Abstract
Background
Sub-anesthetic ketamine has rapid-onset effects for the treatment of major depressive disorder (MDD). However, the mechanism underlying ketamine’s antidepressant properties remains unclear. Recent studies have reported an interrelationship between autophagy and the inflammasome, both of which are involved in the pathophysiology of MDD. In this study, we assess whether ketamine exerts its antidepressant effects via an association with the autophagy-NLRP3 inflammasome pathway.
Methods
We established a depressive-like rat model by treating Wistar Kyoto rats with chronic restraint stress (CRS) for 28 days. Microglial cells from newborn Sprague–Dawley rats were used for in vitro experiments.
Results
We found sub-anesthetic ketamine treatment reversed depressive-like behavior in CRS rats. Ketamine triggered autophagy in the microglia of prefrontal cortex (PFC) and (hippocampus) HPC, with increased levels of LC3B, decreased levels of p62 protein, and elevated autophagosomes both in vivo and in vitro. Moreover, NLRP3 inflammasome activation was also inhibited by ketamine, with reduced expression of NLRP3-ASC-CASP1 assembly and decreased IL-1β levels in cerebrospinal fluid (CSF) as well as in the serum. Increased BDNF levels and synaptophysin levels were detected in the ketamine-treated group. The rapid anti-depressive effects, elevation of autophagy, reduction in NLRP3, and neuroplasticity-related factors induced by ketamine could be significantly blocked by the autophagy inhibitor Baf A1 (0.1 mg/kg).
Conclusions
Our findings demonstrate that sub-anesthetic doses of ketamine exert their antidepressant-like effects by inhibiting inflammation and initiating neuroprotection via autophagy activation. These data might help expand future investigations on the antidepressant properties of ketamine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major depressive disorder (MDD) has the highest disease burden of all mental health disorders (Rehm and Shield 2019). Therefore, the treatment of MDD is an urgent public health problem. However, classical antidepressants require several weeks of daily dosing to be fully effective (Witkin et al. 2019). Patients with delayed response to treatment often have more frequent recurrence, longer disease courses, higher rates of comorbidity, more needs to change treatment regimens, and higher hospitalization rates. Each of these is major contributor to the heavy economic and social burdens of depression (Mrazek et al. 2014). In addition to delayed effects, the existing classical antidepressants, which target monoaminergic receptors, also have other disadvantages, including treatment resistance, high suicidal risk, and high recurrence rates (Lepine et al. 2012; Hillhouse and Porter 2015), suggesting an urgent need to determine new antidepressants with differing mechanisms.
In recent years, sub-anesthetic ketamine has been used to treat MDD, and has rapid effects and a high response rate. Ketamine is associated with significant improvements in depressive symptoms within hours of administration (Berk et al. 2018; Ramadan and Mansour 2020). It can also effectively reduce self-injury behaviors (Vanle et al. 2019). Further, the response rate of patients with treatment-resistant depression to a single sub-anesthetic dose of ketamine infusion is approximately 70% (Serafini et al. 2014). However, ketamine’s antidepressant mechanism remains unclear.
Recent evidence suggests that the inflammasome may act as a mediator between MDD and stress. Preclinical research has shown that the inflammasome is a promising therapeutic target for depression (Alcocer-Gómez and Cordero 2014; Kaufmann et al. 2017). The inflammasome complex typically consists of a NOD-like receptor (NLR), a procaspase-1 precursor, and an apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC or PYCARD) adaptor. Within the NLR family, it has been reported that the NLR pyrin domain-containing protein 3 (NLRP3) is increased in both MDD patients and stress-induced depressive-like rodent models (Alcocer-Gómez et al. 2014, 2016). The inflammatory marker IL-1β, as a key mediator of stress-induced depression-like behavior, is activated by the inflammasome complex, which also appears to be a critical factor in pro-inflammatory responses to psychological stress (Mota et al. 2013). Multiple studies have indicated that ketamine can regulate NLRP3 in a dose-dependent manner. For example, anesthetic doses of ketamine can induce hippocampal pyroptosis in postnatal mice in a caspase-1 dependence manner, whose precursor is a major component of NLRP3 (Ye et al. 2018). On the other hand, sub-anesthetic dose of ketamine has been shown to downregulate NLRP3 inflammasomes in a LPS-induced rodent depressive model (Li et al. 2019). Therefore, NLRP3 is a possible mediator between ketamine and stress, but the precise potential inhibitory mechanism has not been investigated.
Impaired autophagy within the central nervous system (CNS) has been implicated in the pathophysiology of several neurological diseases through the formation of NLRP3 (Kesharwani et al. 2019). Autophagy is the process of degrading impaired intracellular contents. Previous evidence suggests that autophagy activation can inhibit NLRP3 activation, reduce inflammatory responses, and promote neuroplasticity (Ye et al. 2019; Geng et al. 2019; Ali et al. 2020). Deficiencies in autophagy could induce the activation of inflammasomes (mainly NLRP3 in the microglia) (Kesharwani et al. 2019). In addition, ketamine might be involved in the formation of autophagy in several disease models. Previous reports have shown that ketamine can cause autophagy in rodent models’ hippocampi, and that this effect can be attenuated by autophagy inhibitors (Li et al. 2018). Ketamine has also been shown to promote mitochondrial division and activate autophagy in in vitro cultures of neural stem cells (Bai et al. 2013). In a traumatic brain injury model, a sub-anesthetic dose of ketamine exerted neuroprotective effects by attenuating inflammation via autophagy (Wang et al. 2017).
Therefore, autophagy, as a key factor regulating NLRP3 activity, may be linked to ketamine’s pharmacological actions, and related to its antidepressant, anti-inflammatory, and neuroprotective effects. The purpose of this study was to investigate the role of ketamine in the autophagy-NLRP3 inflammasome pathway, and to determine whether blocking autophagy would reduce ketamine’s antidepressant-like effects.
Materials and methods
Depressive-like model and behavior tests
Animals
Our behavioral study employed 40 male Wistar-Kyoto (WKY) rats (8-week, weighting around 180 g). All rats were allowed 7 days of acclimatization to the environment before the behavior study. During the acclimatization phase, rats were housed separately and were subjected to a 12-:12-h light–dark cycle under a constant temperature of 22℃. Water and food were not restricted. Procedures involving laboratory animals were in strict accordance with the Guide for the China National Standards for Laboratory Animal Quality and the Chinese Guidelines for Care and Use of Laboratory Animals.
Chronic restraint stress-induced depression-like model
To induce depressive-like behaviors, a restraint-based chronic stress model was adapted from previous literature (Ampuero et al. 2015; Yang et al. 2018). A total of 32 of the 40 rats were subjected to chronic restraint treatment for 6 h daily for 28 days. When delivering chronic restraint stress (CRS), rats were stressed in adaptable fixers adjusted for subjects’ body sizes, and were housed in a separate room from the control rats. No food or water was allowed during the CRS treatment.
Behavior essays and grouping
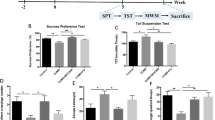
Behavioral essays were performed on animals before the CRS procedures, after the CRS, and 24 h after drug (ketamine, autophagy inhibitor bafilomycin A1, saline, etc.) injections (see Fig. 1). The doses of sub-anesthetic ketamine (Gutian Pharma Co., Ltd.) and bafilomycin A1 (MedChemExpress LLC, HY100558) were based on previous studies (Wang et al. 2011; Yuan et al. 2015). Intraperitoneal administration was used as the route of injection. Behavioral analyses (a sucrose preference test (SPT), an open field test (OFT), and a tail suspension test (TST)) were performed by researchers blinded to experimental conditions.
Chronic restraint stress (CRS) induced depression-like models and behavioral test results after ketamine and autophagy inhibitor injection. A Time scheme of experimental procedures. B Grouping of experimental animals and treatments. C Sucrose preference index of SPT after drug injection. D Total travel distance of OFT after drug injection. E Immobility time during the TST following drug injection. CRS, chronic restraint stress; Baf A1, bafilomycin A1. Data were expressed as mean ± s.e.m. (n = 8–12). *P < 0.05, * *P < 0.01, n.s., not significant
Sucrose preference test
Before the test, rats were acclimatized to the presence of two bottles of water for 48 h. After 12 h acclimatization, the rats were separated one per cage and were allowed 24 h access to either 1% sucrose or water. Solution intake with the containers of water and sucrose was weighed every 6 h. Container positions were switched after each weighing to reduce side bias. The sucrose consumption index was calculated as the sucrose consumption divided by the total consumption volume (Liu et al. 2018).
Open field test
After being acclimatized in the test environment for 1 h, rats were placed separately in the center of an arena (100 cm × 100 cm × 50 cm) for 10 min in a dimly lit environment. A camera, which was set directly above the apparatus, was used to record the total distance traveled by each rat (Any-maze, Stoelting).
Tail suspension test
Rats were suspended individually from their tails using a specialized tail suspension system (YLS-18A, Hunan Shuhang) for measuring behavioral despair. A 4-min test session was recorded in total using a video camera. Immobile time was defined as the time spent in a completely immobile posture for the last 3-min.
Sample collection
Approximately, 36 h after the final acute stress manipulation, and 12 h after the last sets of behavioral tests were completed, rats were sacrificed under anesthesia using sodium pentobarbital (40 mg/kg, intraperitoneally). Serum from peripheral blood samples, CSF, and the PFC and HPC from the whole brain, was collected as described previously (Pan et al. 2014). All samples were stored at − 80 ℃ before analysis.
ELISA analysis
Interleukin 1 beta (IL-1β) levels in serum and CSF were measured using a commercial Enzyme-linked immunosorbent assay (ELISA) kit (KE1433, Immuno Way Biotechnology Co., Ltd.). The ELISA kit was used following the manufacturer’s introductions. Each subject’s mean value was calculated for all analyses.
Isolation, purification, and culture of microglia
Newborn Sprague–Dawley Rats rat were craniotized in a sterile environment, and the brains were stored in pre-chilled D-Hank’s solution. The meninges and blood vessels were carefully separated, cut into pieces, and digested with 0.125% trypsin for 15 min to prepare a single-cell suspension. After that, we adjusted the cell concentration to 108 cell/L, cultured cells in a DMEM/F12 medium for 10 days, discarded the culture solution after the cells were fully stratified, and used 0.05% trypsin Digest while observing (i.e., gently shook the culture flask until the microglia were separated, and stopped the digestion of the digestion solution containing floating microglia with a complete medium). We next centrifuged the solution at 1000 rpm for 5 min, collected the cells, and discarded the supernatant. Finally, a complete culture medium was pipetted again into a cell suspension and planted in a culture flask which had been pre-coated with polylysine. After 1 h, non-adherent cells were removed by changing the medium, and a DMEM/F12 complete medium was added for further culture for 24 h. The cells were plated in a 96-well plate treated with polylysine after passage. Cells were cultured with 100 ng/ml LPS for 3 h, and were then incubated with Baf A1 (10 nM) for 1 h, treated with 0.6 μg/ml ketamine for 3 h, and incubated for 1 h with 5 mM ATP before undergoing further analysis.
Western blot analysis
Proteins were extracted from culture of microglia cells, separated using SDS-PAGE, and transferred onto polyvinylidene fluoride membranes. The membranes were blocked with 5% non-fat milk at room temperature for 1 h. The membranes were incubated with primary antibodies on a shaker for gentle agitation overnight at 4℃, and then incubated with a secondary antibody at room temperature for 1 h. Detection was performed using a LumiGLO chemiluminescent substrate system (KPL). Band intensity was quantified using BandScan software (Glyko).
Transmission electron microscopy
PFC and HPC tissues were fixed in precooled 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.4, at 4 ℃ for 2 h. Tissues were washed in the buffer three times, post-fixed in 1% osmium tetroxide in 0.1 M phosphate buffer for 1 h, and dehydrated and embedded in Epon 812. Ultrathin sections were stained with uranyl acetate and lead citrate and observed under a Hitachi H-7100 electron microscope.
Immunofluorescence
We used xylene and ethanol to deparaffinize and rehydrate the tissue paraffin sections. After microwave repair, we blocked tissue with goat serum for 1 h, centrifuged to separate the blocked serum, incubated it in primary antibody overnight at 4℃, and rewarmed it for 45 min and washed it 3 times with PBS solution. We incubated the secondary antibody for 60 min, washed it 3 times with PBS solution, stained it with DAPI for 10 min, and washed it 3 times with PBS solution. Finally, we mounted the slides with an anti-fluorescence quencher.
Immunohistochemistry
Nestin expression was analyzed using immunochemical staining of paraffin-embedded HPC sections. The sections were deparaffinized, rehydrated, and washed in 1% PBS-Tween 20, and then treated with 2% hydrogen peroxide and blocked with 3% goat serum (Life Technology, 16,210–064) for 1 h at room temperature. Next, cells were incubated with anti-Nestin primary antibodies (4760 T, Cell Signaling Technology, Inc.) at room temperature overnight at 4℃. The slides were then incubated with streptavidin-HRP (Shanghai Gene Company, GK500705) for 40 min, then stained with DAB (Shanghai Gene Company, GK500705) substrate and counter-stained with hematoxylin. Images were acquired by light microscopy (Olympus CKX31).
Statistical analysis
Data were analyzed using GraphPad Prism 8.0 software. Data were presented as mean ± standard error of the mean (SEM) to show the accuracy of the sample mean. The statistical test used for comparison of behavioral data (before drug injection) was unpaired t-test. The statistical test used for comparison of behavioral data (after drug injection), as well as relative morphological and immunochemical densities, was one-way analysis of variance (ANOVA) followed by post hoc Turkey’s tests for multiple comparisons. The western blot data were expressed as the ratio of gray band intensity of the proteins to that of albumin. A probability level of P < 0.05 was accepted as significant.
Results
Rapid anti-depressive effects of ketamine were antagonized by autophagy inhibitors in a CRS model
To study whether blocking autophagy could attenuate the anti-depressive effect of ketamine, forty WKY rats were randomly subjected to CRS (n = 32) or not subjected to CRS (forming a control group; n = 8) for a total of 28 days. Baseline evaluation before CRS treatment showed no significant differences in OFT, SPT, TST, or body weight between the CRS group and the control group (Fig. S1). After 28 days of CRS procedures, thirty-one of the 32 CRS rats remained alive. Behavior testing (Fig. S2) indicated reliable depression-like phenotypes induced by chronic restraint. The mean body weight of CRS rats was also significantly lower than that of controls (208.3 ± 1.606 g vs. 259.6 ± 7.488 g, t = 10.61, P < 0.0001). After a single dose of ketamine infusion, depressive-like behaviors were reversed (i.e., there was a significantly longer total travel length for OFT, and a shorter period of immobile time in TST, in the ketamine treatment group; Fig. 1C, D, E). When the autophagy inhibitor Baf A1 was co-administered with ketamine treatment, the reversion in behavior tests was drastically reduced. Together, these results indicate that the rapid antidepressant effects of ketamine were antagonized by an autophagy inhibitor.
Autophagy activation after ketamine was blocked by the autophagy inhibitor both in vivo and in vitro
There is a strong link between autophagy, neuroinflammation and depression. Therefore, we investigated autophagy in LPS-induced neuroinflammation and depression-like conditions. Western blot analysis showed significantly increased levels of LC3B in vivo and in vitro, suggesting increased autophagy activity after ketamine treatment (Fig. 2A, B, C). Baf A1 significantly reduced LC3B activity. Levels of p62 were reduced after ketamine injection, and increased when autophagy was inhibited by Baf A1, which further indicated that the activation of autophagy in microglia might be regulated by ketamine (Fig. 2A, B, C). NLRP3 and LC3B co-localization was confirmed by immunofluorescence in vitro, reinforcing the notion that ketamine’s autophagy effects were also related to microglia (Fig. 2D). Figure 2E and F showed that significantly decreased autophagy and increased morphological manifestations of cellular damage were present in the PFC and HPC of the CRS groups, which suggests that chronic stress might be related to decreased autophagy and/or structural damage to the CNS. Conversely, increased levels of autophagosomes in the ketamine-treated group could be found using TEM, which suggests that ketamine was associated with the formation of autophagosomes (Fig. 2E, F). Next, autophagy in rats that were primed with the autophagy inhibitor Baf A1 was reduced compared with the ketamine treatment group (Fig. 2E, F), indicating that ketamine’s activation of autophagy and reduction of cell damage could be blocked by an autophagy inhibitor. These results strongly supported ketamine-mediated activation of autophagy both in vivo and in vitro.
The effects of sub-anesthetic doses of ketamine and Baf A1 autophagy. A Autophagy-related markers LC3B (F (3, 56) = 1269, P < 0.0001) and p62 (F (3, 56) = 68.82, P < 0.0001) in the PFC of CRS rates were reduced by ketamine. B Autophagy-related markers LC3B (F (3, 56) = 303, P < 0.0001) and p62 (F (3, 56) = 33.87, P < 0.0001) in the HPC of CRS rats were reduced by ketamine. C Autophagy-related markers LC3B (F (3, 8) = 2626, P < 0.0001) and p62 (F (3, 8) = 127.6, P < 0.0001) in microglia were reduced by ketamine. D NLRP3 (F (3, 28) = 87.95, P < 0.0001) and LC3B (F (3, 28) = 30.95, P < 0.0001) co-location in microglia using immunofluorescence. Blue-DAPI-nucleus; green-LC3-autophagy; red-NLRP3-inflammasome. E Autophagy was increased in the PFC of ketamine-treated CRS rats (F (3, 8) = 39.92, P < 0.001). F Autophagy was increased in the HPC of ketamine-treated CRS rats (F (3, 8) = 41.25, P < 0.001). Data are mean ± s.e.m. *P < 0.05, **P < 0.01, n.s., not significant
Decreased NLRP3 and downstream inflammation after ketamine treatment was blocked by an autophagy inhibitor in both CRS models and microglia
In addition to the antidepressant-like effect of CRS rats, we examined inflammation levels and inflammasome activation both in vivo and in vitro. Before ketamine treatment of CRS rats, IL-1β concentrations were significantly increased in both serum and CSF compared with non-CRS control rats (Fig. 3A, B). After ketamine treatment, concentration of IL-1β in the CSF of CRS rats was significantly decreased (Fig. 3B). Compared to rats treated with only ketamine, rats treated with Baf A1 injections and ketamine showed increased concentrations of IL-1β in both serum and CSF. This suggests that ketamine’s effects on IL-1β are reduced by previous autophagy inhibition. Inflammasome markers NLRP3, ASC, and caspase-1, as well as the inflammatory cytokine IL-1β in the PFC and HPC, showed significant decreases following a single dose of ketamine treatment, and the effects of ketamine were attenuated by Baf A1 (Fig. 3C, D). As shown in Fig. 3E, F, and D, NLRP3 and Iba1 proteins were co-localized in both the PFC and HPC of CRS rats, suggesting that microglia might be the primary location for NLRP3 inflammasome expression. NLRP3 immunofluorescence was significantly lower with treatment of ketamine than that with co-administration with Baf A1 (Fig. 3F). Next, to investigate the effects of ketamine on microglia in vitro, a LPS plus ATP treatment model was used as the NLRP3 inflammasome activator. NLRP3, ASC, caspase-1 and IL-1β were suppressed by ketamine, as exemplified by western blotting (Fig. 3G). Again, it was found that ketamine-mediated inhibition of the inflammasome was significantly blocked by Baf A1. Hence, these data suggested that the stress-related NLRP3 inflammasome might be reduced by ketamine via autophagy activation both in vitro and vivo.
The effects of sub-anesthetic doses of ketamine and Baf A1 on NLRP3-related inflammation levels. A Concentration of IL-1β level in serum (F (3, 35) = 8.074, P < 0.001). B Concentration of IL-1β level in CSF (F (3, 20) = 12.04, P = 0.007). C NLRP3 inflammasome-related markers NLRP3 (F (3, 56) = 42.39, P < 0.0001), ASC (F (3, 56) = 29.78, P < 0.0001), caspase1 p10 (F (3, 56) = 58.96, P < 0.0001), and IL-1β (F (3, 56) = 123.9, P < 0.0001) were reduced by ketamine in the PFC of CRS rats. D NLRP3 inflammasome-related markers NLRP3 (F (3, 56) = 154.6, P < 0.0001), ASC (F (3, 56) = 123.8, P < 0.0001), caspase1 p10 (F (3, 56) = 36.45, P < 0.0001), and IL-1β (F (3, 56) = 87.99, P < 0.0001) were reduced by ketamine in the HPC of CRS rats. E NLRP3 and IBA1 co-location in the PFC using immunofluorescence. Blue-DAPI-nucleus; green-NeuN-neuron; red-NLRP3-inflammasome; pink-IBA1-microglia. F NLRP3 and IBA1 co-location in HPC using immunofluorescence. G NLRP3 inflammasome-related markers NLRP3 (F (3, 8) = 649.2, P < 0.0001), ASC (F (3, 8) = 203.3, P < 0.0001), caspase1 p10 (F (3, 8) = 389.1, P < 0.0001), and IL-1β (F (3, 8) = 926.2, P < 0.0001) were reduced by ketamine in microglia. Data are presented as mean ± s.e.m. *P < 0.05, **P < 0.01, n.s., not significant
Neuroplasticity improvements induced by ketamine were blocked by autophagy inhibitors both in the CRS model and microglia
To test whether ketamine would regulate plasticity in vivo, neurotrophic factors were examined in the CRS group. BDNF and Synaptophysinin expression in the CRS group was significantly lower than that of the control group, as assessed by western blotting (Fig. 4A, B). Further, BDNF and Synaptophysin expression was significantly increased after ketamine treatment. With Baf A1 co-administration, BDNF and Synaptophysin elevation was significantly reduced (Fig. 4A, B).
The effect of sub-anesthetic doses of ketamine and Baf A1 on neuroplasticity-related factors. A Expression of BDNF (F (3, 56) = 197.4, P < 0.001) and synaptophysin (F (3, 56) = 291.9, P < 0.001) was increased by ketamine in the PFC of CRS rats. B Expression of BDNF (F (3, 56) = 258.1, P < 0.001) and Synaptophysin (F (3, 56) = 78.71, P < 0.001) was increased by ketamine with in the HPC of CRS rats. C The Nestin-positive area of IHC staining was increased by ketamine (F (3, 8) = 9.934, P = 0.04). Data are presented as mean ± s.e.m. *P < 0.05, **P < 0.01, n.s., not significant
The neural stem cell marker Nestin (analyzed using IHC staining) showed that the Nestin-positive in the CRS group and Baf A1-treated group was significantly lower than controls, and the Nestin-positive area in the ketamine group was significantly increased compared to the CRS group (Fig. 4C). These results suggest that ketamine can increase the expression levels of neurotrophic-related proteins via autophagy in vivo.
Discussion
In our study, we found that the antidepressant-like effects of ketamine could be antagonized by autophagy inhibitors. Ketamine triggered autophagy in microglia within the PFC and HPC. NLRP3 and downstream inflammation decreased after ketamine was blocked by autophagy inhibitors, both in CRS models and microglia. The elevation of autophagy and neuroplasticity-related factors, and reduction in NLRP3 induced by ketamine, was blocked by an autophagy inhibitor both in the CRS model and microglia.
The first finding of the present study is that the antidepressant-like effects of ketamine could be antagonized by autophagy inhibitors. Studies have suggested that autophagy might serve an important role in the treatment of depression by classical antidepressants, including fluoxetine and venlafaxine (Zschocke et al. 2011), as well as andrographolide, melatonin, and ɑ-tocopherol (Huang et al. 2018; Geng et al. 2019; Ali et al. 2020). In the present study, ketamine effectively improved depressive-like behaviors and activated autophagy in both the PFC and HPC of CRS rats and LPS-treated microglia, as demonstrated by changes in levels of autophagic markers and autophagosomes. Several studies have shown the time-course change of autophagy by ketamine in vivo and intro could be observed within 24 h (Li et al. 2018; Shan et al. 2019). As ketamine has also shown its fast-acting anti-depressive actions within 24 h, it is possible that autophagy might be associated with ketamine’s rapid onset of anti-depressive effect. The suppression of autophagy by Baf A1 reduced the improvements in depressive-like behaviors in CRS rats with ketamine treatment, suggesting that a sub-anesthetic dose of ketamine might produce antidepressant-like effects through the induction of autophagy. Furthermore, the antagonizing effects of Baf A1 against ketamine indicated that ketamine’s autophagy inducing mechanism might have links to the degradation of autophagosomes. In short, the rapid anti-depressive effects of ketamine were regulated by the activation of autophagy pathway.
The second result of this study demonstrated that ketamine could reduce the stress-related NLRP3 inflammasome by evoking autophagy in microglia. Previous studies have suggested the activation of NLRP3 could be an important sensor in stress-induced depressive behavior (Alcocer-Gómez et al. 2016; Zhu et al. 2017), and that microglia were the primary contributor to NLRP3 and depressive-like behaviors. In our study, we also found that the NLRP3 assembly elevation and microglial activation in both PFC and HPC were attributed to chronic stress. It is now widely believed that chronic stress may promote the initiation and development of depressive-like behaviors through inflammatory activation. In this study, we found that NLRP3 and microglia elevation after CRS could be alleviated by ketamine. Furthermore, the alleviation of ketamine was inhibited by autophagy inhibitors. Research suggests that the anti-inflammatory and anti-depressive effects of autophagic agonists might be related to downstream MAPK/NF-κB and AMPK/mTOR signaling (Su et al. 2017; Huang et al. 2018). But whether ketamine’s inhibition of NLRP3 is associated with these pathways needs further study. In addition, co-localization experiments indicated that the activation of NLRP3 took place within microglia, which is consistent with other rodent depression models of chronic stress (Pan et al. 2014; Gong et al. 2019). Therefore, ketamine acts as an antidepressant by activating autophagy, which in turn inhibits NLRP3 and its subsequent inflammation. Inhibition of autophagy-mediated NLRP3 development within microglia might serve as a promising treatment option for patients with stress-related depression.
The third result of our study shows that neuroplasticity associated with neurochemical changes and synaptogenesis in different regions of the brain is affected by ketamine. The PFC was previously speculated to play a central role in the development of depression (Pan et al. 2014). In the present study, both PFC and HPC’s expression of NLRP3 and IL-1β correlated with serum and CSF IL-1β levels, suggesting the contribution of chronic stress to CNS inflammation might not be limited to the PFC. Some researchers have demonstrated the importance of the HPC in hippocampal neurogenesis in relation to stress and depression (Mahar et al. 2014). Our analyses on the neuroplastic effect of ketamine have provided evidence to support the idea that the rapid anti-depressive effects of ketamine might be associated with synaptogenesis in the HPC. Moreover, the association between anti-inflammatory response and neuroprotective effects has been reported in a memory impairment model in vivo. The potential neuroprotective effects of sub-anesthetic dose of ketamine might be beneficial to alleviate the cognitive symptoms of major depressive disorder (Noroozi et al. 2022). Conversely, ketamine at a larger dose significantly reduced cell viability in vitro (Shan et al. 2019), indicating the dosage and frequency of administration for ketamine should be cautiously adjusted for neuroprotection purposes.
However, some potential limitations in our study should be noted. First, we did not assess any dose–response effects of ketamine on autophagy. Prior evidence suggests larger doses of ketamine significantly reduce cell viability in vitro (Shan et al. 2019), indicating that the dosage and frequency of administration for ketamine should be cautiously adjusted for neuroprotection purposes. Second, the long-term antidepressant-like effects of ketamine were not examined in our study. Another study has shown that, compared to a single ketamine treatment, repeated ketamine treatments affected a few local topological properties in models of rat brain networks, suggesting that repeated drug administration might contribute to convergent neuroplasticity (Gass et al. 2020). Third, the effects of autophagy inhibitors without ketamine treatment were not studied. Taken together, it is imperative to expand future investigations on effects of neuroinflammation and autophagy by repeated administration of ketamine. In clinical settings, autophagy and inflammasome-related markers sensitive to ketamine’s anti-depressive effect should be explored for personalized treatment outcome monitoring.
Conclusion
In conclusion, the present study demonstrates that the antidepressant-like effects, inhibition of inflammation, and neuroprotective effects of a sub-anesthetic dose of ketamine were mediated by autophagy activation. The mechanism underlying the activity of ketamine involves autophagy-dependent inactivation of the NLRP3 inflammasome, leading to decreased NLRP3-ASC-CASP1-mediated IL-β secretion from microglia in both the PFC and HPC. Although further research to study the effects of sub-anesthetic ketamine doses on autophagy, inflammasome, neurons, and microglia in a depression model is needed, our results offer primary evidence that autophagy may have a modulatory effect on the anti-inflammatory properties of ketamine.
Data availability
All materials are commercially available, and data are presented in this article are available per the open access policy.
Abbreviations
- AMP:
-
AMP-activated protein kinase
- Baf A1:
-
Bafilomycin A1
- BDNF:
-
Brain-derived neurotrophic factor
- CARD:
-
Caspase-recruitment domain
- CASP1:
-
Caspase 1, apoptosis-related cysteine peptidase
- CRS:
-
Chronic restraint stress
- CSF:
-
Cerebrospinal fluid
- DAPI:
-
4′, 6-Diamidino-2-phenylindole
- HPC:
-
Hippocampus
- IHC:
-
Immunohistochemistry
- IL-1β:
-
Interleukin 1, beta
- LC3B:
-
Microtubule-associated proteins 1A/1B light chain 3B
- LPS:
-
Lipopolysaccharide
- MDD:
-
Major depressive disorder
- mTOR:
-
Mechanistic target of rapamycin
- NF-κB:
-
Nuclear factor-κB
- NLRP3:
-
NLR family, pyrin domain-containing 3
- PFC:
-
Prefrontal cortex
- PYCARD/ASC:
-
PYD and CARD domain containing
References
Alcocer-Gomez E, Cordero MD (2014) NLRP3 inflammasome: a new target in major depressive disorder. CNS Neurosci Ther 20:294–295. https://doi.org/10.1111/cns.12230
Alcocer-Gomez E, de Miguel M, Casas-Barquero N, Nunez-Vasco J, Sanchez-Alcazar JA, Fernandez-Rodriguez A, Cordero MD (2014) NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav Immun 36:111–117. https://doi.org/10.1016/j.bbi.2013.10.017
Alcocer-Gomez E, Ulecia-Moron C, Marin-Aguilar F, Rybkina T, Casas-Barquero N, Ruiz-Cabello J, Ryffel B, Apetoh L, Ghiringhelli F, Bullon P, Sanchez-Alcazar JA, Carrion AM, Cordero MD (2016) Stress-induced depressive behaviors require a functional NLRP3 inflammasome. Mol Neurobiol 53:4874–4882. https://doi.org/10.1007/s12035-015-9408-7
Ali T, Rahman SU, Hao Q, Li W, Liu Z, Ali Shah F, Murtaza I, Zhang Z, Yang X, Liu G, Li S (2020) Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J Pineal Res 69:e12667. https://doi.org/10.1111/jpi.12667
Ampuero E, Luarte A, Santibanez M, Varas-Godoy M, Toledo J, Diaz-Veliz G, Cavada G, Rubio FJ, Wyneken U (2015) Two chronic stress models based on movement restriction in rats respond selectively to antidepressant drugs: Aldolase C as a potential biomarker. Int J Neuropsychopharmacol 18:pyv038. https://doi.org/10.1093/ijnp/pyv038
Bai X, Yan Y, Canfield S, Muravyeva MY, Kikuchi C, Zaja I, Corbett JA, Bosnjak ZJ (2013) Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway. Anesth Analg 116:869–880. https://doi.org/10.1213/ANE.0b013e3182860fc9
Berk M, Loo C, Davey CG, Harvey BH (2018) Ketamine and rapidly acting antidepressants: Breaking the speed of sound or light? Aust N Z J Psychiatry 52:1026–1029. https://doi.org/10.1177/0004867418783567
Gass N, Becker R, Reinwald J, Cosa-Linan A, Sack M, Weber-Fahr W, Vollmayr B, Sartorius A (2020) The influence of ketamine’s repeated treatment on brain topology does not suggest an antidepressant efficacy. Transl Psychiatry 10:56. https://doi.org/10.1038/s41398-020-0727-8
Geng J, Liu J, Yuan X, Liu W, Guo W (2019) Andrographolide triggers autophagy mediated inflammation inhibition and attenuates chronic unpredictable mild stress (CUMS)-induced depressive-like behavior in mice. Toxicol Appl Pharmacol 379:114688. https://doi.org/10.1016/j.taap.2019.114688
Gong W, Zhang S, Zong Y, Halim M, Ren Z, Wang Y, Ma Y, Li B, Ma L, Zhou G, Yu J, Zhang J, Liu Q (2019) Involvement of the microglial NLRP3 inflammasome in the anti-inflammatory effect of the antidepressant clomipramine. J Affect Disord 254:15–25. https://doi.org/10.1016/j.jad.2019.05.009
Hillhouse TM, Porter JH (2015) A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol 23:1–21. https://doi.org/10.1037/a0038550
Huang X, Wu H, Jiang R, Sun G, Shen J, Ma M, Ma C, Zhang S, Huang Z, Wu Q, Chen G, Tao W (2018) The antidepressant effects of a-tocopherol are related to activation of autophagy via the AMPK/mTOR pathway. Eur J Pharmacol 833:1–7. https://doi.org/10.1016/j.ejphar.2018.05.020
Kaufmann FN, Costa AP, Ghisleni G, Diaz AP, Rodrigues ALS, Peluffo H, Kaster MP (2017) NLRP3 inflammasome-driven pathways in depression: Clinical and preclinical findings. Brain Behav Immun 64:367–383. https://doi.org/10.1016/j.bbi.2017.03.002
Kesharwani R, Sarmah D, Kaur H, Mounika L, Verma G, Pabbala V, Kotian V, Kalia K, Borah A, Dave KR, Yavagal DR, Bhattacharya P (2019) Interplay between mitophagy and inflammasomes in neurological disorders. ACS Chem Neurosci 10:2195–2208. https://doi.org/10.1021/acschemneuro.9b00117
Lepine BA, Moreno RA, Campos RN, Couttolenc BF (2012) Treatment-resistant depression increases health costs and resource utilization. Braz J Psychiatry 34:379–388. https://doi.org/10.1016/j.rbp.2012.05.009
Li JM, Liu LL, Su WJ, Wang B, Zhang T, Zhang Y, Jiang CL (2019) Ketamine may exert antidepressant effects via suppressing NLRP3 inflammasome to upregulate AMPA receptors. Neuropharmacology 146:149–153. https://doi.org/10.1016/j.neuropharm.2018.11.022
Li X, Li Y, Zhao J, Li L, Wang Y, Zhang Y, Li Y, Chen Y, Liu W, Gao L (2018) Administration of ketamine causes autophagy and apoptosis in the rat fetal hippocampus and in PC12 cells. Front Cell Neurosci 12:21. https://doi.org/10.3389/fncel.2018.00021
Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, Chen H, Zhu DY, Zhou QG (2018) Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc 13:1686–1698. https://doi.org/10.1038/s41596-018-0011-z
Mahar I, Bambico FR, Mechawar N, Nobrega JN (2014) Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev 38:173–192. https://doi.org/10.1016/j.neubiorev.2013.11.009
Mota R, Gazal M, Acosta BA, de Leon PB, Jansen K, Pinheiro RT, Souza LD, Silva RA, Oses JP, Quevedo L, Lara DR, Ghisleni G, Kaster MP (2013) Interleukin-1beta is associated with depressive episode in major depression but not in bipolar disorder. J Psychiatr Res 47:2011–2014. https://doi.org/10.1016/j.jpsychires.2013.08.020
Mrazek DA, Hornberger JC, Altar CA, Degtiar I (2014) A review of the clinical, economic, and societal burden of treatmentresistant depression: 1996–2013. Psychiatr Serv 65:977–987. https://doi.org/10.1176/appi.ps.201300059
Noroozi N, Shayan M, Maleki A, Eslami F, Rahimi N, Zakeri R, Abdolmaleki Z, Dehpour AR (2022) Protective effects of dapsone on scopolamine-induced memory impairment in mice: Involvement of nitric oxide pathway. Dement Geriatr Cogn Dis Extra 12:43–50. https://doi.org/10.1159/000522163
Pan Y, Chen XY, Zhang QY, Kong LD (2014) Microglial NLRP3 inflammasome activation mediates IL-1beta-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun 41:90–100. https://doi.org/10.1016/j.bbi.2014.04.007
Ramadan AM, Mansour IA (2020) Could ketamine be the answer to treating treatment-resistant major depressive disorder? Gen Psychiatr 33:e100227. https://doi.org/10.1136/gpsych-2020-100227
Rehm J, Shield KD (2019) Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep 21:10. https://doi.org/10.1007/s11920-019-0997-0
Serafini G, Howland RH, Rovedi F, Girardi P, Amore M (2014) The role of ketamine in treatment-resistant depression: a systematic review. Curr Neuropharmacol 12:444–461. https://doi.org/10.2174/1570159X12666140619204251
Shan Z, Wei L, Yu S, Jiang S, Ma Y, Zhang C, Wang J, Gao Z, Wan F, Zhuang G, Wu J, Liu D (2019) Ketamine induces reactive oxygen species and enhances autophagy in SV-HUC-1 human uroepithelial cells. J Cell Physiol 234:2778–2787. https://doi.org/10.1002/jcp.27094
Su WJ, Zhang Y, Chen Y, Gong H, Lian YJ, Peng W, Liu YZ, Wang YX, You ZL, Feng SJ, Zong Y, Lu GC, Jiang CL (2017) NLRP3 gene knockout blocks NF-kappaB and MAPK signaling pathway in CUMS-induced depression mouse model. Behav Brain Res 322:1–8. https://doi.org/10.1016/j.bbr.2017.01.018
Vanle B, Fuller M, Hangiandreou G, Silverman S (2019) Treatment of nonsuicidal self injury disorder with oral ketamine. Heart Mind 3:129. https://doi.org/10.4103/hm.hm_50_19
Wang CQ, Ye Y, Chen F, Han WC, Sun JM, Lu X, Guo R, Cao K, Zheng MJ, Liao LC (2017) Posttraumatic administration of a sub-anesthetic dose of ketamine exerts neuroprotection via attenuating inflammation and autophagy. Neuroscience 343:30–38. https://doi.org/10.1016/j.neuroscience.2016.11.029
Wang J, Goffer Y, Xu D, Tukey DS, Shamir DB, Eberle SE, Zou AH, Blanck TJ, Ziff EB (2011) A single subanesthetic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology 115:812–821. https://doi.org/10.1097/ALN.0b013e31822f16ae
Witkin JM, Martin AE, Golani LK, Xu NZ, Smith JL (2019) Rapid-acting antidepressants. Adv Pharmacol 86:47–96. https://doi.org/10.1016/bs.apha.2019.03.002
Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, Hu H (2018) Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554:317–322. https://doi.org/10.1038/nature25509
Ye JS, Chen L, Lu YY, Lei SQ, Peng M, Xia ZY (2019) Honokiol-mediated mitophagy ameliorates postoperative cognitive impairment induced by surgery/sevoflurane via inhibiting the activation of NLRP3 inflammasome in the hippocampus. Oxid Med Cell Longev 2019:8639618. https://doi.org/10.1155/2019/8639618
Ye Z, Li Q, Guo Q, Xiong Y, Guo D, Yang H, Shu Y (2018) Ketamine induces hippocampal apoptosis through a mechanism associated with the caspase-1 dependent pyroptosis. Neuropharmacology 128:63–75. https://doi.org/10.1016/j.neuropharm.2017.09.035
Yuan N, Song L, Zhang S, Lin W, Cao Y, Xu F, Fang Y, Wang Z, Zhang H, Li X, Wang Z, Cai J, Wang J, Zhang Y, Mao X, Zhao W, Hu S, Chen S, Wang J (2015) Bafilomycin A1 targets both autophagy and apoptosis pathways in pediatric B-cell acute lymphoblastic leukemia. Haematologica 100:345–356. https://doi.org/10.3324/haematol.2014.113324
Zhu W, Cao FS, Feng J, Chen HW, Wan JR, Lu Q, Wang J (2017) NLRP3 inflammasome activation contributes to long-term behavioral alterations in mice injected with lipopolysaccharide. Neuroscience 343:77–84. https://doi.org/10.1016/j.neuroscience.2016.11.037
Zschocke J, Zimmermann N, Berning B, Ganal V, Holsboer F, Rein T (2011) Antidepressant drugs diversely affect autophagy pathways in astrocytes and neurons–dissociation from cholesterol homeostasis. Neuropsychopharmacology 36:1754–1768. https://doi.org/10.1038/npp.2011.57
Acknowledgements
We acknowledge Hui Xiao, M.D., for her technical assistance with figures of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81701344), the Shanghai “Science and Technology Innovation Action Plan,” the Natural Science Foundation of Shanghai (21ZR1455100, 20ZR1448400), the Ministry of Science and Technology of the People’s Republic of China (2016YFC0906300), and the Shanghai Municipal Health Bureau (201740115).
Author information
Authors and Affiliations
Contributions
Dongbin Lyu, Fan Wang, Zezhi Li, and Wu Hong contributes substantially to the design, experiment, analysis, and data interpretation of the study. Dongbin Lyu, Fan Wang, and Mengke Zhang are responsible for drafting and revising the manuscript. Wu Hong, Zezhi Li, and Chen Zhang are responsible for the final approval of manuscript. Weichieh Yang, Haijing Huang, Qinte Huang, and Chenglin Wu are accountable for the acquisition and analysis of data in animal experiments. Nuoshi Qian, Meiti Wang, Jing Chen, and Yingmei Fu are responsible for experiments in vitro. Huanfei Zhang and Sichai Zheng contribute to the dosing and administration of experimental drugs. All authors certify that they have participated sufficiently in this study to take public responsibility for the content.
Corresponding authors
Ethics declarations
Ethics approval
All animal protocols were approved by the ethics committee of Shanghai Mental Health Center (Shanghai, China).
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lyu, D., Wang, F., Zhang, M. et al. Ketamine induces rapid antidepressant effects via the autophagy-NLRP3 inflammasome pathway. Psychopharmacology 239, 3201–3212 (2022). https://doi.org/10.1007/s00213-022-06201-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-022-06201-w