Abstract
Background
Parkinson’s disease (PD) is the second most common neurodegenerative disease, and is characterized by accumulation of α-synuclein (α-syn). Neuroinflammation driven by microglia is an important pathological manifestation of PD. α-Syn is a crucial marker of PD, and its accumulation leads to microglia M1-like phenotype polarization, activation of NLRP3 inflammasomes, and impaired autophagy and phagocytosis in microglia. Autophagy of microglia is related to degradation of α-syn and NLRP3 inflammasome blockage to relieve neuroinflammation. Microglial autophagy and phagocytosis of released α-syn or fragments from apoptotic neurons maintain homeostasis in the brain. A variety of PD-related genes such as LRRK2, GBA and DJ-1 also contribute to this stability process.
Objectives
Further studies are needed to determine how α-syn works in microglia.
Methods
A keyword-based search was performed using the PubMed database for published articles.
Conclusion
In this review, we discuss the interaction between microglia and α-syn in PD pathogenesis and the possible mechanism of microglial autophagy and phagocytosis in α-syn clearance and inhibition of neuroinflammation. This may provide a novel insight into treatment of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease (AD) [1]. Age is the foremost risk factor for PD. With aging of the world population, the prevalence of PD is also on the rise. It is reported that, by 2040, the number of PD patients will double to 14.2 million from 6.9 million in 2015 [2]. However, there is no effective early diagnosis and treatment of PD, and the study of its specific pathogenesis is still in the early stage [3]. Therefore, correctly understand the pathogenesis of PD, develop early diagnostic methods, explore the key markers of disease diagnosis, and develop effective therapeutic drugs are the current research priorities. Microglia are continuously activated throughout the disease, and the neuroinflammation induced by microglia activation plays an important role in the progression of PD, so microglia have the potential to become a marker for the diagnosis and treatment of PD [4]. Pathologically, PD is characterized by the formation of Lewy bodies and the progressive loss of dopaminergic (DA) neurons in the substantia nigra of the midbrain. Specifically, the molecular mechanism shows persistent neuroinflammation, autophagy disorder, oxidative stress, and calcium disorder [5]. Among these, autophagy has become a research hotspot in recent years, but previous reports on autophagy in PD are mostly focused on neurons. The autophagy in microglia has been more attractive to scientists in the recent years, because it is associated with its function of phagocytosis and degradation of α-syn. α-Syn aggregates into oligomers or fibrils. These pathological forms of α-syn are the driving factor of PD pathology, which aggravates DA neuron damage by inducing microglia to activate NLRP3 inflammasomes and promoting neuroinflammation. Pathological α-syn can also damage the autophagy and phagocytosis of microglia, which impairs the degradation of pathological α-syn and NLRP3 inflammasomes, and further exacerbates neuroinflammation in PD. In recent years, some studies have found that the activation of microglial autophagy can inhibit its inflammatory activation by promoting microglial phagocytosis and degradation of α-syn or activated NLRP3 inflammasomes. Autophagy activation degrades proinflammatory cytokines secreted by microglia to avoid their activation, and improves immune function and protects against PD in many clinical trials and preclinical studies. This suggests that autophagy can slow down the progression of PD by inhibiting neuroinflammation induced by microglia. Exploring the relationship between autophagy and inflammation in microglia will provide a promising therapeutic strategy for PD. Here, we review the current research progress on autophagy, phagocytosis and inflammation of microglia in PD pathology, emphasizing the role of α-syn, and further discuss the possible treatment of PD.
PD and α-syn
PD is regarded as a synucleinopathy because its pathological marker is the formation of Lewy bodies (LBs), which are composed of aggregated α-syn. Synucleinopathy also includes multiple system atrophy, pure autonomic failure, and dementia with LBs [6]. In a recent report, researchers extracted α-syn filaments from the brains of six patients with PD, PD with dementia, and dementia with LBs, and reported cryoelectron microscopic structures of these α-syn filaments. They called the ordered core structure Lewy fold, which is formed by residues 31–100 of α-syn [7]. α-Syn is a small protein with three domains composed of 140 amino acids (14 kDa), which is encoded by SNCA gene [8]. SNCA is the first identified gene associated with familial PD and is known to be affected by seven mutations associated with the disease: A30P, E46K, H50Q, G51D, A53V, A53T and A53E [9]. The sequence variation of SNCA regulatory region is related to the increased expression of α-syn and the increased risk of idiopathic PD, which accounts for > 90% of cases [10]. Therefore, SNCA mutation is often regarded as a marker of idiopathic PD.
Formation and aggregation of α-syn
The toxicity of α-syn depends on its forms at different stages. The α-syn monomer is nontoxic and soluble. The aggregation of α-syn is a process of gain of toxic function and probably caused by mutation of its coding gene (SNCA), which plays a core role in PD [9, 11]. Phosphorylated α-syn, supposed to be the pathogenic isoform of α-syn, is more likely to aggregate [12]. In addition, duplications and triplications of SNCA can also increase the aggregation of α-syn [13, 14]. α-Syn fibrils are the main form of α-syn in LBs. The transformation of α-syn monomer aggregation to α-syn fibrils is a complex process, involving multiple cellular and biochemical events, and regulated by a variety of genetic and environmental factors [15, 16]. This process can be simply explained by the imbalance between the synthesis and elimination of α-syn. It is generally believed that the aggregated α-syn first form α-syn oligomers, which contains 30–35 monomers of α-syn with a molecular weight of ~ 440 kDa [6]. They interact abnormally with neuronal membranes, resulting in their disruption [17, 18]. 8300 a-syn monomers form α-syn fibrils with a molecular weight of ~ 120 000 kDa [6]. The toxicity of α-syn fibrils is similar to α-syn oligomers [19]; both of which can spread in the brain and trigger neurodegeneration [20]. Aggregated α-syn can induce a variety of pathological processes, such as mitochondrial dysfunction, calcium homeostasis disorders, neuroinflammation, endoplasmic reticulum stress and lysosomal disorders [21] (Fig. 1).
Although the importance of α-syn has been studied in depth, there is still an important question as to whether the α-syn pathology is a driving factor or an epiphenomenon that is the best observed in the neurodegenerative processes in PD [22]. α-Syn aggregation may represent a pathophysiological hallmark and be an epiphenomenon of neurodegeneration.
PD and microglia
Microglia are the immune cells in the brain and play a key role in providing host defense against pathogens and central nervous system (CNS) diseases. In addition, microglia maintain CNS homeostasis at different stages of growth and development [23]. In the neonatal brain, microglia regulate neurogenesis and neuronal survival by phagocytosis of excess neonatal cells, removal of redundant synaptic connections, and secretion of essential neurotrophic factors during development, such as insulin-like growth factor, transforming growth factor-β and brain-derived neurotrophic factor [24]. In the adult brain, in addition to secretion of the above-mentioned nutritional factors to support neural growth and phagocytosis of excessive metabolites or damaged tissue, microglia also show functions in learning by regulating synaptic pruning and neuronal circuit remodeling [25]. As age is the main risk factor for PD [26], the microglia in the brain of older people present changes in morphology, phenotype, inflammation and overall functional response. Microglia at this stage easily over-react to external stimuli and neurotoxic damage, producing a large number of proinflammatory cytokines to induce neuroinflammation [27]. As early as the 1980s, researchers reported that there was a large number of activated microglia in the substantia nigra of PD patients [28]. Lymphocyte infiltration was also observed in the substantia nigra of PD patients, which could promote the activation of microglia [29]. Persistent overactivation of microglia is considered to be one of the main pathological manifestations of PD. Activated microglia mediate inflammation by releasing a plethora of proinflammatory cytokines, including interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α and NO [30]. These cytokines eventually lead to apoptosis of DA neurons and accelerate progression of PD. Microglia release most of the inflammatory cytokines, which is the most important feature in the pathogenesis of neuroinflammation in PD [31, 32]. In the following part, we discuss the pathological factors that drive microglial inflammation in PD.
Microglial inflammation in PD
Microglia express diverse immune pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) and scavenger receptors (SRs) [33]. PRRs recognize exogenous pathogenic molecules called pathogen-associated molecular patterns (PAMPs) or endogenous host-derived molecules called damage-associated molecular patterns (DAMPs). Microglia are the earliest cells in the CNS in response to PAMPs/DAMPs [34]. Microglia are activated through recognition of PAMPs/DAMPs stimuli via PRRs, causing intracellular cascade reactions, kinases and downstream transcription factor activation, to produce and release proinflammatory cytokines [35]. In the resting state under normal conditions, the microglia exhibit ramified morphology. Once activated, the shape changes to an ameboid-like form. Some studies have described this change in microglia (known as microgliosis) in patients or animal models with PD [36].
According to the difference in secreted cytokines, trigger stimuli and cell surface markers, the classes of microglia are usually divided into M1 and M2 activation. M1 microglia are closely related to PD, and release harmful proinflammatory cytokines and lead to neuronal degeneration and loss [37]. M1 microglia produce proinflammatory cytokines [IL-1β, IL-6, IL-12, interferon (IFN)-γ and TNF-α], chemokines (CCL2, CCL20 and CXCL-100, cytotoxic substances [reactive-oxygen species (ROS), reactive-nitrogen species (RNS), nitric oxide (NO) and excitatory amino acids (EAA)], and prostaglandin E2 [38], and are stimulated by IFN-γ or lipopolysaccharide (LPS) in vivo or in vitro [39,40,41]. M2 microglia can phagocytose and remove fragmented neurons, and release anti-inflammatory factors and neuroprotective cytokines to recover damaged neurons after injury [42, 43]. Similar to macrophages, the M2 microglia can be further divided into three subtypes: M2a, M2b and M2c. [44]. IL-4 or IL-13 can induce M2a activation, and IL-4 can directly stimulate cell surface markers (SRs and CD206) to perform phagocytosis and inhibit inflammation [45,46,47]. The function of M2b is similar to that of M2a. TLRs, activated by fusing with Fcγ receptors, binds to the IgG complex, causing microglia polarization to M2b phenotype, which in turn causes secretion of IL-10 and production of specific cell surface markers (MHC-II and CD86) [48]. M2c can be induced by IL-10 and exhibit an anti-inflammatory effect [38]. Therefore, microglia act as a “double-edged sword” under physiological conditions.
Cyclo-oxygenase 2 and inducible NO synthase are markers of M1 microglia. High expression of cyclo-oxygenase 2 and inducible NO synthase has been observed in the microglia of PD patients [49], indicating that inflammatory injury from microglia is magnified in PD. The DA neurons loss caused by microglia-mediated neuroinflammation in PD has been proved in many studies [50,51,52,53]. LPS, as the ligand of TLR4, can activate nuclear factor (NF)-κB pathway and release proinflammatory cytokines [54]. LPS-induced PD inflammation is demonstrated to compromise DA neurons [55, 56]. In the model of PD inflammation induced by LPS, a significant increase in the level of inflammatory cytokines was observed on day 3, and damage of DA neurons appeared on day 21, indicating that activation of microglia often occurs before the loss of DA neurons [57]. The PD transgenic mouse model (A53T) and neurotoxin (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxydopamine (6-OHDA) or rotenone) model showed the same results as LPS did [58,59,60]. With the progression of PD, the chronic inflammatory environment can promote the continuous activation of microglia, leading to neuroinflammation, oxidative stress and neurotoxicity. Alleviation of neuroinflammation to delay the progression of PD has been regarded as a possible treatment [61].
α-Syn in microglial inflammation
α-Syn is misfolded and accumulated in LBs, and is detected in postmortem brain sections of PD patients. α-Syn promotes progression of PD, but the mechanism is still not fully understood [62]. Most studies have focused on the role of α-syn in neurons, because of its aggregation and high expression in neurons [63]. However, α-syn in microglia is also implicated as the initiating factor of neuroinflammation in PD [64]. Extracellular α-syn activates microglia through PRRs to release inflammatory cytokines. Incompletely removed α-syn leads to chronic neuroinflammation and neuronal damage, which initiates the process of PD neuroinflammation [65, 66]. The neuroinflammation induced by α-syn also occurs ahead of the DA neuronal loss [67].
In the pathological process of PD, α-syn is secreted from neurons into the extracellular space and is detected in the blood and cerebrospinal fluid of PD patients [68, 69]. The released α-syn is aggregated into oligomers or fibrils and then transferred between cells as free floating proteins or via extracellular vesicles [70]. This aggregated α-syn acts as a chemoattractant to direct microglial migration toward damaged neurons [71]. The binding of α-syn to TLRs is an important trigger for the activation of microglia [72]. α-Syn oligomers induce microglia to M1-like phenotype polarization through TLR1/2 signaling, resulting in nuclear translocation of NF-κB and increased production of proinflammatory cytokines through a MyD88-dependent pathway [73]. Some studies have reported that α-syn released from neurons to the extracellular environment is an endogenous agonist of TLR2, which activates inflammation in microglia [74]. In addition to TLR2, TLR4 also mediates α-syn-dependent activation of microglia, including production of ROS and proinflammatory cytokines [75]. Scavenger P2X7 receptor and complement receptor are also involved in α-syn-induced microglial activation [76,77,78].
Evidence of a chronic neuroinflammatory response can be found in PD and other diseases involving α-syn, where microglia are activated in all brain regions with accumulated α-syn [28, 79]. T cells from patients with PD can recognize α-syn, which probably aggravate microglial inflammation [80]. Selective overexpression of α-syn in murine microglia induces apoptosis of DA neurons through oxidative stress [81]. In vitro, exogenous α-syn can directly activate microglia and lead to the release of inflammatory cytokines [18, 82]. In vivo, sustained activation of microglia can be induced by adeno-associated virus overexpression of α-syn or by injection of α-syn preformed fibrils (PFF) into the murine brain (striatum or substantia nigra) [83, 84]. The NF-κB and JAK–STAT pathway are implicated in the activated microglia induced by α-syn [85,86,87]. Therefore, as the main pathogenic factor of PD, α-syn induces microglia to activate and release NO, ROS, proinflammatory cytokines and chemokines, which aggravate CNS inflammation in patients with PD and eventually lead to neuronal death (Fig. 2).
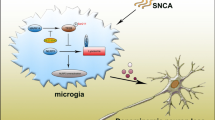
Neuroinflammation induced by aggregated α-syn aggravates neuronal degeneration and loss. a Accumulation of α-syn and neuroinflammation lead to degeneration and loss of DA neurons in substantia nigra. b The dead neurons release α-syn, which promotes activation of microglia, then the activated microglia release proinflammatory cytokines, which in turn aggravate neuronal death. c α-Syn promotes activation of microglia in several ways. Created with BioRender.com
Microglial NLRP3 inflammasomes and PD
Inflammasomes are macromolecular protein complexes that are an important part of the innate immune system. Many inflammasomes, including NLR family pyrin domain (NLRP)1, NLRP2, NLRP3, NLRC4 and AIM2, have been reported in CNS diseases [88]. Among these inflammasome proteins, NLRP3 is a widely studied oligopolyprotein inflammasome complex in activated microglia and is the main molecule that activates caspase-1 and cleaves IL-1β and IL-18 [89]. It was the first inflammasome found and most studied in PD [90]. High expression of NLRP3 has been detected in the brain of PD patients [91]. NLRP3 inflammasomes are found in microglia, astrocytes, neurons and endothelial cells in PD patients [92,93,94].
New evidence suggests that overactivated NLRP3 inflammasomes in microglia are driving factors that aggravate pathology and ultimately accelerate the progression of neurodegenerative diseases [95]. The expression of NLRP3 inflammasomes is increased in activated microglia in the substantia nigra [64], and colocalization of microglia and NLRP3 is found in LPS-induced PD inflammation and MPTP-induced PD models [96]. NLRP3 inflammatory bodies in PD are mainly activated by α-syn in peripheral and CNS-resident immune cells [94]. α-Syn released from neurons is recognized and taken up by surrounding microglia [97], which promotes NLRP3 activation and drives the inflammatory process in microglia through TLR2 and TLR5 [98]. α-Syn promotes the activation of NLRP3 in murine and human microglia, accompanied with the production of IL-1β [82, 99]. The expression of NLRP3, caspase-1 and IL-1β in midbrain was robustly increased in A53T transgenic mice [100]. Inhibition of neuroinflammation in microglia through blocking NLRP3 can alleviate progression of PD. MCC950, a highly selective NLRP3 inhibitor, is a latent therapeutic drug for PD [101]. In murine microglia, α-syn induced the activation of NLRP3 inflammasome and promoted release of IL-1β and apoptosis-associated speck-like protein containing a CARD (ASC). MCC950 blocked this process and significantly ameliorated α-syn PFF-induced motor deficit, DA neuronal damage, and accumulation of α-syn in the substantia nigra and striatum [102]. Several anti-inflammatory drugs inhibited the activation of NLRP3 inflammasomes in PD models, such as melatonin, glibenclamide and andrographolide [103,104,105].
Microglial inflammation facilitated α-syn transmission
The recent studies have shown that the neuroinflammation induced by microglia can aggravate the accumulation and transmission of α-syn to aggravate progression of PD. Coculture of activated microglia with SH-SY5Y cells in vitro demonstrated that activating microglia promoted the phosphorylation and aggregation of α-syn, which led to the shortening of synapses, upregulated TLR2/4 expression, activated p38/JNK, and inhibited autophagic flux [106]. Neurons usually discharge excess α-syn to the extracellular space, which can damage physiological processes of microglia, such as oxidative stress or dysfunction of mitochondria and lysosomes; thus, amplifying the pathological effects [107,108,109]. Microglia activated by α-syn increase their phagocytic activity and transmission of α-syn in a prion-like manner [110, 111]. IL-4 activated M2 microglia effectively reduced extracellular α-syn; thus, reducing α-syn transfer among neurons. In contrast, microglia treated with LPS-exhibited M1 activation, aggravated neuroinflammation, and impaired clearance of α-syn in vivo [110]. Activation of microglia in the olfactory bulb induced by intranasal injection of LPS in mice promoted expression of inflammatory cytokines, which increased accumulation of α-syn in the olfactory bulb and α-syn transmission to the substantia nigra and striatum. Mechanistically, LPS-induced microglia promoted PD pathology through IL-1β/IL-1R1-dependent signal transduction [112]. In summary, microglial inflammation promotes misfolding, aggregation and prion-like transmission of α-syn to induce death of fragile neurons and drive progression of PD [110, 111]; the cause which may be related to the increase in intracellular α-syn accumulation in microglia and acceleration of α-syn secretion into the extracellular space through an exocrine pathway [113].
PD pathogenic genes and microglial inflammation
PD is usually classified as familial or sporadic according to heredity, and familial PD accounts for ~ 15% of cases [114]. To date, rare mutations in > 20 genes have been identified to cause familial PD or PD-related diseases [115]. The common genetic variants of PD are mainly determined by genome-wide association studies, and 90 independent risk signals have been identified [116]. According to the analysis from > 1 million patients and controls, the common genes associated with PD cohorts are PARK16, GBA1, SNCA, LRRK2, GCH1, VPS13C and MAPT [117]. Sporadic PD is a late-onset disease, and its risk is affected by a series of common variants with low penetrance and environmental damage. Genome-wide association studies also identified PD genes including SNCA, GBA and LRRK2 in sporadic PD, indicating that there is a common pathogenic pathway between familial and sporadic PD [116]. Most of the PD pathogenic genes identified are expressed in microglia; some of which play a role in microglia-induced neuroinflammation [118]. Mutations in SNCA can lead to pathological accumulation of α-syn, which directly promotes neuroinflammation induced by microglia [9]. Mutations in the E3-ubiquitin ligase gene Parkin are the most common cause of recessive hereditary PD [119]. Parkin in microglia inhibits NF-κB mediated inflammation, and its mutation aggravates microglia-induced inflammation [120]. The loss of DJ-1 function is associated with 1–2% of autosomal recessive early-onset PD [121]. DJ-1 knockdown in microglia increases the production of inflammatory cytokines induced by LPS [122]. LRRK2 mutation is the most common single genetic cause of familial and sporadic PD. LRRK2 is highly expressed under inflammatory stimulation and enhances α-syn-induced neuroinflammation through the binding of α-syn to TLR2 [123]. G2019S LRRK2 mutant promotes the release of inflammatory cytokines from microglia [124]. These studies have shown that PD-related pathogenic genes SNCA, PARKIN, DJ-1, LRRK2 and GBA play a role in microglial inflammation.
Aging and microglia in PD
Age is the main pathogenic factor of PD, and microglia show age-dependent changes [25]. With the increase in the age, the length, branches and dendritic area of microglia decrease [125]. Although the relationship between microglial quantity and age is still controversial, microglial senescence is associated with functional changes that are likely to contribute to an age-dependent increase of microglial-mediated neuroinflammatory responses [126]. Microglia express a high level of IL-1β with aging, which makes them prone to M1 transition [127]. This inflammatory hypersensitive phenotype is commonly referred to as primed microglia [128], which secrete more inflammatory cytokines after being stimulated by LPS [129]. By collecting the brains of rats of different ages, M1 markers (TNF-α and IL-β) increased while M2 markers (arginase 1 and IL-10) decreased in aged rats, which also indicated the effect of age on microglial polarization [130]. A recent study directly demonstrated the correlation between age and pathology of PD induced by α-syn. After injection of α-syn PFF to induce PD, there were continuously activated microglia in the CNS of old mice, and microglia of young mice showed more abundant branches, which led to a higher inflammatory environment in the brain of old mice [84]. These phenotypic changes affect the ability of microglia to function properly over time, and the accumulation of nonfunctional aging microglia may evoke irreversible and progressive neurodegeneration in PD [131]. α-Syn only induces an aging phenotype in young microglia rather than in aged microglia, which indicates that there is some overlap between aging and α-syn in microglia [84]. Therefore, the relationship between senescence and microglia function in PD seems to be linked through induced pathology.
Autophagy, pathogenesis and PD
Autophagy is an evolutionarily conservative catabolic process that regulates cell homeostasis by degrading or recovering cytoplasmic components such as proteins, aggregates, and damaged organelles. Three types of autophagy have been identified: macrophage, chaperone-mediated autophagy (CMA) and microautophagy [132]. Autophagy generally refers to macrophages; the function of which is involved in regulation of synaptic plasticity, the development of myelin and oligodendrocytes, and the anti-inflammation of glial cells, thus playing a key role in maintaining neuronal health [133]. The accumulation of α-syn is caused by defects in the two main protein catabolism systems, ubiquitin–proteasome system and autophagy–lysosome pathway (ALP) [134]. The correlation between autophagy defect and PD has been confirmed by many reports. The deficiency of autophagy leads to the enhancement of endogenous α-syn and LRRK2 protein levels in vivo, and the deletion of Atg7 alone can induce neurodegeneration [135, 136]. The role of α-syn in autophagy is vital in PD. Pathological aggregation of α-syn disrupts the operation of synaptic proteins and deteriorates autophagic, lysosomal and mitochondrial function [137]. Autophagic dysfunction can also accelerate the intercellular transfer of pathological α-syn [138]. Previous studies on autophagy in PD have mostly focused on neurons, but there have been a few studies on autophagy in glial cells, especially in microglia [139]. However, as mentioned earlier, microglia may initiate the occurrence and development of PD; therefore, the function of microglial autophagy in PD pathogenesis has to be emphasized as research has advanced.
Microglial autophagy in α-syn clearance
ALP plays an important role in the degradation of aggregates [140], abnormal cytoplasmic organelles under physiological conditions [141], and remains to be the critical way to remove pathologic α-syn in PD. ALP dysfunction contributes to the accumulation of α-syn and loss of dopaminergic neurons in PD [142, 143]. α-Syn contains a CMA-targeted motif, which enables α-syn to be selectively transferred to lysosomes through CMA for degradation [144, 145]. Therefore, one of the promising developments for PD is improvement of the metabolism of α-syn by regulating lysosomes or autophagy, or both [146]. Although both neurons and glial cells in the brain can uptake and degrade extracellular α-syn, microglia show the highest efficiency in vitro [97, 147]. Surprisingly, a recent study reported that inhibition of neuronal ALP function decreased the toxicity of extracellular α-syn to neurons [148], suggesting that targeting ALP in microglia is more reasonable for the degradation of α-syn than targeting neurons. The decrease in PD microglial ALP degradation efficiency boosts the accumulation of misfolded α-syn and degeneration of DA neurons. An in vitro study showed that exosomes secreted by neurons overexpressing α-syn inhibited microglial autophagy [149]. The accumulation of α-syn has also been shown to inhibit the level of autophagy in microglia, demonstrated by the upregulation of autophagy marker SQSTM1/p62 [150], and conversely, autophagy blockade can enhance the release and intercellular transfer of α-syn in microglia [151]. The clearance of α-syn by microglia is mediated by TLR4/NF-κB signal transduction through upregulation of autophagy receptor p62/SQSTM1 transcription [147]. Inhibition of autophagy in the whole brain induces neurodegeneration [152], while the specific loss of microglial autophagy does not lead to death or activation of microglia under physiological conditions. However, mice deficient in microglial autophagy showed more obvious motor disorders, α-syn deposition and loss of tyrosine hydroxylase (TH) neurons under the stimulation of α-syn [139, 147]. This suggests that the loss of microglial autophagy aggravates α-syn aggregation and the vulnerability of DA neurons to α-syn.
Microglial phagocytosis of α-syn
Autophagy-associated ATG proteins also regulate phagocytosis [153]. Phagocytosis is a cellular process by which cells recognize, phagocytize and digest large particles (> 0.5 μm) [154], and functions in developing normal neural circuits and maintaining balance in the CNS. Microglia are considered to be the main phagocytes in the brain, continuously removing synapses, apoptotic cells and debris to maintain neural function [155]. Microglia improve the neural network by removing the synapses overproduced by the CNS during development, which contributes to learning and memory [156], and antagonize central infection through direct phagocytosis of bacteria and viruses [157]. In PD, phagocytosis is involved in promoting the clearance of α-syn and apoptotic neurons. Phagocytosis of microglia is mainly accomplished by the complement system and some receptors, including TLRs, scavenger receptor CD14, TAM (Tyro3, Axl and Mer) receptor and triggering receptor expressed on myeloid cells-2 (TREM2) [158,159,160,161,162].
According to the formation of phagosomes, phagocytosis can be divided into three types: LC3-dependent phagocytosis, LC3-independent phagocytosis and xenophagy [163]. LC3-dependent phagocytosis mainly activates three kinds of signal complexes [164]. The first signal, LC3-associated protein, is directly coupled to the phagosome membrane and necessary for the effective elimination of pathogens, dead cells and misfolded proteins [165, 166]. The second signal complex, Beclin-1, Rubicon, vacuolar protein sorting 34 (Vps34) and UV radiation resistance-associated gene (UVRAG) are involved in the production of phosphatidylinositol 3-phosphate (PI3P), which is necessary and functions in phagosome maturation [167]. The third signal, nicotinamide adenine dinucleotide phosphate oxidase 2 (NOX2), which promotes the production of superoxides, is involved in phagocytosis and autophagy [168].
Microglial phagocytosis is beneficial to tissue homeostasis, preventing the overflow of proinflammatory and neurotoxic molecules by rapidly removing dying cells through receptor-mediated signaling [163, 169]. For example, microglia specifically expressing TREM2 can enhance the phagocytosis of microglia and attenuate inflammation by negatively regulating activation of NF-κB signal transduction mediated by TLR4 and transferring M1-like to M2-like phenotype polarization [170, 171]. Some studies have used the proportion of M2/M1 microglia to indirectly represent the phagocytosis of microglia [172] because of the scavenging effect of M2 microglia on α-syn in PD [173]. The apoptotic neurons by microglial phagocytosis mediated by TREM2 receptor is associated with the reduction of proinflammatory cytokines [162]. α-Syn serves as both regulator and effector of microglial phagocytosis [174]. The exogenous monomer α-syn promotes the phagocytosis of microglia, while aggregated α-syn impairs this function [175, 176]. Aggregated α-syn inhibits the phagocytosis of microglia through the FCγ RIIB/SHP-1 pathway and mediates the release of TNF-α and IL-1β from microglia [177]. Prostaglandin E receptor subtype 2 regulates α-syn phagocytosis and CD11b-mediated microglial activation [178]. α-Syn interacts with CD11b to activate NOX2 through extracellular signal-regulated kinase 1/2 kinase activation and the RhoA-dependent pathway, leading to microglial migration [179, 180].
Other nonclassical proteins, such as lymphocyte-activation gene 3, are also involved in regulating the phagocytosis of α-syn by microglia [181]. Although low-density lipoprotein receptor-related protein (LRP)1 is necessary for neurons to phagocytize α-syn monomers and oligomers [182], and enhancement of LRP1 in microglia significantly activates phagocytosis of microglia through upregulating Beclin1 and LC3 upon inflammation, it is not clear whether it can promote the phagocytosis of α-syn by microglia [183].
The lack of some pathogenic genes such as DJ-1 in PD has been shown to impair autophagy and reduce the phagocytosis of α-syn by microglia [184]. LRRK2 is also associated with autophagy and phagocytosis of microglia, and the imbalance of the LRRK2/ALP axis can lead to the accumulation of α-syn [185] (Fig. 3).
Microglial autophagy in NLRP3 inflammasome clearance
Growing evidence indicates that activation of inflammasomes is associated with autophagy and that they interact with each other [186, 187]. Overactivated NLRP3 inflammasomes in microglia aggravate PD pathology, and accelerate neuronal death and progression of neurodegenerative diseases [95]. Autophagy activation in microglia decreases expression of NLRP3 protein and inactivates NLRP3 inflammasomes. Drug-stimulated microglial autophagy reduces NLRP3 inflammasome activation induced by LPS, which can be blocked by ATG5 or knockout [188]. Microglial autophagy in clearance of NLRP3 inflammasomes represent a way in which autophagy regulates inflammation in microglia [94]. Knockout of ATG5 in BV2 cells or primary microglia leads to autophagic disorders and increases NLRP3 levels [189]. Ketamine, an antidepressant, induces autophagy of primary microglia cultured from prefrontal cortex and hippocampus. Autophagy induced by ketamine suppresses the activation of NLRP3 inflammasomes in microglia induced by LPS and ATP, and this effect is blocked by autophagy inhibitor bafilomycin A1 (BafA1) [190].
We summarized the recent studies on autophagy and NLRP3 inflammasomes in microglia in different disease models (Table 1), to prove that promoting autophagy is an important way to inhibit NLRP3 inflammasomes.
Interplay of autophagy and inflammation in microglia of PD
α-Syn mediates the double-edged sword role of microglia in PD pathology. In the early stage, α-syn promoted activation of microglia, which degraded α-syn through phagocytosis and autophagy. However, during disease progression, α-syn oligomers or fibrils aggregated from α-syn monomers in the extracellular matrix impair the autophagy and phagocytosis of microglia, induce M1 microglia and NLRP3 inflammasome activation, and release of inflammatory factors that promote M1 microglia change from M2 phenotype. Together with some pathogenic genes of PD, a positive feedback is created to aggravate the accumulation of α-syn in PD pathology and progression of PD. In the following section, we review the interaction between autophagy and inflammation in microglia.
Microglial autophagy in inflammation inhibition
Autophagy inhibition of chronic neuroinflammation may be related to the pathogenesis of PD and AD, while in cerebral ischemia, brain injury and other diseases that trigger acute neuroinflammation, the enhancement of autophagy may aggravate disease progression [194, 212, 213]. In the acute phase of intracerebral hemorrhage with ventricular extension, activated NLRP3 in microglia promotes the extracellular release of IL-1β, which leads to excess autophagy and neuronal apoptosis in vivo and in vitro through the AMPK/Beclin-1 pathway [194]. These different results from PD may depend on the time and degree of autophagic activation, so the correct fine-tuning of time and intensity is vital for clinical application.
Exploring the relationship between autophagy and inflammation in microglia is helpful to clarify the pathogenesis of PD. Activated microglia have been detected in the brains of patients with PD [28], as well as in a variety of PD animal models [214, 215]. Similarly, low autophagic levels of microglia have been detected in different PD models, accompanied by increased inflammation induced by microglia [216]. Several studies have also proved that autophagy in microglia appears to be reciprocally regulated with inflammation. For example, when autophagy of microglia is halted, α-syn induces an increase in NLRP3 inflammasome and IL-1β, suggesting that autophagic disorders of microglia promote α-syn-induced inflammation [217]. TLRs are directly related to inflammatory signals in microglia [218], and their activation arrests microglial autophagy. Neuroinflammation derived from activated microglia induces neuronal TLR2/4-p38/JNK activation to disrupt autophagy and aggravate accumulation of α-syn [106]. p38 inhibits autophagy but increases microglial inflammation by regulating ULK1 phosphorylation [219]. The use of rapamycin, an autophagy agonist, also results in microglial autophagy and inflammation [220]. In addition, transient receptor potential vanilloid 1 is associated with microglia autophagy in PD, and its activation upregulates expression of ATG5 in microglia, and activates Ca2+/CaMKK2/AMPK/mTOR signaling pathways to facilitate autophagy by elevating AMP-activated kinase (AMPK) protein phosphorylation [212]. AMPK involvement in the autophagy pathway modulation of microglial inflammation has also been demonstrated [221].
Autophagy activation in microglia can improve neuroinflammation, which is a potential therapeutic target of PD.
α-Syn and NLRP3 degradation by microglial autophagy in inhibition of inflammation
α-Syn in PD regulates the production of NLRP3 inflammasomes and impairs the ability of microglial autophagy and phagocytosis. Therefore, targeting autophagy in microglia to promote the degradation of α-syn and NLRP3 in PD is a promising therapeutic target.
The increased level of autophagy promotes the phagocytic ability of BV2 cells and degrades exogenous α-syn [163], suggesting that microglia degrade α-syn by autophagy promotion, thus reducing neuroinflammation caused by microglial activation. Some drugs aimed at this mechanism have been developed and were effective in PD models. PD180970, a small molecular inhibitor of tyrosine kinase, induces autophagy in a mammalian target of rapamycin (mTOR)-dependent manner and inhibits the release of IL-6 and monocyte chemoattractant protein (MCP)-1 by reducing TLR4-mediated NF-κB activation to improve α-syn-mediated neurotoxicity. [222]. Caffeic acid activates JNK/Bcl-2-mediated autophagy, promotes the degradation of aggregated α-syn, and shows neuroprotective activity in PD [223].
In PD, NLRP3 in microglia is mainly activated by α-syn, and its degradation is also related to autophagy. Inhibition of autophagy aggravates neuroinflammation and neurodegeneration mediated by NLRP3 inflammasomes [224]. Small molecule kaempferol induces selective autophagy of NLRP3 protein degradation by ubiquitin to inactivate NLRP3 inflammasomes, showing a significant protective effect in various murine PD models, reducing the loss of TH [188]. MCC950 (NLRP3 inhibitor) can impair mTOR-mediated autophagy and increase degradation of α-syn [225].
Activation of NLRP3 inflammasomes and neuroinflammation induced by α-syn accumulation can cause CNS dysfunction, and autophagy impairment aggravates neuroinflammation and accumulation of α-syn, leading to a vicious cycle. Autophagy of microglia directly promotes the degradation of α-syn and NLRP3, thus alleviating PD pathology and maintaining the internal environment of the CNS.
Application of microglial autophagy-targeting drugs
At present, PD treatment can only alleviate the symptoms but cannot delay progression. Therefore, there is an urgent need for developing new method to treat PD, and combined with what we have described above, we believe that targeting microglia autophagy is a potential therapeutic target. We summarize the current drugs targeting microglial autophagy in PD. These drugs can enhance microglial autophagy and reduce neuroinflammation in PD (Table 2), showing the potential capability to remove α-syn for the treatment of PD, but they still need to be verified experimentally.
Targeted microglial autophagy also shows latent therapeutic effects in other disease models. Metformin promotes the phenotypic transformation of microglia from M1 to M2, enhances the fusion of autophagosomes and lysosomes by inhibiting the AMPK–mTOR signaling pathway, and significantly slows down the inflammatory response in the spinal cord [226]. PNU-282987 is an agonist of α-7nAChR, which promotes the polarization of microglia into anti-inflammatory subtype (CD206) and the level of autophagy in microglia [227]. AMPK agonist AICAR, resveratrol alleviates inflammation in microglia by potentiating autophagy [228]. Pien-Tze-Huang, a Chinese patent formula, regulates AMPK/mTOR/ULK-related pathways to increase autophagy of microglia and inhibit NLRP3 activity [202].
In summary, we believe that the mediation of autophagy in microglia can inhibit PD neuroinflammation and clear α-syn, which is a potential target for the treatment of PD.
Concluding remarks
The risk factors associated with PD are usually accompanied by an increase in inflammatory response, such as α-syn aggregation and mutations in some related genes. At the same time, studies have reported defective autophagy in PD, which is related to the accumulation of α-syn. The accumulation of α-syn can directly damage microglial autophagy and phagocytosis, which compromises clearance of α-syn. Loss or mutation of DJ-1, GBA or LRRK2 also damages microglial autophagy and phagocytosis, which aggravates progression of PD. In summary, under physiological conditions, endogenous α-syn maintains the phagocytosis of microglia, and microglial phagocytosis of apoptotic neurons and their release of exogenous α-syn maintain the stability of the internal environment. Once environmental factors are induced or related genetic mutations occur, accumulated α-syn impairs the autophagy and phagocytosis of microglia and promotes the transformation of microglia to M1 phenotype, which represents stronger neuroinflammation. In this process, M1 microglia with phagocytized α-syn promote the release of inflammatory cytokines. The increase of inflammatory cytokines further promotes the transformation of microglia from M2 to M1 phenotype. Aging also aggravates the transformation of microglia to proinflammatory phenotype, and genetic mutations further aggravate the above-mentioned pathological process.
Inhibition of neuroinflammation in PD is an effective way to delay progression of PD. In recent years, some researchers have tried to verify the role of autophagy-targeting microglial inflammation in PD. In most studies, activation of autophagy reduced the activation of microglia, manifested by a decrease in inflammatory cytokines and increase in M2 microglia, which to some extent represents enhancement of microglial phagocytosis. The effects of autophagy and its inducers on the inhibition of neuroinflammation are focused on the degradation of PD-related pathogenic factors. On the one hand, microglial autophagy is involved in the degradation of NLRP3 inflammasomes, which is an important step in the relief of neuroinflammation. On the other hand, microglial autophagy is involved in the degradation of α-syn, which functions in microglial and NLRP3 activation. Therefore, these protective effects may be due to the phagocytosis and degradation of α-syn by microglia.
Autophagy and phagocytosis of microglia work together in the degradation of α-syn. For example, LC3 and Beclin1, which are needed for phagocytosis, are also major proteins in autophagy. Some receptors dependent on phagocytosis, such as TLR4 and TREM2, are involved in microglial autophagy. However, the direct relationship between autophagy and phagocytosis has not been well studied, and the degradation of α-syn in microglia in PD is generally described as autophagy. Further exploration of the connection and difference between the two would be helpful to fully understand the pathology of PD.
Availability of data and materials
Not applicable.
References
Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397(10291):2284–303.
Dorsey ER, Bloem BR. The Parkinson pandemic-a call to action. JAMA Neurol. 2018;75(1):9–10.
Tolosa E, Garrido A, Scholz SW, Poewe W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021;20(5):385–97.
Zhu B, Yin D, Zhao H, Zhang L. The immunology of Parkinson’s disease. Semin Immunopathol. 2022;44(5):659–72.
Fakih W, Zeitoun R, AlZaim I, Eid AH, Kobeissy F, Abd-Elrahman KS, et al. Early metabolic impairment as a contributor to neurodegenerative disease: Mechanisms and potential pharmacological intervention. Obesity (Silver Spring). 2022;30(5):982–93.
Chavarria C, Ivagnes R, Souza JM. Extracellular alpha-synuclein: mechanisms for glial cell internalization and activation. Biomolecules. 2022;12(5):655.
Yang Y, Shi Y, Schweighauser M, Zhang X, Kotecha A, Murzin AG, et al. Structures of alpha-synuclein filaments from human brains with Lewy pathology. Nature. 2022;610(7933):791–5.
Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14(1):38–48.
Bras IC, Outeiro TF. Alpha-synuclein: mechanisms of release and pathology progression in synucleinopathies. Cells. 2021;10(2):375.
Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet. 2014;46(9):989–93.
Chiti F, Dobson CM. Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu Rev Biochem. 2017;86:27–68.
Samuel F, Flavin WP, Iqbal S, Pacelli C, Sri Renganathan SD, Trudeau LE, et al. Effects of serine 129 phosphorylation on alpha-synuclein aggregation, membrane association, and internalization. J Biol Chem. 2016;291(9):4374–85.
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841.
Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364(9440):1167–9.
Bridi JC, Hirth F. Mechanisms of alpha-Synuclein induced synaptopathy in Parkinson’s disease. Front Neurosci. 2018;12:80.
Vidović M, Rikalovic MG. Alpha-synuclein aggregation pathway in Parkinson’s disease: current status and novel therapeutic approaches. Cells. 2022;11(11):1732.
Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, et al. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27(34):9220–32.
Angelova PR, Choi ML, Berezhnov AV, Horrocks MH, Hughes CD, De S, et al. Alpha synuclein aggregation drives ferroptosis: an interplay of iron, calcium and lipid peroxidation. Cell Death Differ. 2020;27(10):2781–96.
Bigi A, Cascella R, Chiti F, Cecchi C. Amyloid fibrils act as a reservoir of soluble oligomers, the main culprits in protein deposition diseases. BioEssays News Rev Mol Cell Dev Biol. 2022;44(11): e2200086.
Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, et al. alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522(7556):340–4.
Serratos IN, Hernandez-Perez E, Campos C, Aschner M, Santamaria A. An update on the critical role of alpha-synuclein in Parkinson’s disease and other synucleinopathies: from tissue to cellular and molecular levels. Mol Neurobiol. 2022;59(1):620–42.
Killinger BA, Kordower JH. Spreading of alpha-synuclein - relevant or epiphenomenon? J Neurochem. 2019;150(5):605–11.
Andoh M, Koyama R. Microglia regulate synaptic development and plasticity. Dev Neurobiol. 2021;81(5):568–90.
Mosser CA, Baptista S, Arnoux I, Audinat E. Microglia in CNS development: shaping the brain for the future. Prog Neurobiol. 2017;149–150:1–20.
Wendimu MY, Hooks SB. Microglia phenotypes in aging and neurodegenerative diseases. Cells. 2022;11(13):2091.
Kouli A, Williams-Gray CH. Age-related adaptive immune changes in Parkinson’s disease. J Parkinsons Dis. 2022;12(s1):S93–104.
Russo T, Riessland M. Age-related midbrain inflammation and senescence in Parkinson’s disease. Front Aging Neurosci. 2022;14: 917797.
McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38(8):1285–91.
Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119(1):182–92.
Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson’s disease. Mov Disord. 2011;26(6):1049–55.
Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med. 2006;38(4):333–47.
Lenz KM, Nelson LH. Microglia and beyond: innate immune cells as regulators of brain development and behavioral function. Front Immunol. 2018;9:698.
Doens D, Fernandez PL. Microglia receptors and their implications in the response to amyloid beta for Alzheimer’s disease pathogenesis. J Neuroinflamm. 2014;11:48.
Becher B, Prat A, Antel JP. Brain-immune connection: immuno-regulatory properties of CNS-resident cells. Glia. 2000;29(4):293–304.
Broggi A, Granucci F. Microbe- and danger-induced inflammation. Mol Immunol. 2015;63(2):127–33.
Halliday GM, Stevens CH. Glia: initiators and progressors of pathology in Parkinson’s disease. Mov Disord. 2011;26(1):6–17.
Guo S, Wang H, Yin Y. Microglia polarization from M1 to M2 in neurodegenerative diseases. Front Aging Neurosci. 2022;14: 815347.
Liu CY, Wang X, Liu C, Zhang HL. Pharmacological targeting of microglial activation: new therapeutic approach. Front Cell Neurosci. 2019;13:514.
Shen H, Pei H, Zhai L, Guan Q, Wang G. Salvianolic acid C improves cerebral ischemia reperfusion injury through suppressing microglial cell M1 polarization and promoting cerebral angiogenesis. Int Immunopharmacol. 2022;110: 109021.
Lee JH, Han JH, Woo JH, Jou I. 25-Hydroxycholesterol suppress IFN-gamma-induced inflammation in microglia by disrupting lipid raft formation and caveolin-mediated signaling endosomes. Free Radic Biol Med. 2022;179:252–65.
Liu Q, Zhang J, Xiao C, Su D, Li L, Yang C, et al. Akebia saponin D protects hippocampal neurogenesis from microglia-mediated inflammation and ameliorates depressive-like behaviors and cognitive impairment in mice through the PI3K-Akt pathway. Front Pharmacol. 2022;13: 927419.
Li S, Wernersbach I, Harms GS, Schafer MKE. Microglia subtypes show substrate- and time-dependent phagocytosis preferences and phenotype plasticity. Front Immunol. 2022;13: 945485.
Dang R, Yang M, Cui C, Wang C, Zhang W, Geng C, et al. Activation of angiotensin-converting enzyme 2/angiotensin (1–7)/mas receptor axis triggers autophagy and suppresses microglia proinflammatory polarization via forkhead box class O1 signaling. Aging Cell. 2021;20(10): e13480.
Bell-Temin H, Culver-Cochran AE, Chaput D, Carlson CM, Kuehl M, Burkhardt BR, et al. Novel molecular insights into classical and alternative activation states of microglia as revealed by stable isotope labeling by amino acids in cell culture (SILAC)-based proteomics. Mol Cell Proteomics. 2015;14(12):3173–84.
Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176(1):287–92.
Subramaniam SR, Federoff HJ. Targeting microglial activation states as a therapeutic avenue in Parkinson’s disease. Front Aging Neurosci. 2017;9:176.
Joers V, Tansey MG, Mulas G, Carta AR. Microglial phenotypes in Parkinson’s disease and animal models of the disease. Prog Neurobiol. 2017;155:57–75.
Franco R, Fernandez-Suarez D. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol. 2015;131:65–86.
Knott C, Stern G, Wilkin GP. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci. 2000;16(6):724–39.
Zhu Y, Tang X, Cheng Z, Dong Q, Ruan G. The anti-inflammatory effect of preventive intervention with ketogenic diet mediated by the histone acetylation of mGluR5 promotor region in rat Parkinson’s disease model: a dual-tracer PET study. Parkinson’s Dis. 2022;2022:3506213.
Yang QY, Li XW, Yang R, Qin TY, Long H, Zhang SB, et al. Effects of intraperitoneal injection of lipopolysaccharide-induced peripheral inflammation on dopamine neuron damage in rat midbrain. CNS Neurosci Ther. 2022;28(10):1624–36.
Patel M, Singh S. Apigenin attenuates functional and structural alterations via targeting NF-kB/Nrf2 signaling pathway in LPS-induced parkinsonism in experimental rats: Apigenin attenuates LPS-induced Parkinsonism in experimental rats. Neurotox Res. 2022;40(4):941–60.
Cankara FN, Kus MS, Gunaydin C, Safak S, Bilge SS, Ozmen O, et al. The beneficial effect of salubrinal on neuroinflammation and neuronal loss in intranigral LPS-induced hemi-Parkinson disease model in rats. Immunopharmacol Immunotoxicol. 2022;44(2):168–77.
Qian L, Li JZ, Sun X, Chen JB, Dai Y, Huang QX, et al. Safinamide prevents lipopolysaccharide (LPS)-induced inflammation in macrophages by suppressing TLR4/NF-kappaB signaling. Int Immunopharmacol. 2021;96: 107712.
Muhammad T, Ikram M, Ullah R, Rehman SU, Kim MO. Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-kappaB signaling. Nutrients. 2019;11(3):648.
Zhang FX, Xu RS. Juglanin ameliorates LPS-induced neuroinflammation in animal models of Parkinson’s disease and cell culture via inactivating TLR4/NF-kappaB pathway. Biomed Pharmacother. 2018;97:1011–9.
Dong AQ, Yang YP, Jiang SM, Yao XY, Qi D, Mao CJ, et al. Pramipexole inhibits astrocytic NLRP3 inflammasome activation via Drd3-dependent autophagy in a mouse model of Parkinson’s disease. Acta Pharmacol Sin. 2022. https://doi.org/10.1038/s41401-022-00951-1.
Karikari AA, McFleder RL, Ribechini E, Blum R, Bruttel V, Knorr S, et al. Neurodegeneration by alpha-synuclein-specific T cells in AAV-A53T-alpha-synuclein Parkinson’s disease mice. Brain Behav Immun. 2022;101:194–210.
He D, Hu G, Zhou A, Liu Y, Huang B, Su Y, et al. Echinocystic acid inhibits inflammation and exerts neuroprotective effects in MPTP-induced Parkinson’s disease model mice. Front Pharmacol. 2021;12: 787771.
Grotemeyer A, McFleder RL, Wu J, Wischhusen J, Ip CW. Neuroinflammation in Parkinson’s disease - putative pathomechanisms and targets for disease-modification. Front Immunol. 2022;13: 878771.
Cinar E, Tel BC, Sahin G. Neuroinflammation in Parkinson’s disease and its treatment opportunities. Balkan Med J. 2022;39(5):318–33.
Marotta NP, Ara J, Uemura N, Lougee MG, Meymand ES, Zhang B, et al. Alpha-synuclein from patient Lewy bodies exhibits distinct pathological activity that can be propagated in vitro. Acta Neuropathol Commun. 2021;9(1):188.
Guo YJ, Xiong H, Chen K, Zou JJ, Lei P. Brain regions susceptible to alpha-synuclein spreading. Mol Psychiatry. 2022;27(1):758–70.
Li Y, Xia Y, Yin S, Wan F, Hu J, Kou L, et al. Targeting microglial alpha-Synuclein/TLRs/NF-kappaB/NLRP3 inflammasome axis in Parkinson’s disease. Front Immunol. 2021;12: 719807.
Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–68.
Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflamm. 2005;2:14.
Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci. 2009;29(11):3365–73.
Majbour NK, Vaikath NN, Eusebi P, Chiasserini D, Ardah M, Varghese S, et al. Longitudinal changes in CSF alpha-synuclein species reflect Parkinson’s disease progression. Mov Disord. 2016;31(10):1535–42.
Tokuda T, Qureshi MM, Ardah MT, Varghese S, Shehab SA, Kasai T, et al. Detection of elevated levels of alpha-synuclein oligomers in CSF from patients with Parkinson disease. Neurology. 2010;75(20):1766–72.
Ingelsson M. Alpha-Synuclein oligomers-neurotoxic molecules in Parkinson’s disease and other Lewy body disorders. Front Neurosci. 2016;10:408.
Wang S, Chu C-H, Stewart T, Ginghina C, Wang Y, Nie H, et al. α-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation. Proc Natl Acad Sci U S A. 2015;112(15):E1926–35.
Kouli A, Horne CB, Williams-Gray CH. Toll-like receptors and their therapeutic potential in Parkinson’s disease and alpha-synucleinopathies. Brain Behav Immun. 2019;81:41–51.
Daniele SG, Beraud D, Davenport C, Cheng K, Yin H, Maguire-Zeiss KA. Activation of MyD88-dependent TLR1/2 signaling by misfolded alpha-synuclein, a protein linked to neurodegenerative disorders. Sci Signal. 2015;8(376):ra45.
Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, et al. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4:1562.
Hughes CD, Choi ML, Ryten M, Hopkins L, Drews A, Botia JA, et al. Picomolar concentrations of oligomeric alpha-synuclein sensitizes TLR4 to play an initiating role in Parkinson’s disease pathogenesis. Acta Neuropathol. 2019;137(1):103–20.
Jiang T, Hoekstra J, Heng X, Kang W, Ding J, Liu J, et al. P2X7 receptor is critical in alpha-synuclein–mediated microglial NADPH oxidase activation. Neurobiol Aging. 2015;36(7):2304–18.
Klegeris A, McGeer PL. Complement activation by islet amyloid polypeptide (IAPP) and alpha-synuclein 112. Biochem Biophys Res Commun. 2007;357(4):1096–9.
Christensen DP, Ejlerskov P, Rasmussen I, Vilhardt F. Reciprocal signals between microglia and neurons regulate alpha-synuclein secretion by exophagy through a neuronal cJUN-N-terminal kinase-signaling axis. J Neuroinflamm. 2016;13(1):59.
Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, et al. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006;21(2):404–12.
Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, et al. T cells from patients with Parkinson’s disease recognize alpha-synuclein peptides. Nature. 2017;546(7660):656–61.
Bido S, Muggeo S, Massimino L, Marzi MJ, Giannelli SG, Melacini E, et al. Microglia-specific overexpression of alpha-synuclein leads to severe dopaminergic neurodegeneration by phagocytic exhaustion and oxidative toxicity. Nat Commun. 2021;12(1):6237.
Pike AF, Varanita T, Herrebout MAC, Plug BC, Kole J, Musters RJP, et al. α-Synuclein evokes NLRP3 inflammasome-mediated IL-1β secretion from primary human microglia. Glia. 2021;69(6):1413–28.
Decressac M, Mattsson B, Bjorklund A. Comparison of the behavioural and histological characteristics of the 6-OHDA and alpha-synuclein rat models of Parkinson’s disease. Exp Neurol. 2012;235(1):306–15.
Iba M, McDevitt RA, Kim C, Roy R, Sarantopoulou D, Tommer E, et al. Aging exacerbates the brain inflammatory micro-environment contributing to α-synuclein pathology and functional deficits in a mouse model of DLB/PD. Mol Neurodegener. 2022;17(1):60.
Lashgari NA, Roudsari NM, Momtaz S, Sathyapalan T, Abdolghaffari AH, Sahebkar A. The involvement of JAK/STAT signaling pathway in the treatment of Parkinson’s disease. J Neuroimmunol. 2021;361: 577758.
Dutta D, Jana M, Majumder M, Mondal S, Roy A, Pahan K. Selective targeting of the TLR2/MyD88/NF-kappaB pathway reduces alpha-synuclein spreading in vitro and in vivo. Nat Commun. 2021;12(1):5382.
Qin H, Buckley JA, Li X, Liu Y, Fox TH 3rd, Meares GP, et al. Inhibition of the JAK/STAT pathway protects against alpha-Synuclein-induced neuroinflammation and dopaminergic neurodegeneration. J Neurosci. 2016;36(18):5144–59.
Chiarini A, Armato U, Gui L, Dal Pra I. “Other than NLRP3” inflammasomes: multiple roles in brain disease. Neuroscientist. 2022. https://doi.org/10.1177/10738584221106114.
Anderson FL, Biggs KE, Rankin BE, Havrda MC. NLRP3 inflammasome in neurodegenerative disease. Transl Res. 2022. https://doi.org/10.1016/j.trsl.2022.08.006.
Nguyen LTN, Nguyen HD, Kim YJ, Nguyen TT, Lai TT, Lee YK, et al. Role of NLRP3 inflammasome in Parkinson’s disease and therapeutic considerations. J Parkinsons Dis. 2022;12(7):2117–33.
von Herrmann KM, Salas LA, Martinez EM, Young AL, Howard JM, Feldman MS, et al. NLRP3 expression in mesencephalic neurons and characterization of a rare NLRP3 polymorphism associated with decreased risk of Parkinson’s disease. NPJ Parkinsons Dis. 2018;4:24.
Liu HD, Li W, Chen ZR, Hu YC, Zhang DD, Shen W, et al. Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem Res. 2013;38(10):2072–83.
Yang F, Wang Z, Wei X, Han H, Meng X, Zhang Y, et al. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab. 2014;34(4):660–7.
Li Y, Xia Y, Yin S, Wan F, Hu J, Kou L, et al. Targeting microglial α-Synuclein/TLRs/NF-kappaB/NLRP3 inflammasome axis in Parkinson’s disease. Front Immunol. 2021;12: 719807.
Haque ME, Akther M, Jakaria M, Kim IS, Azam S, Choi DK. Targeting the microglial NLRP3 inflammasome and its role in Parkinson’s disease. Mov Disord. 2020;35(1):20–33.
Javed H, Thangavel R, Selvakumar GP, Dubova I, Schwartz N, Ahmed ME, et al. NLRP3 inflammasome and glia maturation factor coordinately regulate neuroinflammation and neuronal loss in MPTP mouse model of Parkinson’s disease. Int Immunopharmacol. 2020;83: 106441.
Lee HJ, Suk JE, Bae EJ, Lee SJ. Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem Biophys Res Commun. 2008;372(3):423–8.
Scheiblich H, Bousset L, Schwartz S, Griep A, Latz E, Melki R, et al. Microglial NLRP3 inflammasome activation upon TLR2 and TLR5 ligation by distinct alpha-Synuclein assemblies. J Immunol. 2021;207(8):2143–54.
Pike AF, Longhena F, Faustini G, van Eik JM, Gombert I, Herrebout MAC, et al. Dopamine signaling modulates microglial NLRP3 inflammasome activation: implications for Parkinson’s disease. J Neuroinflamm. 2022;19(1):50.
Zhou Y, Lu M, Du RH, Qiao C, Jiang CY, Zhang KZ, et al. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol Neurodegener. 2016;11:28.
Huang S, Chen Z, Fan B, Chen Y, Zhou L, Jiang B, et al. A selective NLRP3 inflammasome inhibitor attenuates behavioral deficits and neuroinflammation in a mouse model of Parkinson’s disease. J Neuroimmunol. 2021;354: 577543.
Gordon R, Albornoz EA, Christie DC, Langley MR, Kumar V, Mantovani S, et al. Inflammasome inhibition prevents alpha-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med. 2018. https://doi.org/10.1126/scitranslmed.aah4066.
Zheng R, Ruan Y, Yan Y, Lin Z, Xue N, Yan Y, et al. Melatonin attenuates neuroinflammation by down-regulating NLRP3 inflammasome via a SIRT1-dependent pathway in MPTP-induced models of Parkinson’s disease. J Inflamm Res. 2021;14:3063–75.
Qiu X, Wang Q, Hou L, Zhang C, Wang Q, Zhao X. Inhibition of NLRP3 inflammasome by glibenclamide attenuated dopaminergic neurodegeneration and motor deficits in paraquat and maneb-induced mouse Parkinson’s disease model. Toxicol Lett. 2021;349:1–11.
Ahmed S, Kwatra M, Ranjan Panda S, Murty USN, Naidu VGM. Andrographolide suppresses NLRP3 inflammasome activation in microglia through induction of parkin-mediated mitophagy in in-vitro and in-vivo models of Parkinson disease. Brain Behav Immun. 2021;91:142–58.
Chung LY, Lin YT, Liu C, Tai YC, Lin HY, Lin CH, et al. Neuroinflammation upregulated neuronal toll-like receptors 2 and 4 to drive synucleinopathy in neurodegeneration. Front Pharmacol. 2022;13: 845930.
Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30(20):6838–51.
Jang A, Lee HJ, Suk JE, Jung JW, Kim KP, Lee SJ. Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem. 2010;113(5):1263–74.
Pacheco C, Aguayo LG, Opazo C. An extracellular mechanism that can explain the neurotoxic effects of alpha-synuclein aggregates in the brain. Front Physiol. 2012;3:297.
George S, Rey NL, Tyson T, Esquibel C, Meyerdirk L, Schulz E, et al. Microglia affect alpha-synuclein cell-to-cell transfer in a mouse model of Parkinson’s disease. Mol Neurodegener. 2019;14(1):34.
Olanow CW, Savolainen M, Chu Y, Halliday GM, Kordower JH. Temporal evolution of microglia and alpha-synuclein accumulation following foetal grafting in Parkinson’s disease. Brain. 2019;142(6):1690–700.
Niu H, Wang Q, Zhao W, Liu J, Wang D, Muhammad B, et al. IL-1beta/IL-1R1 signaling induced by intranasal lipopolysaccharide infusion regulates alpha-Synuclein pathology in the olfactory bulb, substantia nigra and striatum. Brain Pathol. 2020;30(6):1102–18.
Xia Y, Zhang G, Han C, Ma K, Guo X, Wan F, et al. Microglia as modulators of exosomal alpha-synuclein transmission. Cell Death Dis. 2019;10(3):174.
Tran J, Anastacio H, Bardy C. Genetic predispositions of Parkinson’s disease revealed in patient-derived brain cells. NPJ Parkinsons Dis. 2020;6:8.
Blauwendraat C, Nalls MA, Singleton AB. The genetic architecture of Parkinson’s disease. Lancet Neurol. 2020;19(2):170–8.
Nalls MA, Blauwendraat C, Vallerga CL, Heilbron K, Bandres-Ciga S, Chang D, et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 2019;18(12):1091–102.
Nishioka K, Imai Y, Yoshino H, Li Y, Funayama M, Hattori N. Clinical manifestations and molecular backgrounds of Parkinson’s disease regarding genes identified from familial and population studies. Front Neurol. 2022;13: 764917.
Dzamko N, Geczy CL, Halliday GM. Inflammation is genetically implicated in Parkinson’s disease. Neuroscience. 2015;302:89–102.
Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–8.
Dionisio PEA, Oliveira SR, Amaral J, Rodrigues CMP. Loss of microglial Parkin inhibits necroptosis and contributes to neuroinflammation. Mol Neurobiol. 2019;56(4):2990–3004.
Abou-Sleiman PM, Healy DG, Quinn N, Lees AJ, Wood NW. The role of pathogenic DJ-1 mutations in Parkinson’s disease. Ann Neurol. 2003;54(3):283–6.
Trudler D, Weinreb O, Mandel SA, Youdim MB, Frenkel D. DJ-1 deficiency triggers microglia sensitivity to dopamine toward a pro-inflammatory phenotype that is attenuated by rasagiline. J Neurochem. 2014;129(3):434–47.
Ho DH, Nam D, Seo M, Park SW, Seol W, Son I. LRRK2 inhibition mitigates the neuroinflammation caused by TLR2-specific alpha-synuclein and alleviates neuroinflammation-derived dopaminergic neuronal loss. Cells. 2022;11(5):861.
Ho DH, Lee H, Son I, Seol W. G2019s LRRK2 promotes mitochondrial fission and increases TNFalpha-mediated neuroinflammation responses. Anim Cells Syst (Seoul). 2019;23(2):106–11.
Davies DS, Ma J, Jegathees T, Goldsbury C. Microglia show altered morphology and reduced arborization in human brain during aging and Alzheimer’s disease. Brain Pathol. 2017;27(6):795–808.
Spittau B. Aging microglia-phenotypes, functions and implications for age-related neurodegenerative diseases. Front Aging Neurosci. 2017;9:194.
Sheng JG, Mrak RE, Griffin WS. Enlarged and phagocytic, but not primed, interleukin-1 alpha-immunoreactive microglia increase with age in normal human brain. Acta Neuropathol. 1998;95(3):229–34.
Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2013;39(1):19–34.
Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55(4):412–24.
Wang G, Zhou Y, Wang Y, Li D, Liu J, Zhang F. Age-associated dopaminergic neuron loss and midbrain glia cell phenotypic polarization. Neuroscience. 2019;415:89–96.
Conde JR, Streit WJ. Microglia in the aging brain. J Neuropathol Exp Neurol. 2006;65(3):199–203.
Klionsky DJ. The molecular machinery of autophagy: unanswered questions. J Cell Sci. 2005;118(Pt 1):7–18.
Kesidou E, Lagoudaki R, Touloumi O, Poulatsidou KN, Simeonidou C. Autophagy and neurodegenerative disorders. Neural Regen Res. 2013;8(24):2275–83.
Xilouri M, Brekk OR, Stefanis L. alpha-Synuclein and protein degradation systems: a reciprocal relationship. Mol Neurobiol. 2013;47(2):537–51.
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441(7095):880–4.
Friedman LG, Lachenmayer ML, Wang J, He L, Poulose SM, Komatsu M, et al. Disrupted autophagy leads to dopaminergic axon and dendrite degeneration and promotes presynaptic accumulation of alpha-synuclein and LRRK2 in the brain. J Neurosci. 2012;32(22):7585–93.
Picca A, Guerra F, Calvani R, Romano R, Coelho-Junior HJ, Bucci C, et al. Mitochondrial dysfunction, protein misfolding and neuroinflammation in Parkinson’s disease: roads to biomarker discovery. Biomolecules. 2021;11(10):1508.
Lee HJ, Cho ED, Lee KW, Kim JH, Cho SG, Lee SJ. Autophagic failure promotes the exocytosis and intercellular transfer of alpha-synuclein. Exp Mol Med. 2013;45: e22.
Tu HY, Yuan BS, Hou XO, Zhang XJ, Pei CS, Ma YT, et al. alpha-synuclein suppresses microglial autophagy and promotes neurodegeneration in a mouse model of Parkinson’s disease. Aging Cell. 2021;20(12): e13522.
Sepulveda D, Cisternas-Olmedo M, Arcos J, Nassif M, Vidal RL. Contribution of autophagy-lysosomal pathway in the exosomal secretion of alpha-synuclein and its impact in the progression of Parkinson’s disease. Front Mol Neurosci. 2022;15: 805087.
Schmidt MF, Gan ZY, Komander D, Dewson G. Ubiquitin signalling in neurodegeneration: mechanisms and therapeutic opportunities. Cell Death Differ. 2021;28(2):570–90.
Parekh P, Sharma N, Gadepalli A, Shahane A, Sharma M, Khairnar A. A cleaning crew: the pursuit of autophagy in Parkinson’s disease. ACS Chem Neurosci. 2019;10(9):3914–26.
Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278(27):25009–13.
Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295(5556):865–8.
Wu JZ, Ardah M, Haikal C, Svanbergsson A, Diepenbroek M, Vaikath NN, et al. Dihydromyricetin and Salvianolic acid B inhibit alpha-synuclein aggregation and enhance chaperone-mediated autophagy. Transl Neurodegener. 2019;8:18.
Fields CR, Bengoa-Vergniory N, Wade-Martins R. Targeting alpha-Synuclein as a therapy for Parkinson’s disease. Front Mol Neurosci. 2019;12:299.
Choi I, Zhang Y, Seegobin SP, Pruvost M, Wang Q, Purtell K, et al. Microglia clear neuron-released alpha-synuclein via selective autophagy and prevent neurodegeneration. Nat Commun. 2020;11(1):1386.
Guiney SJ, Adlard PA, Lei P, Mawal CH, Bush AI, Finkelstein DI, et al. Fibrillar alpha-synuclein toxicity depends on functional lysosomes. J Biol Chem. 2020;295(51):17497–513.
Zhou T, Lin D, Chen Y, Peng S, Jing X, Lei M, et al. alpha-synuclein accumulation in SH-SY5Y cell impairs autophagy in microglia by exosomes overloading miR-19a-3p. Epigenomics. 2019;11(15):1661–77.
Tu H-Y, Yuan B-S, Hou X-O, Zhang X-J, Pei C-S, Ma Y-T, et al. α-synuclein suppresses microglial autophagy and promotes neurodegeneration in a mouse model of Parkinson’s disease. Aging Cell. 2021;20(12): e13522.
Minakaki G, Menges S, Kittel A, Emmanouilidou E, Schaeffner I, Barkovits K, et al. Autophagy inhibition promotes SNCA/alpha-synuclein release and transfer via extracellular vesicles with a hybrid autophagosome-exosome-like phenotype. Autophagy. 2018;14(1):98–119.
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885–9.
Munz C. The macroautophagy machinery in MHC restricted antigen presentation. Front Immunol. 2021;12: 628429.
Galloway DA, Phillips AEM, Owen DRJ, Moore CS. Phagocytosis in the brain: homeostasis and disease. Front Immunol. 2019;10:790.
Jung YJ, Chung WS. Phagocytic roles of glial cells in healthy and diseased brains. Biomol Ther (Seoul). 2018;26(4):350–7.
Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25(2):269–78.
Nau R, Ribes S, Djukic M, Eiffert H. Strategies to increase the activity of microglia as efficient protectors of the brain against infections. Front Cell Neurosci. 2014;8:138.
Heidari A, Yazdanpanah N, Rezaei N. The role of toll-like receptors and neuroinflammation in Parkinson’s disease. J Neuroinflamm. 2022;19(1):135.
Arcuri C, Mecca C, Bianchi R, Giambanco I, Donato R. The pathophysiological role of microglia in dynamic surveillance, phagocytosis and structural remodeling of the developing CNS. Front Mol Neurosci. 2017;10:191.
Owlett LD, Karaahmet B, Le L, Belcher EK, Dionisio-Santos D, Olschowka JA, et al. Gas6 induces inflammation and reduces plaque burden but worsens behavior in a sex-dependent manner in the APP/PS1 model of Alzheimer’s disease. J Neuroinflamm. 2022;19(1):38.
Fourgeaud L, Traves PG, Tufail Y, Leal-Bailey H, Lew ED, Burrola PG, et al. TAM receptors regulate multiple features of microglial physiology. Nature. 2016;532(7598):240–4.
Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201(4):647–57.
Janda E, Boi L, Carta AR. Microglial phagocytosis and its regulation: a therapeutic target in Parkinson’s disease? Front Mol Neurosci. 2018;11:144.
Rickman AD, Hilyard A, Heckmann BL. Dying by fire: noncanonical functions of autophagy proteins in neuroinflammation and neurodegeneration. Neural Regen Res. 2022;17(2):246–50.
Martinez J, Malireddi RKS, Lu Q, Cunha LD, Pelletier S, Gingras S, et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat Cell Biol. 2015;17(7):893–906.
Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, et al. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc Natl Acad Sci U S A. 2011;108(42):17396–401.
Wong SW, Sil P, Martinez J. Rubicon: LC3-associated phagocytosis and beyond. FEBS J. 2018;285(8):1379–88.
Mortimer PM, Mc Intyre SA, Thomas DC. Beyond the extra respiration of phagocytosis: NADPH oxidase 2 in adaptive immunity and inflammation. Front Immunol. 2021;12: 733918.
Wolf SA, Boddeke HW, Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol. 2017;79:619–43.
Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4(4): e124.
Zhang Y, Feng S, Nie K, Li Y, Gao Y, Gan R, et al. TREM2 modulates microglia phenotypes in the neuroinflammation of Parkinson’s disease. Biochem Biophys Res Commun. 2018;499(4):797–802.
Zheng ZV, Lyu H, Lam SYE, Lam PK, Poon WS, Wong GKC. The dynamics of microglial polarization reveal the resident neuroinflammatory responses after subarachnoid hemorrhage. Transl Stroke Res. 2020;11(3):433–49.
Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014;11:98.
Ho MS. Microglia in Parkinson’s disease. Adv Exp Med Biol. 2019;1175:335–53.
Austin SA, Floden AM, Murphy EJ, Combs CK. Alpha-synuclein expression modulates microglial activation phenotype. J Neurosci. 2006;26(41):10558–63.
Park JY, Paik SR, Jou I, Park SM. Microglial phagocytosis is enhanced by monomeric alpha-synuclein, not aggregated alpha-synuclein: implications for Parkinson’s disease. Glia. 2008;56(11):1215–23.
Choi YR, Kang SJ, Kim JM, Lee SJ, Jou I, Joe EH, et al. FcgammaRIIB mediates the inhibitory effect of aggregated alpha-synuclein on microglial phagocytosis. Neurobiol Dis. 2015;83:90–9.
Jin J, Shie FS, Liu J, Wang Y, Davis J, Schantz AM, et al. Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated alpha-synuclein. J Neuroinflamm. 2007;4:2.
Hou L, Bao X, Zang C, Yang H, Sun F, Che Y, et al. Integrin CD11b mediates alpha-synuclein-induced activation of NADPH oxidase through a Rho-dependent pathway. Redox Biol. 2018;14:600–8.
Wang S, Chu CH, Stewart T, Ginghina C, Wang Y, Nie H, et al. alpha-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation. Proc Natl Acad Sci U S A. 2015;112(15):E1926–35.
Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y, et al. Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science. 2016. https://doi.org/10.1126/science.aah3374.
Chen K, Martens YA, Meneses A, Ryu DH, Lu W, Raulin AC, et al. LRP1 is a neuronal receptor for alpha-synuclein uptake and spread. Mol Neurodegener. 2022;17(1):57.
Qiu WQ, Pan R, Tang Y, Zhou XG, Wu JM, Yu L, et al. Lychee seed polyphenol inhibits Abeta-induced activation of NLRP3 inflammasome via the LRP1/AMPK mediated autophagy induction. Biomed Pharmacother. 2020;130: 110575.
Nash Y, Schmukler E, Trudler D, Pinkas-Kramarski R, Frenkel D. DJ-1 deficiency impairs autophagy and reduces alpha-synuclein phagocytosis by microglia. J Neurochem. 2017;143(5):584–94.
Schapansky J, Nardozzi JD, LaVoie MJ. The complex relationships between microglia, alpha-synuclein, and LRRK2 in Parkinson’s disease. Neuroscience. 2015;302:74–88.
Netea-Maier RT, Plantinga TS, van de Veerdonk FL, Smit JW, Netea MG. Modulation of inflammation by autophagy: consequences for human disease. Autophagy. 2016;12(2):245–60.
Yong YY, Zhang L, Hu YJ, Wu JM, Yan L, Pan YR, et al. Targeting autophagy regulation in NLRP3 inflammasome-mediated lung inflammation in COVID-19. Clin Immunol. 2022;244: 109093.
Han X, Sun S, Sun Y, Song Q, Zhu J, Song N, et al. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: implications for Parkinson disease. Autophagy. 2019;15(11):1860–81.
Wang HQ, Song KY, Feng JZ, Huang SY, Guo XM, Zhang L, et al. Caffeine inhibits activation of the NLRP3 inflammasome via autophagy to attenuate microglia-mediated neuroinflammation in experimental autoimmune encephalomyelitis. J Mol Neurosci. 2022;72(1):97–112.
Lyu D, Wang F, Zhang M, Yang W, Huang H, Huang Q, et al. Ketamine induces rapid antidepressant effects via the autophagy-NLRP3 inflammasome pathway. Psychopharmacology. 2022;239:3201–12.
Li Y, Lei Z, Ritzel RM, He J, Li H, Choi HMC, et al. Impairment of autophagy after spinal cord injury potentiates neuroinflammation and motor function deficit in mice. Theranostics. 2022;12(12):5364–88.
La Rosa F, Zoia CP, Bazzini C, Bolognini A, Saresella M, Conti E, et al. Modulation of MAPK- and PI3/AKT-dependent autophagy signaling by stavudine (D4T) in PBMC of Alzheimer’s disease patients. Cells. 2022;11(14):2180.
Liang L, Wang H, Hu Y, Bian H, Xiao L, Wang G. Oridonin relieves depressive-like behaviors by inhibiting neuroinflammation and autophagy impairment in rats subjected to chronic unpredictable mild stress. Phytother Res. 2022;36(8):3335–51.
Zhang Z, Guo P, Huang S, Jia Z, Chen T, Liu X, et al. Inhibiting microglia-derived NLRP3 alleviates subependymal edema and cognitive dysfunction in posthemorrhagic hydrocephalus after intracerebral hemorrhage via AMPK/Beclin-1 pathway. Oxid Med Cell Longev. 2022;2022:4177317.
Zhou J, Wang F, Jia L, Chai R, Wang H, Wang X, et al. 2,4-dichlorophenoxyacetic acid induces ROS activation in NLRP3 inflammatory body-induced autophagy disorder in microglia and the protective effect of Lycium barbarum polysaccharide. Environ Toxicol. 2022;37(5):1136–51.
Qiu J, Chen Y, Zhuo J, Zhang L, Liu J, Wang B, et al. Urolithin A promotes mitophagy and suppresses NLRP3 inflammasome activation in lipopolysaccharide-induced BV2 microglial cells and MPTP-induced Parkinson’s disease model. Neuropharmacology. 2022;207: 108963.
Chen J, Mao K, Yu H, Wen Y, She H, Zhang H, et al. p38-TFEB pathways promote microglia activation through inhibiting CMA-mediated NLRP3 degradation in Parkinson’s disease. J Neuroinflamm. 2021;18(1):295.
Zhao X, Sun J, Yuan Y, Lin S, Lin J, Mei X. Zinc promotes microglial autophagy through NLRP3 inflammasome inactivation via XIST/miR-374a-5p axis in spinal cord injury. Neurochem Res. 2022;47(2):372–81.
Ge X, Wang Y, Yu S, Cao X, Chen Y, Cheng Q, et al. Anti-inflammatory activity of a polypeptide fraction from achyranthes bidentate in amyloid beta oligomers induced model of Alzheimer’s disease. Front Pharmacol. 2021;12: 716177.
Yang S, Zhang X, Zhang H, Lin X, Chen X, Zhang Y, et al. Dimethyl itaconate inhibits LPSinduced microglia inflammation and inflammasomemediated pyroptosis via inducing autophagy and regulating the Nrf2/HO1 signaling pathway. Mol Med Rep. 2021. https://doi.org/10.3892/mmr.2021.12311.
Shao S, Xu CB, Chen CJ, Shi GN, Guo QL, Zhou Y, et al. Divanillyl sulfone suppresses NLRP3 inflammasome activation via inducing mitophagy to ameliorate chronic neuropathic pain in mice. J Neuroinflamm. 2021;18(1):142.
Huang Z, Zhou X, Zhang X, Huang L, Sun Y, Cheng Z, et al. Pien-Tze-Huang, a Chinese patent formula, attenuates NLRP3 inflammasome-related neuroinflammation by enhancing autophagy via the AMPK/mTOR/ULK1 signaling pathway. Biomed Pharmacother. 2021;141: 111814.
Zhang L, Xiao F, Zhang J, Wang X, Ying J, Wei G, et al. Dexmedetomidine mitigated NLRP3-mediated neuroinflammation via the ubiquitin-autophagy pathway to improve perioperative neurocognitive disorder in mice. Front Pharmacol. 2021;12: 646265.
Du Y, Lu Z, Yang D, Wang D, Jiang L, Shen Y, et al. MerTK inhibits the activation of the NLRP3 inflammasome after subarachnoid hemorrhage by inducing autophagy. Brain Res. 2021;1766: 147525.
Han B, Jiang W, Cui P, Zheng K, Dang C, Wang J, et al. Microglial PGC-1alpha protects against ischemic brain injury by suppressing neuroinflammation. Genome Med. 2021;13(1):47.
Farre-Alins V, Narros-Fernandez P, Palomino-Antolin A, Decouty-Perez C, Lopez-Rodriguez AB, Parada E, et al. Melatonin reduces NLRP3 inflammasome activation by increasing alpha7 nAChR-mediated autophagic flux. Antioxidants (Basel). 2020;9(12):1299.
Lin JQ, Tian H, Zhao XG, Lin S, Li DY, Liu YY, et al. Zinc provides neuroprotection by regulating NLRP3 inflammasome through autophagy and ubiquitination in a spinal contusion injury model. CNS Neurosci Ther. 2021;27(4):413–25.
Espinosa-Garcia C, Atif F, Yousuf S, Sayeed I, Neigh GN, Stein DG. Progesterone attenuates stress-induced NLRP3 inflammasome activation and enhances autophagy following ischemic brain injury. Int J Mol Sci. 2020;21(11):3740.
Fu C, Zhang X, Lu Y, Wang F, Xu Z, Liu S, et al. Geniposide inhibits NLRP3 inflammasome activation via autophagy in BV-2 microglial cells exposed to oxygen-glucose deprivation/reoxygenation. Int Immunopharmacol. 2020;84: 106547.
You M, Miao Z, Tian J, Hu F. Trans-10-hydroxy-2-decenoic acid protects against LPS-induced neuroinflammation through FOXO1-mediated activation of autophagy. Eur J Nutr. 2020;59(7):2875–92.
Shao BZ, Wei W, Ke P, Xu ZQ, Zhou JX, Liu C. Activating cannabinoid receptor 2 alleviates pathogenesis of experimental autoimmune encephalomyelitis via activation of autophagy and inhibiting NLRP3 inflammasome. CNS Neurosci Ther. 2014;20(12):1021–8.
Yuan J, Liu H, Zhang H, Wang T, Zheng Q, Li Z. Controlled activation of TRPV1 channels on microglia to boost their autophagy for clearance of alpha-synuclein and enhance therapy of Parkinson’s disease. Adv Mater. 2022;34(11): e2108435.
Cheng X, Wei Y, Qian Z, Han L. Autophagy balances neuroinflammation in Alzheimer’s disease. Cell Mol Neurobiol. 2022. https://doi.org/10.1007/s10571-022-01269-6.
Rui WJ, Li S, Yang L, Liu Y, Fan Y, Hu YC, et al. Microglial AIM2 alleviates antiviral-related neuro-inflammation in mouse models of Parkinson’s disease. Glia. 2022;70(12):2409–25.
Mendes-Pinheiro B, Soares-Cunha C, Marote A, Loureiro-Campos E, Campos J, Barata-Antunes S, et al. Unilateral intrastriatal 6-hydroxydopamine lesion in mice: a closer look into non-motor phenotype and glial response. Int J Mol Sci. 2021;22(21):11530.
Yao L, Zhu Z, Wu J, Zhang Y, Zhang H, Sun X, et al. MicroRNA-124 regulates the expression of p62/p38 and promotes autophagy in the inflammatory pathogenesis of Parkinson’s disease. FASEB J. 2019;33(7):8648–65.
Cho MH, Cho K, Kang HJ, Jeon EY, Kim HS, Kwon HJ, et al. Autophagy in microglia degrades extracellular beta-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy. 2014;10(10):1761–75.
Dzamko N, Gysbers A, Perera G, Bahar A, Shankar A, Gao J, et al. Toll-like receptor 2 is increased in neurons in Parkinson’s disease brain and may contribute to alpha-synuclein pathology. Acta Neuropathol. 2017;133(2):303–19.
He Y, She H, Zhang T, Xu H, Cheng L, Yepes M, et al. p38 MAPK inhibits autophagy and promotes microglial inflammatory responses by phosphorylating ULK1. J Cell Biol. 2018;217(1):315–28.
Movahedpour A, Vakili O, Khalifeh M, Mousavi P, Mahmoodzadeh A, Taheri-Anganeh M, et al. Mammalian target of rapamycin (mTOR) signaling pathway and traumatic brain injury: a novel insight into targeted therapy. Cell Biochem Funct. 2022;40(3):232–47.
Yao ZA, Xu L, Jin LM, Wang TS, Wang BX, Li JZ, et al. kappa-Carrageenan oligosaccharides induce microglia autophagy through AMPK/ULK1 pathway to regulate their immune response. Int J Biol Macromol. 2022;194:198–203.
Sn S, Pandurangi J, Murumalla R, Dj V, Garimella L, Acharya A, et al. Small molecule modulator of aggrephagy regulates neuroinflammation to curb pathogenesis of neurodegeneration. EBioMedicine. 2019;50:260–73.
Zhang Y, Wu Q, Zhang L, Wang Q, Yang Z, Liu J, et al. Caffeic acid reduces A53T alpha-synuclein by activating JNK/Bcl-2-mediated autophagy in vitro and improves behaviour and protects dopaminergic neurons in a mouse model of Parkinson’s disease. Pharmacol Res. 2019;150: 104538.
Qin Y, Qiu J, Wang P, Liu J, Zhao Y, Jiang F, et al. Impaired autophagy in microglia aggravates dopaminergic neurodegeneration by regulating NLRP3 inflammasome activation in experimental models of Parkinson’s disease. Brain Behav Immun. 2021;91:324–38.
Ren Y, Wang Q, Yang Z, Feng L, Zhang Y. MCC950 ameliorates the dementia symptom at the early age of line M83 mouse and reduces hippocampal α-synuclein accumulation. Biochem Biophys Res Commun. 2022;611:23–30.
Wu YQ, Xiong J, He ZL, Yuan Y, Wang BN, Xu JY, et al. Metformin promotes microglial cells to facilitate myelin debris clearance and accelerate nerve repairment after spinal cord injury. Acta Pharmacol Sin. 2022;43(6):1360–71.
Su Y, Zhang W, Zhang R, Yuan Q, Wu R, Liu X, et al. Activation of cholinergic anti-inflammatory pathway ameliorates cerebral and cardiac dysfunction after intracerebral hemorrhage through autophagy. Front Immunol. 2022;13: 870174.
Wan L, Jia RM, Ji LL, Qin XM, Hu L, Hu F, et al. AMPK-autophagy-mediated inhibition of microRNA-30a-5p alleviates morphine tolerance via SOCS3-dependent neuroinflammation suppression. J Neuroinflamm. 2022;19(1):25.
Zhang Q, Zhou J, Shen M, Xu H, Yu S, Cheng Q, et al. Pyrroloquinoline quinone inhibits rotenone-induced microglia inflammation by enhancing autophagy. Molecules. 2020;25(19):4359.
Lopez-Lopez A, Villar-Cheda B, Quijano A, Garrido-Gil P, Garcia-Garrote M, Diaz-Ruiz C, et al. NADPH-oxidase, rho-kinase and autophagy mediate the (pro)renin-induced pro-inflammatory microglial response and enhancement of dopaminergic neuron death. Antioxidants (Basel). 2021;10(9):1340.
Huang R, Gao Y, Chen J, Duan Q, He P, Zhang J, et al. TGR5 agonist INT-777 alleviates inflammatory neurodegeneration in Parkinson’s disease mouse model by modulating mitochondrial dynamics in microglia. Neuroscience. 2022;490:100–19.
Liang Y, Zheng D, Peng S, Lin D, Jing X, Zeng Z, et al. Rifampicin attenuates rotenone-treated microglia inflammation via improving lysosomal function. Toxicol In Vitro. 2020;63: 104690.
Liang Y, Zhou T, Chen Y, Lin D, Jing X, Peng S, et al. Rifampicin inhibits rotenone-induced microglial inflammation via enhancement of autophagy. Neurotoxicology. 2017;63:137–45.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82071420), Jiangsu Provincial Key R&D Program (BE2018658), Suzhou Technology Development Programme (SLJ2021010), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
QL wrote the manuscript; KT contributed to figure generation; XW contributed to table generation; XY, MP and JL contributed to conception of the study; FW and CL was involved in the project design and supervision, and manuscript revision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Consent for publication
Not applicable.
Ethical approval and consent to participate
Not applicable.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lv, QK., Tao, KX., Wang, XB. et al. Role of α-synuclein in microglia: autophagy and phagocytosis balance neuroinflammation in Parkinson’s disease. Inflamm. Res. 72, 443–462 (2023). https://doi.org/10.1007/s00011-022-01676-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-022-01676-x