4 % for FROZ-6, and 41 % for FROZ-9. In contrast, the mean mechanical properties of cryopreserved grafts showed no strain increase at the 200th cycle compared with FRESH for CRYO-3 and CRYO-6, and only a 5 % difference in CRYO-9. All of the cryopreserved grafts had lower elongation rates compared to their frozen counterparts at all storage times after both cyclic loading protocols (Table 2). However, statistically significant differences were observed only at six-month storage after first and second cycling.
There were two modes of graft failure present: a tendon mid-substance rupture or tendon avulsion from the tibial osseous block. A mid-substance rupture occurred in the majority of FRESH (70 %) and cryopreserved grafts [CRYO-3 (70 %), CRYO-6 (75 %), and CRYO-9 (67 %)], but less often in frozen grafts [FROZ-3 (55.6 %), FROZ-6 (44.4 %), and FROZ-9 (37.5 %)]. The LTF testing induced a wider variation in results of all the measured quantities of the frozen grafts. The results of testing cryopreserved grafts were more consistent and revealed similar ultimate elongation, uniformly higher ultimate stress, and higher ultimate stiffness compared with FRESH (both non-significant). The ultimate stress was higher in the cryopreserved grafts compared to the frozen grafts (Fig. 3c): 17.2 % after 3 months, 47.7 % after 6 months, and 13.3 % after 9 months. The ultimate elongation (except for FROZ-3 vs. CRYO-3) and ultimate stiffness (except for FROZ-6 vs. CRYO-6; Fig. 3d) were lower in the frozen than the cryopreserved grafts. The numerical details are given in Table 2.
Discussion
The most important findings of the presented study on cadaveric material were improved biomechanical characteristics of glycerol cryopreserved BTB allografts in comparison to fresh frozen storage. Their lower elongation rates during cyclic loading and their higher ultimate stress and stiffness during LTF were recorded.
Several previous studies have examined the mechanical properties of non-sterilised BTB grafts during LTF testing. The reported values vary immensely due to the different methods of preservation, graft processing, testing configurations, and the age of included donors [31]. Almquist et al. and Scheffler et al. used an uniaxial testing configuration comparable to our study. Their results are consistent with our findings in terms of ultimate stiffness levels from 168 ± 13°N/mm for frozen and 194 ± 57°N/mm for fresh BTB allografts [1, 31]. On the other hand, cryopreserved grafts in our study showed approximately 17.1 % (10.8–23 %) better ultimate stiffness levels compared to Scheffler et al. and 35.3 % (28–42.2 %) higher ultimate stiffness levels relative to Almqvist et al. for fresh frozen BTB grafts. The gathered stiffness differences between our data and the studies above can partly be attributed to a higher mean donor age of 59 years (16–82 years) [1, 31]. The testing protocol of Hoburg et al. who evaluated the effects of high-dose electron beam irradiation resembles ours. They found similar results in terms of ultimate stress, but they observed higher overall strain values, as they used a different definition of strain [13, 14].
To the authors’ knowledge, only the study by Kamiski et al. studied the effect of glycerolisation on BTB allografts [18]. They demonstrated inferior biomechanical properties for glycerolisation in comparison to fresh frozen grafts. Their study protocol included only LTF testing on a small number (2–3 per each group) of donors with a high variability in age (17–84 years), and therefore, the strength of evidence from that study is limited. Theories of freezing injury suggest that damage to cells occurs due to extracellular ice formed during the freezing process, which exerts an osmotic disequilibrium on intracellular water [10]. Permeating cryoprotectants, such as glycerol, act by penetrating the cell membrane, thereby reducing the intracellular amount of ice formed, and cause the concentration of electrolytes to decrease by a given temperature during the freezing process, thus minimising slow freezing damage [10, 23].
A discussion on the mechanical testing parameters used for ligaments requires special consideration; as vast discrepancies in force intervals, number of cycles and test frequency are reported in the literature [31]. Although a single load-to-failure testing of grafts provides information regarding graft fixation, such testing does not address the mechanical behaviour of the graft during early rehabilitation with repetitive graft loading [32, 33]. Barber-Westin et al. performed sequential arthrometer testing after ACL reconstruction surgery using a BTB allograft and noted that 62 % of the abnormal displacement was seen before the 24th week, much earlier than study subjects returned to full sports activities [4]. Load range for the first cycling loading in our study was set between 50°N, which represents a reasonable level of minimum force in the graft from pre-tensioning, and 250°N, which is presumably the peak force during early rehabilitation [34]. The upper loading limit of 500°N was chosen within the range of previous studies [24, 27, 34]. A cyclic loading with two cyclic modes of 200 cycles was based on the results of Markolf et al. They demonstrated that the major length increase occurred during the first 6 out of 2,000 loading cycles [21]. A comparable study by Honl et al. found that the predominant elongation occurred within the first 600 of 60,000 cycles [15]. Prior to testing, we performed a pilot study on a small number of frozen ligaments using 500 loading cycles in the 50–250°N and another 500 cycles in the 150–500°N interval. The unpublished data demonstrated that the majority of graft elongation occurred before the 50th cycle, and the elongation was stabilised between 150th and 200th cycles. An additional technical detail in our ligament testing protocol needs to be highlighted—a simple potting method in polyurethane of fragile bone block. Clamping of the hardened bone resin allowed safe and reproducible measurements without any slippage from the fixation device during cyclic and LTF testing.
Limitations of our study are the short duration of preservation, a relatively small number of specimens in each group, and, seemingly, a high donor age. As the number of available donors was limited, the age limit of 65 years was defined, because it does not significantly affect the initial biomechanical properties of tendons [11]. However, with increasing demand and biomechanical studies indicating no correlation between donor age and graft tensile strength, the spectrum of donor age has expanded, creating the potential for a large disparity in age between the tissue donor and recipient [19]. Irrespective of the rather short preservation period, 9 months proved to be sufficient for an estimation of early behaviour of BTB grafts. Grafts in tissue banks are typically used within 6 months of storage; therefore, the limit of 9 months mirrors the real situation [2].
Cryopreservation with glycerol solution seems to be an improved method for preservation of soft-tissue allografts in an attempt to further improve the outcome of knee reconstructive surgery.
Conclusions
The results of this study demonstrate that in vitro mechanical properties, such as strain during cyclic measurements, ultimate stress, and ultimate stiffness at failure, are statistically superior in cryopreserved grafts compared with frozen grafts within the preservation interval of 9 months. Therefore, cryopreservation with glycerol solution seems to be an improved method to deep-freezing for preservation of soft-tissue allografts. This in vitro study only allowed an assessment of the mechanical properties at the time of graft implantation. No final conclusions can be drawn on the mechanical properties of the BTB grafts during biological healing and graft incorporation.
References
Almqvist KF, Jan H, Vercruysse C, Verbeeck R, Verdonk R (2007) The tibialis tendon as a valuable anterior cruciate ligament allograft substitute: biomechanical properties. Knee Surg Sports Traumatol Arthrosc 15:1326–1330
American Association of Tissue Banks: Standards for Tissue Banking—12th edn. McLean: American Association of Tissue Banks, 2008. Available at: http://www.aatb.org
Attarian H, Feng Z, Buckner CD, MacLeod B, Rowley SD (1996) Long-term cryopreservation of bone marrow for autologous transplantation. Bone Marrow Transplant 17:425–430
Barber-Westin SD, Noyes FR, Heckmann TP, Shaffer BL (1999) The effect of exercise and rehabilitation on anterior-posterior knee displacements after anterior cruciate ligament autograft reconstruction. Am J Sports Med 27:84–93
Barbour SA, King W (2003) The safe and effective use of allograft tissue-an update. Am J Sports Med 31:791–797
Bonamo JJ, Krinick RM, Sporn AA (1984) Rupture of the patellar ligament after use of its central third for anterior cruciate reconstruction. A report of two cases. J Bone Joint Surg Am 66:1294–1297
Buchmann S, Musahl V, Imhoff AB, Brucker PU (2008) Allografts for cruciate ligament reconstruction. Orthopade 37:772–778
Castagnoli C, Alotto D, Cambieri I et al (2003) Evaluation of donor skin viability: fresh and cryopreserved skin using tetrazolioum salt assay. Burns 29:759–767
Cohen SB, Sekiya JK (2007) Allograft safety in anterior cruciate ligament reconstruction. Clin Sports Med 26:597–605
Fowler A, Toner M (2005) Cryo-injury and biopreservation. Ann NY Acad Sci 1066:119–135
Greaves LL, Hecker AT, Brown CH Jr (2008) The effect of donor age and low-dose gamma irradiation on the initial biomechanical properties of human tibialis tendon allografts. Am J Sports Med 36:1358–1366
Hamada M, Shino K, Mitsuoka T, Abe N, Horibe S (1998) Cross-sectional area measurement of the semitendinosus tendon for anterior cruciate ligament reconstruction. Arthroscopy 14:696–701
Hoburg AT, Keshlaf S, Schmidt T, Smith M, Gohs U, Perka C, Pruss A, Scheffler S (2010) Effect of electron beam irradiation on biomechanical properties of patellar tendon allografts in anterior cruciate ligament reconstruction. Am J Sports Med 38:1134–1140
Hoburg A, Keshlaf S, Schmidt T, Smith M, Gohs U, Perka C, Pruss A, Scheffler S (2011) Fractionation of high-dose electron beam irradiation of BPTB grafts provides significantly improved viscoelastic and structural properties compared to standard gamma irradiation. Knee Surg Sports Traumatol Arthrosc 19:1955–1961
Honl M, Carrero V, Hille E, Schneider E, Morlock MM (2002) Bone-patellar tendon-bone grafts for anterior cruciate ligament reconstruction: an in vitro comparison of mechanical behavior under failure tensile loading and cyclic submaximal tensile loading. Am J Sports Med 30:549–557
Indelli PF, Dillingham MF, Fanton GS, Schurman DJ (2004) Anterior cruciate ligament reconstruction using cryopreserved allografts. Clin Orthop Relat Res 420:268–275
Jackson DW, Corsetti J, Simon TM (1996) Biologic incorporation of allograft anterior cruciate ligament replacements. Clin Orthop Relat Res 324:126–133
Kamiński A, Gut G, Marowska J, Lada-Kozłowska M, Biwejnis W, Zasacka M (2008) Mechanical properties of radiation-sterilised human bone-tendon-bone grafts preserved by different methods. Cell Tissue Bank 45:122–129
Kang RW, Strauss EJ, Barker JU, Bach BR Jr (2011) Effect of donor age on bone mineral density in irradiated bone-patellar tendon-bone allografts of the anterior cruciate ligament. Am J Sports Med 39:380–383
Lind M, Lund B, Faunø P, Said S, Miller LL, Christiansen SE (2012) Medium to long-term follow-up after ACL revision. Knee Surg Sports Traumatol Arthrosc 20:166–172
Markolf KL, Zemanovic JR, McAllister DR (2002) Cyclic loading of posterior cruciate ligament replacements fixed with tibial tunnel and tibial inlay methods. J Bone Joint Surg Am 84-A:518–524
Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD (2010) Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy 26(9 Suppl):S58–S66
Mazur P (2004) Principles of cryobiology. In: Fuller BJ, Lane N, Benson EE (eds) Life in the frozen state. CRC Press, Boca Raton, pp 3–65
Noyes FR, Butler DL, Grood ES, Zernicke RF, Hefzy MS (1984) Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Joint Surg Am 66:344–352
Olson EJ, Harner CD, Fu FH, Silbey MB (1992) Clinical use of fresh, frozen soft tissue allografts. Orthopedics 15:1225–1232
Park HJ, Urabe K, Naruse K, Onuma K, Nemoto N, Itoman M (2009) The effect of cryopreservation or heating on the mechanical properties and histomorphology of rat bone-patellar tendon-bone. Cell Tissue Bank 10:11–18
Paulos LE, Karistinos A, Walker J (2006) “Criteria”-based rehabilitation of surgically reconstructed and nonsurgically treated anterior cruciate ligament injuries. In: Scott WN (ed) Insall & scott surgery of the knee, 4th edn. Elsevier Churchill Livingstone, Philadelphia, pp 693–714
Rodeo SA, Seneviratne A, Suzuki K, Felker K, Wickiewicz TL, Warren RF (2000) Histological analysis of human meniscal allografts. A preliminary report. J Bone Joint Surg Am 82-A:1071–1082
Rodrigo JJ, Jackson DW, Simon TM (1993) The immune response to freeze dried bone tendon bone allografts in humans. Am J Knee Surg 6:347–353
Rosenberg TD, Franklin JL, Baldwin GN, Nelson KA (1992) Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med 20:519–525
Scheffler SU, Scherler J, Pruss A, von Versen R, Weiler A (2005) Biomechanical comparison of human bone-patellar tendon-bone grafts after sterilization with peracetic acid ethanol. Cell Tissue Bank 6:109–115
Scheffler SU, Sudkamp NP, Gockenjan A, Hoffmann RF, Weiler A (2002) Biomechanical comparison of hamstring and patellar tendon graft anterior cruciate ligament reconstruction techniques: the impact of fixation level and fixation method under cyclic loading. Arthroscopy 18:304–315
Seil R, Rupp S, Krauss PW, Benz A, Kohn DM (1998) Comparison of initial fixation strength between biodegradable and metallic interference screws and a press-fit fixation technique in a porcine model. Am J Sports Med 26:815–819
Shelburne KB, Torry MR, Pandy MG (2005) Muscle, ligament, and joint-contact forces at the knee during walking. Med Sci Sports Exerc 37:1948–1956
Shelton WR, Treacy SH, Dukes AD, Bomboy AL (1998) Use of allografts in knee reconstruction: I. Basic science aspects and current status. J Am Acad Orthop Surg 6:165–168
Vuola J, Pipping D (2002) Maintaining a glycerolized skin bank-a practical approach. Burns 28(Suppl 1):S31–S33
Wingenfeld C, Egli RJ, Hempfing A, Ganz R, Leunig M (2002) Cryopreservation of osteochondral allografts: dimethyl sulfoxide promotes angiogenesis and immune tolerance in mice. J Bone Joint Surg Am 84-A:1420–1429 (Erratum in: J Bone Joint Surg Am 84-A:1855)
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suhodolčan, L., Brojan, M., Kosel, F. et al. Cryopreservation with glycerol improves the in vitro biomechanical characteristics of human patellar tendon allografts. Knee Surg Sports Traumatol Arthrosc 21, 1218–1225 (2013). https://doi.org/10.1007/s00167-012-1954-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-012-1954-1