Abstract
Rational microbial chassis design and engineering for improving production of amino acids have attracted a considerable attention. l-glutamate, l-lysine, l-threonine and l-tryptophan are the main amino acids demanded in the food industry. Systems metabolic engineering and synthetic biology engineering generally are believed as the comprehensive engineering approaches to obtain rationally designed strains and construct high-performance platforms for amino acids. The strategies focus on microbial chassis characterization optimization, precise metabolic engineering such as promoter engineering, modular pathway engineering, transporter engineering, and dynamic switch systems application, and global genome streamline engineering to reduce cell burden. In this review, we summarized the efficient engineering strategies to optimize Corynebacterium glutamicum and Escherichia coli cell factories for improving the production of l-glutamate, l-lysine, l-threonine, and l-tryptophan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amino acids in food industrial have numerous applications, and the market demand for them is likely to grow. l-glutamate, l-lysine, l-threonine, and l-tryptophan are the main food industry fermented amino acids in the market demand [1]. These amino acids are the main precursors for many other important chemical products. For examples, and l-glutamate is the precursor for poly-(l-glutamic acid) (PLGA) which is a hydrogel material that is hydrophilic, biocompatible, biodegradable, and non-immunogenic [2], and l-glutamate is the direct precursor of γ-amino butyric acid (GABA) which is also the monomer of nylon 4 [3]; l-lysine can be further converted into valuable chemicals such as poly(l-lysine)-based structures as novel antimicrobials for diabetic foot infections [4], 1,5-diamino-2-hydroxy-pentane as a new type of aliphatic amino alcohol [5], GABAb antagonist 5-aminovalleric acid (5-AVA) [6], glutaric acid [7, 8], cis-3-hydroxypipecolic acid as a key structural component of tetrapeptide antibiotic GE81112 [9], and so on; the l-threonine could be converted to 2-hydroxybutyrate, one of the important monomers of polyhydroxyalkanoates (PHAs), through sequential reactions of several enzymes [10,11,12]; the l-tryptophan can be a potential precursor for melanin synthesis in life forms [13]. Therefore, optimizing the production of l-glutamate, l-lysine, l-threonine, and l-tryptophan are crucial.
The microbial chassis fermentation is the main efficient approach for amino acids production [14,15,16], because excellent microbial cell factories own economical and environmentally friendly. Therefore, development of rational design and efficiently engineering strategies increasingly attracting a considerable attention [15, 17]. Exploiting the inherent cellular synthetic capacity of a microorganism is an efficient strategy for achieving amino acids production. To enhance the synthetic capacity of microorganisms, systems metabolic engineering and synthetic biology approaches have been successfully applied in the microbial production of amino acids, including microbial chassis characterization optimization, precise metabolic engineering such as promoter engineering, modular pathway engineering, transporter engineering, and dynamic switch systems application to maximize metabolic flux to target amino acid, and global genome streamline to reduce cell burden. Although these engineering strategies are highly effective, improving the synthetic capacity remains a major challenge, due to the lack of adequate understanding of complex cellular regulations and difficulties for maximization of conversion rate from substrates. In fact, more and more high-performance microbial cell factories have been constructed. In this review, recent technical advances improving the production of food amino acids are discussed. In addition, existing challenges and potential strategies for increasing the output of these amino acids are described [14].
The microbial production of amino acids used in food industries is a large area where synthetic biology and systems metabolic engineering strategies have been successfully applied, mainly in two important producing microorganisms: Corynebacterium glutamicum and Escherichia coli. According to the market demand, the amino acids produced by fermentation and used in the food industries mainly include l-glutamate (3,210,000 tons), l-lysine (2,600,000 tons), l-threonine (700,000 tons), l-tryptophan (41,000 tons), l-arginine (1200 tons), l-valine (500 tons), l-leucine (500 tons), and l-isoleucine (400 tons) [1]. l-Glutamate is the major bulk amino acid covering nearly two thirds of the amino acid market. Then, the market demand of l-lysine ranks just next to l-glutamate and is mainly produced by microbial fermentation employing mutant strains of bacteria, such as Corynebacterium sp. and Escherichia sp. [1]. The l-threonine is currently the third amino acid produced by microbial fermentation second only to l-glutamate and l-lysine. Unlike the l-glutamate and l-lysine produced by fermentation of C. glutamicum, l-threonine and l-tryptophan are mainly produced by E. coli [18]. While, the production of l-arginine, l-valine, l-leucine, and l-isoleucine by C. glutamicum or E. coli fermentation is still at a low level.
Systems metabolic engineering and synthetic biology generally are believed as the comprehensive engineering approaches to obtain rationally designed strains and construct high-performance platforms for amino acids. Synthetic biology emphasizes design and redesign to build new biological systems, which often brings unexpected effects. Combining the advantages of different approaches, it is promising to dig out the maximum potentials of microbial cell factories. Therefore, understanding the reported new strategies are important for establishing new design ideas and efficient approaches to optimize microbial cell factories. In this review, engineering strategies for the main amino acids including l-glutamate, l-lysine, l-threonine, and l-tryptophan used in food industries are summarized, mainly focusing on the two major industrial production microorganisms: C. glutamicum and E. coli.
Brief summary of microbial chassis design and engineering strategies for amino acids production
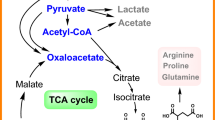
Amino acid production by fermentation is a success story of biotechnology. In C. glutamicum and E. coli, amino acids are generally synthesized from intermediate metabolites derived from glycolysis, pentose phosphate pathway and TCA cycle (Fig. 1). As shown Fig. 1, the intermediate oxaloacetate (OAA), α-ketoglutarate (α-KG), and 4-phosphate-erythrose (E4P) are the precursors of l-lysine and l-threonine, l-glutamate and l-tryptophan, respectively. Therefore, microbial chassis design and engineering is an emerging discipline that combines the concepts of synthetic engineering, systems’ metabolic engineering and evolutionary engineering [17]. With the development of high-throughput technologies, synthetic biology has become much more mature and applicable, and has already manifested its giant potential in providing genome-wide information and clues for engineering. Various strain breeding approaches have been developed, whilst genetically defined metabolic strategies have gradually taken the place of the conventional random mutagenesis-selection method and become the mainstream.
Recent reports have described metabolic engineering methods for the rational design of amino acid-producing host cells [16]. Common strategies for the design of amino acid-producing strains are (i) enhancement of biosynthesis pathway enzymes for the target amino acids, (ii) enhancement of the precursors synthesis for target amino acid and reduction of the precursors consumption, (iii) reduction of by-products formation, (iv) release of feedback regulation of key enzymes by the target amino acid, (v) increased or replaced the supply of reducing equivalents such as NADPH and ATP, (vi) optimize the uptake of carbon source, (vii) increased export of target amino acids out of the cells. However, because of cellular metabolic complexity, metabolic engineering modifications often resulted in unbalance between cell growth and chemicals production, or unbalanced distribution of metabolic flux, which increasingly become the major limitation for further improving amino acids production. To dissolve this, researchers created rational dynamic switch regulation systems aimed at realizing the production efficiency maximum of the target amino acids from glucose or other substrates [19]. In addition, genome reduction has been applied to C. glutamicum and E. coli to improve amino acids [15], e.g. for lysine production strain C. glutamicum GRLys1 [20, 21]; and for l-threonine production strains E. coli MGF-01 [22] and E. coli MDS42 [23]. In addition, our recent studies suggested that cell envelope simplification also influence intracellular metabolism, which showed potential advantages for amino acids production [24,25,26], especially for the l-glutamate [27] and l-threonine [28, 29]. Omics-based metabolic engineering techniques and various evolution approaches when no obvious target genes are known also inspire us to design new engineering strategies [26, 27, 30]. Furthermore, several studies suggested that the introduction of gene cluster phaCAB for poly-3-hydroxybutyrate (PHB) biosynthesis from acetyl-CoA facilitates the amino acids production including l-glutamate [31], l-threonine [32], and l-tryptophan [33], which suggested that stimulating cellular carbon flux to acetyl-CoA benefits amino acids biosynthesis. In this review, the detailed rational design and engineering strategies of microbial chassis for l-glutamate, l-lysine, l-threonine, and l-tryptophan production are analyzed and discussed below.
The amino acids derived from the intermediates of glycolysis, TCA cycle and PP pathway. l-Glutamate derives from α-ketoglutarate and exported by McsCG, l-lysine and l-threonine derive from OAA, and exported by LysE and RhtABC, respectively, and l-tryptophan derives from E4P and exported by YddG. PTS: phosphotransferase system; Glc: glucose; PP Pathway: pentose phosphate pathway; TCA cycle: tricarboxylic acid cycle; ATP: adenosine triphosphate; G6P: glucose 6-phosphate; R5P: ribulose 5-phosphate; F6P: fructose 6-phosphate; F1P: fructose 1-phosphate; FBP: fructose 1,6-bisphosphate; E4P: erithrose 4-phosphate; G3P: glucose 3-phosphate; Acetyl-CoA (AcCoA), acetyl-coenzyme A; PEP: phosphoenol pyruvate; Ser: serine; Gly: glycine; Cys: cysteine; Trp: tryptophan; Phe: phenylalanine; Tyr: tyrosine; Val: valine; Leu: leucine; Lys: lysine; Thr: threonine; Ile: isoleucine; Gln: glutamine; Glu: glutamate; Arg: arginine; Pro: proline; GABA: γ-amino butyric acid; l-Asp: l-aspartic acid; l-Aspartyl-P: l-aspartyl-phosphate.
Microbial chassis design and engineering for production of l-glutamate
l-Glutamate, as the most demanded in the global market, is one of the most intensely studied food ingredients in the food supply and has been found safe [34]. The production of l-glutamate is mainly based on the fermentation of C. glutamicum and reaches up to 130 g/L [35, 36]. In C. glutamicum, l-glutamate derives from 2-oxoglutarate of TCA cycle under the catalysis of glutamate dehydrogenase (GDH). For synthesis of 1 mol of l-glutamate from 1 mol of glucose, 2 mol of NADH are generated and 2 mol of NADPH are consumed. Current findings are correlated with the change of intracellular metabolism [17, 37] and the structural and functional variation of cell envelope [27, 38, 39] (Fig. 2). To improve l-glutamate production, metabolic engineering strategies including metabolic pathway engineering, regulatory engineering, transport system engineering and in C. glutamicum were established [17, 40] (Fig. 2 and Table 1). Meanwhile, glycerol-auxotrophic, fatty acid-auxotrophic, temperature-sensitive and mycolate defective C. glutamicum strains have been constructed [27, 41, 42].
For intracellular metabolic pathway engineering, the enhancement of the anaplerotic pathway of the PEP-pyruvate-OAA node has been proved to be an effective approach to increase the carbon flux for l-glutamate. As PEP is needed for the anaplerotic pathway, the replacement of the PEP-dependent phosphotransferase system (PTS) with non-PTS could save more PEP. The introduction of the key non-oxidative glycolytic (NOG) pathway enzyme, phosphoketolase (PKT) with (T2A/I6T/H260) with improved specific activity, into heterologous C. glutamicum Z188 resulted in 16.67% improvement in l-glutamate titer, compared with the wild-type BA-PKT [43]. Then, the overexpression of pyc gene encoding PEP carboxylase and pyruvate carboxylase (PCx) in l-glutamate producer strains increased the supply of OAA for l-glutamate synthesis and decreased byproduct excretion at the pyruvate node [44]. Meanwhile, Yao et al. reported that double disruption of dtsR1 (encoding a subunit of acetyl-CoA carboxylase complex) and pyc (encoding pyruvate carboxylase) caused increased activity of phosphoenolpyruvate carboxylase (PEPC) encoded by ppc, then facilitated efficient overproduction of l-glutamate in C. glutamicum ATCC13032 [45]. The above two strategies suggested that the increased OAA benefit l-glutamate production. Besides, the decrease in the 2-oxoglutarate dehydrogenase complex (ODHC, encoded by odhA) activity and the simultaneous increase of GDH (encoded by gltAB) activity is crucial and essential for the l-glutamate production [37]. In addition, the deletion of gene ldhA responsible for the accumulation of the byproduct l-lactate made 11.61% higher l-glutamate (70.7 g/L) and 58.50% lower l-alanine production in C. glutamicum [46].
Additionally, rational engineering regulation factors could efficiently improve l-glutamate. The gdh and gltBD genes encoding l-glutamate dehydrogenase and l-glutamate synthase, respectively, are negatively regulated by ArgR, the expression of gdh is controlled by the transcription regulator FarR (fatty acyl-responsive regulator), accordingly, it was suggested that the ArgR protein in combination with FarR of C. glutamicum contributes to the transcriptional control of l-glutamate biosynthesis pathways [15]. Li et al. identified a crucial transcription factor RosR in C. glutamicum G01 and demonstrated that RosR regulated l-glutamate metabolic network by binding to the promoters of glnA, pqo, ilvB, ilvN, ilvC, ldhA, odhA, dstr1, fas, argJ, ak, and pta, then overexpression of RosR in G01 resulted in significantly decreased by-products yield and improved l-glutamate titer (130.6 g/L) and yield (0.541 g/g from glucose) in fed-batch fermentation [36], which provided a good reference not only for other C. glutamicum platforms to further improve l-glutamate production but also for optimizing other amino acid biosynthesis in C. glutamicum strains.
For transport system engineering, l-glutamate synthesized intracellularly is exported through mechanosensitive transmembrane channel proteins (MscCG and MscCG2, encoded by mscCG and mscCG2) activated by the force-from-lipids [47, 48], and the sodium-coupled secondary l-glutamate uptake system encoded by gltS has also been defined [49]; the studies showed that the overexpression of mscCG or mscCG2 but deletion of gltS could all increase l-glutamate [47,48,49]. Notably, a high capacity export system capable of expelling the surplus of synthesized l-glutamate could be activated by higher temperature to 39 °C [41]. The higher temperature results in a rapid attenuation in oxoglutarate dehydrogenase complex (ODHC) activity and an increase from 28% to more than 90% of the isocitrate dehydrogenase flux split toward glutamate synthesis [41]. Therefore, a mutation in the C. glutamicum ltsA gene responsible for temperature-sensitive growth facilitated higher l-glutamate production at higher temperatures [42]. Further study suggested that the fluidity of the C. glutamicum mycomembrane plays an important role in glutamate excretion during the temperature-triggered process [50].
Notably, researchers established several efficient strategies based on synthetic biology approaches to improve l-glutamate production. Lin et al. established an efficient and stable microfluidic artificial photosynthetic system for reduced nicotinamide adenine dinucleotide (NADH) regeneration and l-glutamate synthesis under visible light, resulting that the NADH regeneration rate of the system reached 56.03%, the maximum production rate of l-glutamate was 98.3% [51]. Chen et al. found that the l-glutamate production could be increased 39–68% by the expression of PHB synthesis genes in C. glutamicum, with less intermediate metabolites or by-products including α-ketoglutarate, l-glutamine and lactate [31]. The overexpression of vgb gene encoding vitreoscilla hemoglobin (VHb) in C. glutamicum promoted 23% more l-glutamate production and 30% more cell density [52]. Heterologous expression of the araBAD operon from E. coli in the wild-type and in an l-lysine producing strain of C. glutamicum was shown to enable production of l-glutamate and l-lysine, respectively, from arabinose as sole carbon source [53].
Our recent study suggested that engineering the envelope of C. glutamicum could improve the l-glutamate production [27]. C. glutamicum has a specific mycolate outer membrane containing mycolic acid (MA) [54]. In C. glutamicum, l-glutamate production can be induced by biotin limitation [55], addition of β-lactam antibiotics [38, 56], and addition of fatty acid ester surfactants [57]. These conditions could all lead to decreased mycolic acid (MA) formation, suggesting that MA is related to l-glutamate production. In C. glutamicum, the lack of Pks13, which catalyzes the formation of MA, results to MA lack [58]. MA can be attached to glucose, forming keto glucose monoketo-corynomycolate (keto-GMCM), and the keto group of keto-GMCM is then reduced by ketoacyl reductase CmrA (encoded by cmrA) to form GMCM (Fig. 2). The cmrA mutant reduced the level of MA by 80% [59]. We found that the deletion of cmrA in C. glutamicum ATCC13869 promoted 10.77-fold more l-glutamate production than the control ATCC13869 [27]. Further transcriptomic analysis showed that the mutant cmrA causes the up-regulation of mscCG and the down-regulation of the genes relevant to l-arginine biosynthesis. However, the complete block of MA by deleting Pks13 resulted in the hindered cell growth and defective cell separation [58], which is not advisable. It suggested that the reasonable membrane engineering is a new synthetic biology strategy to design and optimize the C. glutamicum chassis.
As shown in Fig. 2 and Table 1, the efficient engineering strategies for l-glutamate focused not only on intracellular metabolism but also on the cell envelope, especially for the enhancement of the anaplerotic pathway of the PEP–pyruvate–OAA node, and the reduction of MA in cell envelope. However, there is still no report so far on a system biology engineering approach to combine these effective strategies for optimizing l-glutamate production in C. glutamicum.
The l-glutamate is derived from the intermediate α-ketoglutarate of TCA cycle and exported by McsCG or McsCG2, but uptake by GltS. PTS: phosphotransferase system; Glc: glucose; PP Pathway: pentose phosphate pathway; TCA cycle: tricarboxylic acid cycle; ATP: adenosine triphosphate; G6P: glucose 6-phosphate; G3P: glucose 3-phosphate; Acetyl-CoA (AcCoA), acetyl-coenzyme A; PEP: phosphoenol pyruvate; Glu: glutamate. The gay font and red X represent the interrupted routes. The green arrow represents the enhanced reactions.
Microbial chassis design and engineering for bioproduction of l-lysine
l-Lysine is mainly produced by C. glutamicum, and the reported highest production reaches 220 g/L by systematically engineered C. glutamicum [60]. Meanwhile, engineered E. coli MG1655 mutant could produce 125 g/L of l-lysine in a recent study report [61]. It suggested that microbial fermentation is an excellent approach for l-lysine production in food industry. l-Lysine is derived from OAA of TCA cycle, and OAA is mainly from PEP or pyruvate of glycolysis. One lysine synthesis needs 4 NADPH and 1 ATP, and the supply of succinyl-CoA and l-glutamate, then efflux or exported by LysE to extracellular (Fig. 3). Rational metabolic engineering strategies have been successfully applied in the breeding of l-lysine high-producing strains (Fig. 3 and Table 2). Metabolic engineering strategies to enhance carbon flux to l-lysine biosynthesis pathway are efficient to improve l-lysine production. Oxaloacetate (OAA) and l-glutamate are essential precursors for the biosynthesis of l-lysine. The sufficient biomass, OAA, l-glutamate, succinyl-CoA, and fed-batch biotin are important to improve l-lysine production [62, 63], and recent reports further confirmed that the related strategies could effectively improve l-lysine production [60, 61, 64, 65], such as overexpression of the PEP-Pyr-OAA node's genes, decrease of TCA cycle and OAA consumption, decrease in the activity of isocitrate dehydrogenase and citrate synthase, decrease of the pyruvate dehydrogenase complex (PDHC) activity. Reconstructing the diaminopimelic acid (DAP) pathway by deleting the nitrogen source regulatory protein AmtR is also functional for improvement of l-lysine [66]. These studies suggested that promoter engineering is efficient for rational enhancement or weaken of target genes. Here, we summarized l-lysine high-producing strains and their engineering strategies in Fig. 3 and Table 2.
The lysine biosynthetic pathways include two NADPH-dependent reactions that are catalyzed by aspartic semialdehyde dehydrogenase (encoded by asd) and dihydrodipicolinate reductase (encoded by dapB) [16]. Therefore, rationally optimizing the NADPH supply pathway is another efficient strategy for improving l-lysine. Besides traditionally enhancing PP pathway and NADPH regeneration reactions to improve NADPH supply [64, 67], researchers constructed or introduced some novel reactions to resolve the problem of NADPH limit, for examples, deleting the negative regulator Cgl2680 for NADPH regeneration [67]; replacing NAD+-dependent glyceraldehyde-3-phosphate dehydrogenase (GAPDH) with NADP+-dependent GAPDH from Streptococcus mutans [60, 64, 68]; overexpressing the membrane-bound transhydrogenase pntAB together with codon-optimized gapN, encoding NADPH-dependent glyceraldehyde 3-phosphate dehydrogenase, and mak, encoding fructokinase [69].
Apart from the pathway engineering, researchers found that rationally engineering glucose uptake systems could also functionally improve l-lysine production. The deletion of arabitol repressor AtlR made the l-lysine biosynthesis from mannitol in C. glutamicum [69]. Optimizing the expression of Pt dehydrogenase in the exeR genome locus with a competitive advantage over common contaminating microbes when grown on media containing Pt as a phosphorus source instead of phosphate could efficiently utilize xenobiotic phosphite (Pt) and establish a more robust fermentation process in C. glutamicum, resulting in 41.00 g/L l-lysine under nonsterile conditions [70]. C. glutamicum with starch utilization could be constructed by temperature-induced adaptive evolution, optimizing amylases expression, especially co-expression of alpha-amylase (AA) and glucoamylase (GA), and introducing AA-GA fusion protein, resulting 23.9 g/L l-lysine [71]. Rational modification of glucose uptake systems could improve the efficiency of l-lysine production through increasing the participation of non-PTSGlc in glucose utilization [72, 73]. In C. glutamicum, PTS transport systems for glucose, fructose and sucrose are different [74], due to the presence of two additional EII genes scrB and NCgl1861 for sucrose and fructose besides that for glucose, respectively [75, 76]. Furthermore, overexpression of inositol permeases (IolT1 and IolT2, encoded by iolT1, iolT2) [77] and ppgK resulted 201.6 g/L of l-lysine production with a productivity of 5.04 g/L/h and carbon yield of 0.65 g/g glucose in fed-batch culture [78]. Then introducing fructokinase from Clostridium acetobutylicum for a functional metabolic pathway of sucrose and fructose [75], as well as the replacement of phosphoenolpyruvate-dependent glucose and fructose uptake system including PTSGlc and PTSFru by inositol permeases (IolT1 and IolT2) with the deletion of negative regulator iolR, further resulted a significant improvement of l-lysine production [60].
In addition, the l-lysine production could be enhanced by engineering transport system in C. glutamicum. The yield of l-lysine was increased by 9.0%, 12.3%, and 10.0% after the deletion of three amino acid transmembrane transporters, namely, GluE, BrnE/BrnF, and LysP, respectively, in C. glutamicum 23,604 [79]. The yield, titer, and the specific production of l-lysine in an industrial C. glutamicum strain were enhanced by 7.8%, 9.5%, and 12% by introduction of a novel exporter MglE defined by Sailesh Malla based on the construction of Cow fecal metagenomic DNA library [80].
Here, we summarized three typical l-lysine high-production cell platforms C. glutamicum LYS-12, C. glutamicum strain K-8 and E. coli LATR11/pWG-DCSMASMBHc.gLP to study the rational design and engineering strategies for optimization of cell factories. J. Becker et al. constructed a genetically defined strain C. glutamicum LYS-12 hyperproducing 120 g/L l-lysine by systems metabolic engineering, with implementation of only 12 defined genome-based changes in genes encoding central metabolic enzymes redirected major carbon fluxes as desired toward the optimal pathway usage predicted by in silico modeling [64]. The engineered C. glutamicum strain K-8 produced highest l-lysine production of 221.3 g/L, with the highest productivity of 5.53 g/L/h and carbon yield of 0.71 g/g glucose in fed-batch fermentation [60]. The K-8 was constructed through four main engineering steps: enhancing the l-lysine biosynthesis pathway with the insertion of genes ppc and pyc in the genes pck and odx loci, deletion of the P1 promoter of gene gltA, and the introduction of Ptac-M promoter for gene gdh; introduction of fructokinase from Clostridium acetobutylicum for a functional metabolic pathway of sucrose and fructose; replacement of phosphoenolpyruvate-dependent glucose and fructose uptake system (PTS(Glc) and PTS(Fru)) by inositol permeases (IolT1 and IolT2) and ATP-dependent glucokinase (ATP-GlK); co-expression of bifunctional ADP-dependent glucokinase (ADP-GlK/PFK) and NADH dehydrogenase (NDH-2) as well as to inactivate SigmaH factor (SigH) to reduce the consumption of ATP and increasing ATP regeneration [60].
In addition to C. glutamicum, E. coli is another important producer for l-lysine, the metabolic engineering strategies in E. coli were similar to that in C. glutamicum. An l-lysine high-yielding strain was developed from E. coli LATR11 via overexpression of ppc, lysCT344M, asd, dapAH56K, dapB, and lysA combined with heterologous expression of C. glutamicum ddh, resulting 125.6 g/L of l-lysine with QP of 3.14 g/L/h and glucose conversion rate of 58.97% [61]. Another l-lysine high-producing E. coli ec_iML1515 model was constructed by optimizing the expression of the 20 top-demanded proteins, and then adjusting NH4 ( +) and dissolved oxygen levels to regulate the synthesis rate of energy metabolism-related proteins, causing lysine titers and glucose yields to increase to 193.6 g/L and 0.74 g/g, respectively. The ec_iML1515 model provides insight into how enzymes required for the biosynthesis of certain products are distributed between and within metabolic pathways [81]. Moreover, using a newly developed high-throughput screening method (fluorescence-activated cell sorting from a 10-million-mutant library generated from a l-lysine high-producing E. coli strain) also evolved two l-lysine high-producing mutants MU-1 and MU-2 with 136.51 and 133.2 9 g/L of lysine, respectively [82]. We found that the efficient strategies for designing and constructing excellent microbial chassis for l-lysine mainly were developed in recent reports, which brought a good reference for microbial chassis design and construction for other amino acids production.
The l-lysine is derived from the intermediate OAA of TCA cycle, and exported by diffusion or LysE, but uptake by LysP. PTS: phosphotransferase system; Glc: glucose; PP Pathway: pentose phosphate pathway; TCA cycle: tricarboxylic acid cycle; ATP: adenosine triphosphate; G6P: glucose 6-phosphate; G3P: glucose 3-phosphate; R5P: ribulose 5-phosphate; F6P: fructose 6-phosphate; F1P: fructose 1-phosphate; FBP: fructose 1,6-bisphosphate; E4P: erithrose 4-phosphate; G3P: glucose 3-phosphate; Acetyl-CoA (AcCoA), acetyl-coenzyme A; PEP: phosphoenol pyruvate. The red font and lines represent the extrinsic routes, whereas the gay font and red X represent the interrupted routes. The green arrow represents the enhanced reactions. The blue font represents the crucial immediate for l-lysine production.
Microbial chassis design and engineering for bioproduction of l-threonine
l-Threonine, as a typical oxaloacetate derivative, is an essential amino acid and is produced by E. coli and C. glutamicum [84]. Rationally engineered E. coli producers could produce l-threonine up to 130 g/L, while the C. glutamicum mutants produce much lower production, only 11 g/L. l-Threonine is biosynthesized from OAA of TCA cycle (Fig. 4), and requires multiple cofactors (NADPH and ATP) [85]. To date, there have been a lot of efforts to improve l-threonine productivity in engineered microbes [86,87,88,89].
In E. coli, to optimize l-threonine production, researchers tried many efforts on the entire metabolic pathways and regulatory systems, and obtained many excellent l-threonine high producers (Table 3 and Fig. 4). As like the construction of l-lysine producer, developing an l-threonine-producing strain from wild-type E. coli generally is focused on modifying the glucose uptake, glyoxylate shunt, l-threonine biosynthetic pathway, regulators, transport system and cofactors supply [90]. Lee et al. constructed the first genetically defined producing strain from the basal strain E. coli WL3110, a lacI-mutant strain of W3110 [91]. They rewired regulatory and metabolic circuits for the development of an initial threonine producer TH07 (pBRThrABC) by removing the feedback inhibition for thrA, metL, and blocking the competing pathways of l-threonine by deleting lysA and tdh, mutant ilvAC290T, then overexpressing ppc (phosphoenolpyruvate carboxylase), enhancing l-threonine exporter system and the glyoxylate shunt; then reducing the by-products acetic acid and overexpressing the acs gene to synthesize more acetyl-CoA, resulting in a final l-threonine producing strain TH28C with an l-threonine titer of 82.4 g/L [91]. The l-threonine production could be improved by elimination of transporters ProP and ProVWX in E. coli [92]. In addition, the betaine supplementation also benefits to improve l-threonine production [92, 93].
As description above, metabolic engineering cooperated with metabolic regulation has been developed to maximize production of natural chemicals [94], especially large-volume amino acid products [14, 16]. However, unbalanced cellular metabolic flux distributions between cell growth and target products have long limited product yield and cell productivity [95]. Traditional techniques, such as blocking the bypass pathways and overexpressing the key genes of metabolic pathways, cannot address the challenges associated with more complex carbon distribution [96], for which it requires multiple cofactors to participate in product synthesis [97]. Recently, researchers designed and constructed dynamic regulation systems to balance the cell growth and l-threonine production, resulting the excellent l-threonine producers E. coli EC125 (105.3 g/L), E. coli TRFC (124.57 g/L from sucrose), E. coli THPE5 (70.8 g/L), and E. coli TWF106/pFT24rp, as shown in Table 2. Especially for the TWF106/pFT24rp, a maximized conversion efficiency of 82% from glucose was reported [19]. To reduce metabolic burden on the host during cell growth stage, a thermal switch system was designed and applied to divide the whole fermentation process into two stages: growth and production [19], redistributing metabolic intermediate ratio between pyruvate and oxaloacetate through cooperating thermal decarboxylation of oxaloacetate with overexpression of pyruvate carboxylase [19]. The thermal switch system was then employed to switch off the l-alanine synthesis pathway, resulting in the highest l-threonine yield of 124.03%, which exceeds the best reported yield (87.88%) and the maximum available theoretical value of l-threonine production (122.47%) [19].
In addition to the metabolic engineering strategies, synthetic biology-based engineering approaches also efficiently improve l-threonine production. Wang et al. found that the overexpression of gene cluster phaCAB from R. eutropha in l-threonine-producing E. coli TFW001, the resulting strain TWF001/pFW01-phaCAB could produce 96.4-g/L l-threonine in 3-L fermenter and 133.5-g/L l-threonine in 10-L fermenter, respectively [32]. The genome reduction is also functional in optimizing l-threonine production. The genome-reduced E. coli strain MDS42 which lacks about 700 genes or 14.3% of its genome as compared to the wild-type E. coli strain MG1655, produced 40 g/L of l-threonine with a yield of 0.4 g/g glucose, increasing about 80% than the mutant strain derived from the wild-type strain MG1655 by the same metabolic engineering strategy [23]. Notably, our latest studies showed that outer membrane simplification benefits the l-threonine production. The 12 genes responsible for the biosynthesis of the enterobacterial common antigen and 50 genes responsible for flagellar biosynthesis in outer membrane were deleted in E. coli MG1655, resulting in WQM022 [28], and all 12 chaperone-usher operons including 64 genes were deleted in MG1655, resulting in the fimbria-lacking strain WQM026 [29]. Mutants WQM022 and WQM026 grew better and could synthesize more l-threonine than MG1655. The knowledge gathered from this study may be applied to the development of superior chassis microorganisms.
We found that genetically defined E. coli chassis produced high production of l-threonine, while C. glutamicum produce much lower l-threonine. In C. glutamicum ATCC13869, the l-threonine production could be improved by blocking l-lysine production and efflux (deleting ddh enconding diaminopimelate dehydrogenase and lysE), and overexpressing lysC, hom and thrB, while the resulting strain IDW103 only produced 7.7 g/L l-threonine [98]. In addition, strain C. glutamicum ATCC21799/pGC42 produced 11.8 g/L l-threonine after enhancing biosynthesis pathway [99], and strain C. glutamicum MH20-22B-(homr-thrB) (pEC-T18mob2-thrE) produce 8.1 g/L l-threonine after enhancing l-threonine biosynthesis pathway and l-threonine efflux in MH20-22B [100]. Notably, the two engineered C. glutamicum strains for l-threonine were both derived from l-lysine producers. As we know, the production of l-lysine by C. glutamicum could reach above 220 g/L, while the l-threonine production of C. glutamicum showed much lower level, which suggested that there are still many difficulties in designing and engineering C. glutamicum chassis for improving l-threonine production. Although it is promising to make better l-threonine C. glutamicum chassis by taking more advantages of the current excellent lysine producers as the start strains. The complex and unbalanced metabolic regulation and low efficiency of efflux in C. glutamicum are the major limitation for l-threonine biosynthesis. Maybe, the dynamic metabolic regulation system to dissolve the unbalance and improve the efflux efficiency would be functional for improving l-threonine production in C. glutamicum.
The l-threonine is derived from the intermediate OAA of TCA cycle and exported by RhtABC, but uptake by TdcC or SstT. PTS: phosphotransferase system; Glc: glucose; PP Pathway: pentose phosphate pathway; TCA cycle: tricarboxylic acid cycle; ATP: adenosine triphosphate; G6P: glucose 6-phosphate; G3P: glucose 3-phosphate; R5P: ribulose 5-phosphate; F6P: fructose 6-phosphate; F1P: fructose 1-phosphate; FBP: fructose 1,6-bisphosphate; E4P: erithrose 4-phosphate; G3P: glucose 3-phosphate; Acetyl-CoA (AcCoA), acetyl-coenzyme A; PEP: phosphoenol pyruvate. The red font and lines represent the extrinsic routes, whereas the gay font and red X represent the interrupted routes. The green arrow represents the enhanced reactions. The blue font represents the crucial immediate for l-threonine production.
Microbial chassis design and engineering for production of l-tryptophan
As one of the three important aromatic amino acids, l-tryptophan has been widely used in food ingredients. Besides Bacillus subtilis [104] and yeast [105], E. coli and C. glutamicum are mainly used as chassis bacteria for l-tryptophan production [106, 107]. The highest l-tryptophan titer of 58 g/L was achieved after 80 h fed-batch fermentation by a recombinant C. glutamicum strain with increasing transketolase activity [107]. Recently, most engineered high-producing microbial cell factories for l-tryptophan are genetically defined E. coli mutants (Fig. 5 and Table 4), because E. coli owns favorable characteristics, such as genetic tractability, metabolic plasticity as well as various existing and on-going tools for genetic engineering [106].
Genetically defined E. coli mutants are the general microbial platforms for l-tryptophan production, the rational design and engineering strategies facilitated l-tryptophan production to 40–55 g/L with conversion efficiency of 18–23% from glucose. E4P of PP pathway and PEP of glycolysis are the important precursors of l-tryptophan; meanwhile, additional precursors such as l-serine, PRPP, and glutamine are required (Fig. 5). In order to avoid carbon loss and increase the E4P supply, the gene zwf (encoding glucose 6-phosphate dehydrogenase) was deleted while the gene tktA (encoding transketolase) was overexpressed [108]. As shown in Fig. 5, the feedback inhibition steps exist in the biosynthesis pathway of l-tryptophan in E. coli. Feedback inhibition by l-tryptophan is exerted on DAHP synthase (encoded by aroH) and anthranillate synthase, AS (encoded by trpED) in E. coli. Furthermore, the genes of trpLEDCBA operon are repressed by TrpR, another regulation is made by the attenuation through trpL [108]. Strategies based on the regulatory mechanisms in l-tryptophan biosynthetic pathway for increasing precursor pools, relieving the feedback inhibition, repression and attenuation, trimming the competing and degradation pathways are functional to improve l-tryptophan production [106, 108, 109]. Metabolic engineering approaches have been attempted so far (Fig. 5 and Table 3). In the l-tryptophan biosynthesis, the overexpression of aroGfbr, aroFfbr, and trpEfbr [110, 111], as well as the tryptophan synthetic pathway genes, trpEDCBA must be considered [110, 111]. Furthermore, the deletion of trpR and trpL genes, and the block of by-products including acetate and indole accumulation are effective [33, 110,111,112,113,114,115]. Moreover, engineering transporters Mtr, TnaB, AroP, and YddG to increase l-tryptophan secretion into extracellular [108, 116, 117].
Wang et al. reported that the genetically defined E. coli TRTH0709/pMEL03 produced 48.86 g/L l-tryptophan with a highest glucose conversion rate of 0.2187 g/g [117], this high producing strain chassis was rationally designed and engineered from E. coli MG1655 by deleting trpR, tnaA, pta, mtr and overexpression of yddG, aroGfbrtrpEfbr DCBA, serA, tktA, and ppsA [117]. For another example, Du et al. rationally designed a “bottom-up” metabolic engineering strategy and gradually modified the l-l-tryptophan biosynthetic pathway, the central metabolic pathway and citric acid transport system with methods of CRISPR–CAS9 and promoter engineering in E. coli W3110, to obtain an l-tryptophan high-producing strain E. coli TRP07 (W3110ΔlacIΔtnaAΔmtrPtrc-trpE*::trpLE Ptrc-aroG*::tyrR ΔpykAΔppc Ppck-pck::ycjV PcitT-citT::poxB Plac-acnB-acnA-icd::ygh Plac-pyc::yjiV)), which produces 49 g/L l-tryptophan with glucose conversion efficiency of 0.186 g/g glucose [113]. In strain E. coli TRP07, the tryptophan degradation pathway and transport system were down-regulated by deleting tnaA and mtr, then the branch acid synthesis pathway was enhanced by integrating Ptrc-trpE (S40F) into the trpLE locus, then the shikimic acid pathway was up-regulated by integrating Ptrc-aroG (S211F) was into the tyrR locus, then the PEP metabolic pathway was down-regulated by deleting pykA and ppc, but the PEP synthesis pathway was enhanced by up-regulated pck and pps, at last, the citric acid transport and TCA cycle were enhanced by up-regulating citT, acnAB, icd, and pyc and deleting ycjV, poxB, and yjiV [113]. In addition, Liu et al. pointed out that modification of phosphoenolpyruvate glucose phosphotransferase system increases the conversion rate between glucose and l-l-tryptophan in E. coli [118]. Moreover, similar to l-glutamate and l-threonine, the co-production of PHB by introducing phaCAB operon in E. coli is also functional on improving l-tryptophan [33].
However, the effect of the reported studies are still far from the theoretical maximum, which means that there is still much room to improve the yield of l-tryptophan by balancing the ratio of biomass formation and l-tryptophan production. The development of dynamic regulation system to balance cell growth and metabolic flux to produce l-tryptophan would be a promising strategy in the near future study. In addition, neither B. subtilis nor C. glutamicum owns favorable characteristics, such as genetic tractability, metabolic plasticity as well as various existing and on-going tools for genetic engineering [106]. Therefore, how to improve the robustness and streamline of strains is very important for strain breeding and fermentation process controlling. Moreover, unlike l-threonine, there have been few reports on genome reduction to construct l-tryptophan high-producing strain. In addition, the C. glutamicum is a food-safety microbial cell factory for producing l-tryptophan, while the C. glutamicum KY9218/pIK9960 is the only reported genetically defined C. glutamicum. To promote the l-tryptophan production in food industry, the better optimized and metabolic engineered C. glutamicum chassis should be designed and developed in the future.
l-tryptophan is derived from the intermediates PEP of glycolysis and E4P of PP pathway. In addition, additional precursors such as PRPP, l-glutamine, and l-serine are required. l-tryptophan is exported by YddG and YedA, but uptake to intracellular by Mtr, TnaB, and AroP. The biosynthesis pathway of l-tryptophan could be regulated by many inhibition regulators, and the inhibition steps were marked as red dotted lines. PTS: phosphotransferase system; Glc: glucose; PP Pathway: pentose phosphate pathway; TCA cycle: tricarboxylic acid cycle; ATP: adenosine triphosphate; G6P: glucose 6-phosphate; R5P: ribulose 5-phosphate; F6P: fructose 6-phosphate;F1P: fructose 1-phosphate; FBP: fructose 1,6-bisphosphate; E4P: erithrose 4-phosphate; G3P: glucose 3-phosphate; Acetyl-CoA (AcCoA), acetyl-coenzyme A; PEP: phosphoenol pyruvate; Ser: serine; Trp: tryptophan; Gln: glutamine; Glu: glutamate; PHB: poly-3-hydroxybutyrate; DAHP: 3-deoxy-darabino-heptulosonate 7-phosphate; GAP: glyceraldehyde 3-phosphate; DHQ: 3-dehydroquinate; DHS: 3-dehydoshikimate; SA: shikimic acid; S3P: shikimate 3-phosphate; SHK: shikimic acid kinase; EPSP: 5-enolpyruvateshikimate 3-phosphate; CHA: chorismic acid; ANT: anthranilate; PRA: phosphoribosyl-anthranilate; CDRP: carboxyphenylamino deoxyribulose phosphate; IGP: indole glycerol phosphate. In C. glutamicum, two types of DAHPSs exist. One is an l-tyrosine sensitive, while another one (Type II) is l-phenylalanine and l-tyrosine sensitive. Others are similar as stated above for E. coli. The red font and lines represent the feedback inhibition regulators and steps, whereas the gay font and red X represent the interrupted routes. The green arrow represents the enhanced reactions. The blue font represents the crucial immediate for l-threonine production.
Perspectives
Systems metabolic engineering and synthetic biology engineering have become efficient and necessary ways to perform strain improvement and bioproduction of amino acids. With the development of advanced techniques of systems biology and synthetic biology, more excellent strains will be undoubtedly generated in the future. However, it is far from ideal productivity for many amino acids fermentation according to the current studies. Moreover, there are still many challenges for amino acids production by C. glutamicum and E. coli, such as the low productivity for l-threonine and l-tryptophan by C. glutamicum platforms; lack of a systematic approach combining different engineering strategies established in different studies; understanding limitations of global and useful information such as omics data or unknown regulation networks, leading to poor integration of useful information into engineering strategies; and food-safety hazards existed in the amino acids production by E. coli. Therefore, we still need lots of efforts to overcome these difficulties for optimizing the production of amino acids used in food industries in the future.
We believed that engineering strategies for improving different amino acids can be learned from each other. For example, some engineering strategies for optimizing C. glutamicum to produce l-lysine can be used in the construction of C. glutamicum producers to synthesize l-threonine or l-tryptophan; in addition, some rational design and efficient approaches based on novel technologies developed in E. coli should be rationally designed and developed in C. glutamicum, such as dynamic switch systems or promoter regulation systems, which could better realize the balance between cell growth and chemicals production or the balance of metabolic flux distribution. Notably, it should be considered to try our best to combine the advantages of different efficient engineering strategies and integrated them into a genetically defined and excellent microbial chassis. For example, researchers have found that rational genome-scale optimization [22, 23], simplification of cell envelope or cell membranes [24,25,26, 28, 29, 119], engineering on cell lifespan [120] and so on, have significant positive influences on cellular metabolism including the amino acids biosynthesis, thus the potential and efficient engineering strategies should be attempted when designing and engineering the microbial chassis for amino acids production. Moreover, engineering strategies for optimizing substrate sugars utilization could also be considered for the production of different amino acids. Carbohydrate uptake and phosphorylation are mainly executed by phosphoenolpyruvate-carbohydrate PTS consisting of 1 membrane-bound carbohydrate-specific EIIABC component (EII) and 2 cytoplasmic components (i.e., enzyme I (EI) and histidine protein (HPr). Studies suggested that enhance PTS-independent carbohydrate uptake systems (i.e., non-PTS) also benefit for cell growth and production of target amino acids; i.e., introduction of fructokinase from Clostridium acetobutylicum for a functional metabolic pathway of sucrose and fructose, and replacement of the phosphoenolpyruvate-dependent glucose and fructose uptake system PTSGlc and PTSFru by inositol permeases IolT1 and IolT2, could both realize the utility of other substrates sucrose and fructose to form amino acids [60]. Until now, biosensor has also been developed to control target gene expression, export system on amino acid, and amino acid-producing strain screening. For example, the utility of biosensor-enabled high-throughput screening facilitated higher production of beta-alanine [121], which suggested that biosensor engineering will be a perspective strategy. In addition, process engineering strategies such as optimization of substrate spectrum and process robustness by rational and evolutive strategies have been applied to efficiently increase amino acid titers, yields, and productivities [122]. Notably, as Gram-negative bacteria, E. coli owns less safety comparing with C. glutamicum. Therefore, we should pay attention to how to minimize the potential safety risk of E. coli to produce amino acids used in food industries. Notably, in our previously published review or paper, it was specifically pointed out that the safety hazards can be reduced by modifying the outer membrane of E. coli [119, 123], including biofilm, multiple antibiotic resistance, pathogenicity or toxicity of lipopolysaccharides, common antigen, pili, virulence factors or peptides and capsular polysaccharides. Therefore, rational cell envelope modification strategies, including simplification of lipopolysaccharide to the minimal structure Kdo2-lipid A, and deletion of other above membrane structures, are expected to be carried out in future modified strains. With all these efforts, systems metabolic engineering and synthetic biology engineering will contribute more to developing excellent microbial chassis for the production of amino acids used in food industries.
Abbreviations
- PTS:
-
Phosphotransferase system
- Glc:
-
Glucose
- PP pathway:
-
Pentose phosphate pathway
- TCA cycle:
-
Tricarboxylic acid cycle
- ATP:
-
Adenosine triphosphate
- G6P:
-
Glucose 6-phosphate
- R5P:
-
Ribulose 5-phosphate
- F6P:
-
Fructose 6-phosphate
- F1P:
-
Fructose 1-phosphate
- FBP:
-
Fructose 1,6-bisphosphate
- E4P:
-
Erithrose 4-phosphate
- G3P:
-
Glucose 3-phosphate
- Acetyl-CoA (AcCoA):
-
Acetyl-coenzyme A
- α-KG:
-
α-Ketoglutaric acid
- Ser:
-
Serine
- Gly:
-
Glycine
- Cys:
-
Cysteine
- Trp:
-
Tryptophan
- Phe:
-
Phenylalanine
- Tyr:
-
Tyrosine
- Val:
-
Valine
- Leu:
-
Leucine
- Lys:
-
Lysine
- Thr:
-
Threonine
- Ile:
-
Isoleucine
- Gln:
-
Glutamine
- Glu:
-
Glutamate
- Arg:
-
Arginine
- Pro:
-
Proline
- GABA:
-
γ-Amino butyric acid
- GMCM:
-
Glucose monocorynomycolate
- Keto-GMCM:
-
Keto-glucose monocorynomycolate
- MA:
-
Mycolic acid
- l-Asp:
-
L-aspartic acid
- l-Aspartyl-P:
-
L-aspartyl-phosphate
- PEP:
-
Phosphoenol pyruvate
- OAA:
-
Oxaloacetate
- Pyr:
-
Pyruvate
- PHB:
-
Poly-3-hydroxybutyrate
- DAHP:
-
3-Deoxy-darabino-heptulosonate 7-phosphate
- GAP:
-
Glyceraldehyde 3-phosphate
- DHQ:
-
3-Dehydroquinate
- DHS:
-
3-Dehydoshikimate
- SA:
-
Shikimic acid
- S3P:
-
Shikimate 3-phosphate
- SHK:
-
Shikimic acid kinase
- EPSP:
-
5-Enolpyruvateshikimate 3-phosphate
- CHA:
-
Chorismic acid
- ANT:
-
Anthranilate
- PRA:
-
Phosphoribosyl-anthranilate
- CDRP:
-
Carboxyphenylamino deoxyribulose phosphate
- IGP:
-
Indole glycerol phosphate
- HPr:
-
Histidine phosphorylatable protein
- EI:
-
Enzyme I of PTS
- EII:
-
Enzyme II of PTS
References
Wendisch VF. Metabolic engineering advances and prospects for amino acid production. Metab Eng. 2020;58:17–34.
Viray CM, van Magill B, Zreiqat H, Ramaswamy Y. Stereolithographic visible-light printing of poly(l-glutamic acid) hydrogel scaffolds. ACS Biomater Sci Eng. 2022;8:1115–31.
Park SJ, Kim EY, Noh W, Oh YH, Kim HY, Song BK, Cho KM, Hong SH, Lee SH, Jegal J. Synthesis of nylon 4 from gamma-aminobutyrate (GABA) produced by recombinant Escherichia coli. Bioprocess Biosyst Eng. 2013;36:885–92.
Grace A, Murphy R, Dillon A, Smith D, Cryan SA, Heise A, Fitzgerald-Hughes D. Modified poly(L-lysine)-based structures as novel antimicrobials for diabetic foot infections, an in-vitro study. HRB Open Res. 2022;5:4.
Li Y, Zhang A, Hu S, Chen K, Ouyang P. Efficient and scalable synthesis of 1,5-diamino-2-hydroxy-pentane from l-lysine via cascade catalysis using engineered Escherichia coli. Microb Cell Fact. 2022;21:142.
Darbin O, Risso JJ, Weiss M, Rostain JC. Pallidal administrations of gabazine and 5-AVA affect pressure-induced behavioral disorders in rats. Pharmacol Biochem Behav. 2002;71:319–24.
Han T, Kim GB, Lee SY. Glutaric acid production by systems metabolic engineering of an l-lysine-overproducing Corynebacterium glutamicum. Proc Natl Acad Sci USA. 2020;117:30328–34.
Hong YG, Moon YM, Choi TR, Jung HR, Yang SY, Ahn JO, Joo JC, Park K, Kim YG, Bhatia SK, et al. Enhanced production of glutaric acid by NADH oxidase and GabD-reinforced bioconversion from l-lysine. Biotechnol Bioeng. 2019;116:333–41.
Hu S, Li Y, Zhang A, Li H, Chen K, Ouyang P. Designing of an efficient whole-cell biocatalyst system for converting l-lysine into cis-3-hydroxypipecolic acid. Front Microbiol. 2022;13: 945184.
Carlier JP, Henry C, Lorin V, Rouffignat K. Conversion of dl-threonine, d-threonine and 2-oxobutyrate into propionate and 2-hydroxybutyrate by Fusobacterium species. Lett Appl Microbiol. 1997;25:371–4.
Choi GG, Kim MW, Kim JY, Rhee YH. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with high molar fractions of 3-hydroxyvalerate by a threonine-overproducing mutant of Alcaligenes sp. SH-69. Biotechnol Lett. 2003;25:665–70.
Wang Q, Liu X, Qi Q. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from glucose with elevated 3-hydroxyvalerate fraction via combined citramalate and threonine pathway in Escherichia coli. Appl Microbiol Biotechnol. 2014;98:3923–31.
Ahmad S, Mohammed M, Mekala LP, Chintalapati S, Chintalapati VR. Tryptophan, a non-canonical melanin precursor: new l-tryptophan based melanin production by Rubrivivax benzoatilyticus JA2. Sci Rep. 2020;10:8925.
Zhao K, Liu J, Gao C, Liu J, Chen X, Liu L, Guo L. Advances in microbial production of feed amino acid. Adv Appl Microbiol. 2022;119:1–33.
Lee JH, Wendisch VF. Production of amino acids—genetic and metabolic engineering approaches. Bioresour Technol. 2017;245:1575–87.
Hirasawa T, Shimizu H. Recent advances in amino acid production by microbial cells. Curr Opin Biotechnol. 2016;42:133–46.
Ma Q, Zhang Q, Xu Q, Zhang C, Li Y, Fan X, Xie X, Chen N. Systems metabolic engineering strategies for the production of amino acids. Synth Syst Biotechnol. 2017;2:87–96.
Dong X, Quinn PJ, Wang X. Microbial metabolic engineering for l-threonine production. Subcell Biochem. 2012;64:283–302.
Fang Y, Wang J, Ma W, Yang J, Zhang H, Zhao L, Chen S, Zhang S, Hu X, Li Y, Wang X. Rebalancing microbial carbon distribution for l-threonine maximization using a thermal switch system. Metab Eng. 2020;61:33–46.
Unthan S, Baumgart M, Radek A, Herbst M, Siebert D, Bruhl N, Bartsch A, Bott M, Wiechert W, Marin K, et al. Chassis organism from Corynebacterium glutamicum—a top-down approach to identify and delete irrelevant gene clusters. Biotechnol J. 2015;10:290–301.
Perez-Garcia F, Peters-Wendisch P, Wendisch VF. Engineering Corynebacterium glutamicum for fast production of l-lysine and l-pipecolic acid. Appl Microbiol Biotechnol. 2016;100:8075–90.
Mizoguchi H, Sawano Y, Kato J, Mori H. Superpositioning of deletions promotes growth of Escherichia coli with a reduced genome. DNA Res. 2008;15:277–84.
Lee JH, Sung BH, Kim MS, Blattner FR, Yoon BH, Kim JH, Kim SC. Metabolic engineering of a reduced-genome strain of Escherichia coli for l-threonine production. Microb Cell Fact. 2009;8:2.
Wang J, Ma W, Wang Y, Lin L, Wang T, Wang Y, Li Y, Wang X. Deletion of 76 genes relevant to flagella and pili formation to facilitate polyhydroxyalkanoate production in Pseudomonas putida. Appl Microbiol Biotechnol. 2018;102:10523–39.
Wang J, Ma W, Fang Y, Zhang H, Liang H, Li Y, Wang X. Truncating the structure of lipopolysaccharide in Escherichia coli can effectively improve poly-3-hydroxybutyrate production. ACS Synth Biol. 2020;9:1201–15.
Wang J, Ma W, Fang Y, Zhang H, Liang H, Liu H, Wang T, Chen S, Ji J, Wang X. Engineering the outer membrane could facilitate better bacterial performance and effectively enhance poly-3-hydroxybutyrate accumulation. Appl Environ Microbiol. 2021;87: e0138921.
Li H, Xu D, Liu Y, Tan X, Qiao J, Li Z, Qi B, Hu X, Wang X. Preventing mycolic acid reduction in Corynebacterium glutamicum can efficiently increase l-glutamate production. Biochem Eng J. 2022;177: 108255.
Qiao J, Tan X, Ren H, Wu Z, Hu X, Wang X. Construction of an Escherichia coli strain lacking fimbriae by deleting 64 genes and its application for efficient production of poly(3-hydroxybutyrate) and l-threonine. Appl Environ Microbiol. 2021;87: e0038121.
Qiao J, Tan X, Huang D, Li H, Wang Z, Ren H, Hu X, Wang X. Construction and application of an Escherichia coli strain lacking 62 genes responsible for the biosynthesis of enterobacterial common antigen and flagella. J Agric Food Chem. 2021;69:4153–63.
Kromer JO, Sorgenfrei O, Klopprogge K, Heinzle E, Wittmann C. In-depth profiling of lysine-producing Corynebacterium glutamicum by combined analysis of the transcriptome, metabolome, and fluxome. J Bacteriol. 2004;186:1769–84.
Liu Q, Ouyang SP, Kim J, Chen GQ. The impact of PHB accumulation on l-glutamate production by recombinant Corynebacterium glutamicum. J Biotechnol. 2007;132:273–9.
Wang J, Ma W, Fang Y, Yang J, Zhan J, Chen S, Wang X. Increasing l-threonine production in Escherichia coli by overexpressing the gene cluster phaCAB. J Ind Microbiol Biotechnol. 2019;46:1557–68.
Gu P, Kang J, Yang F, Wang Q, Liang Q, Qi Q. The improved l-tryptophan production in recombinant Escherichia coli by expressing the polyhydroxybutyrate synthesis pathway. Appl Microbiol Biotechnol. 2013;97:4121–7.
Jinap S, Hajeb P. Glutamate. Its applications in food and contribution to health. Appetite. 2010;55:1–10.
Hermann T. Industrial production of amino acids by coryneform bacteria. J Biotechnol. 2003;104:155–72.
Li X, Bao T, Osire T, Qiao Z, Liu J, Zhang X, Xu M, Yang T, Rao Z. MarR-type transcription factor RosR regulates glutamate metabolism network and promotes accumulation of l-glutamate in Corynebacterium glutamicum G01. Bioresour Technol. 2021;342: 125945.
Asakura Y, Kimura E, Usuda Y, Kawahara Y, Matsui K, Osumi T, Nakamatsu T. Altered metabolic flux due to deletion of odhA causes l-glutamate overproduction in Corynebacterium glutamicum. Appl Environ Microbiol. 2007;73:1308–19.
Radmacher E, Stansen KC, Besra GS, Alderwick LJ, Maughan WN, Hollweg G, Sahm H, Wendisch VF, Eggeling L. Ethambutol, a cell wall inhibitor of Mycobacterium tuberculosis, elicits l-glutamate efflux of Corynebacterium glutamicum. Microbiology (Reading). 2005;151:1359–68.
Hirasawa T, Wachi M. Glutamate fermentation-2: mechanism of l-glutamate overproduction in Corynebacterium glutamicum. Adv Biochem Eng Biotechnol. 2017;159:57–72.
Sheng Q, Wu XY, Xu X, Tan X, Li Z, Zhang B. Production of l-glutamate family amino acids in Corynebacterium glutamicum: physiological mechanism, genetic modulation, and prospects. Synth Syst Biotechnol. 2021;6:302–25.
Uy D, Delaunay S, Goergen JL, Engasser JM. Dynamics of glutamate synthesis and excretion fluxes in batch and continuous cultures of temperature-triggered Corynebacterium glutamicum. Bioprocess Biosyst Eng. 2005;27:153–62.
Hirasawa T, Wachi M, Nagai K. L-glutamate production by lysozyme-sensitive Corynebacterium glutamicum ltsA mutant strains. BMC Biotechnol. 2001;1:9.
Dele-Osibanjo T, Li Q, Zhang X, Guo X, Feng J, Liu J, Sun X, Wang X, Zhou W, Zheng P, et al. Growth-coupled evolution of phosphoketolase to improve l-glutamate production by Corynebacterium glutamicum. Appl Microbiol Biotechnol. 2019;103:8413–25.
Guo X, Wang J, Xie X, Xu Q, Zhang C, Chen N. Enhancing the supply of oxaloacetate for l-glutamate production by pyc overexpression in different Corynebacterium glutamicum. Biotechnol Lett. 2013;35:943–50.
Yao W, Deng X, Zhong H, Liu M, Zheng P, Sun Z, Zhang Y. Double deletion of dtsR1 and pyc induce efficient l-glutamate overproduction in Corynebacterium glutamicum. J Ind Microbiol Biotechnol. 2009;36:911–21.
Zhang D, Guan D, Liang J, Guo C, Xie X, Zhang C, Xu Q, Chen N. Reducing lactate secretion by ldhA deletion in l-glutamate-producing strain Corynebacterium glutamicum GDK-9. Braz J Microbiol. 2014;45:1477–83.
Kawasaki H, Martinac B. Mechanosensitive channels of Corynebacterium glutamicum functioning as exporters of l-glutamate and other valuable metabolites. Curr Opin Chem Biol. 2020;59:77–83.
Wang Y, Cao G, Xu D, Fan L, Wu X, Ni X, Zhao S, Zheng P, Sun J, Ma Y. A Novel Corynebacterium glutamicum l-Glutamate Exporter. Appl Environ Microbiol. 2018;84: e02691.
Trotschel C, Kandirali S, Diaz-Achirica P, Meinhardt A, Morbach S, Kramer R, Burkovski A. GltS, the sodium-coupled l-glutamate uptake system of Corynebacterium glutamicum: identification of the corresponding gene and impact on l-glutamate production. Appl Microbiol Biotechnol. 2003;60:738–42.
Bokas D, Uy D, Grattepanche F, Duportail G, Guedon E, Delaunay S, Goergen JL. Cell envelope fluidity modification for an effective glutamate excretion in Corynebacterium glutamicum 2262. Appl Microbiol Biotechnol. 2007;76:773–81.
Lin HC, Liu Y, Yang CH, Zhao GZ, Song J, Zhang TY, Huang XW. Microfluidic artificial photosynthetic system for continuous NADH regeneration and l-glutamate synthesis. Catal Sci Technol. 2022;12:4057–65.
Liu Q, Zhang J, Wei XX, Ouyang SP, Wu Q, Chen GQ. Microbial production of l-glutamate and l-glutamine by recombinant Corynebacterium glutamicum harboring Vitreoscilla hemoglobin gene vgb. Appl Microbiol Biotechnol. 2008;77:1297–304.
Schneider J, Niermann K, Wendisch VF. Production of the amino acids l-glutamate, l-lysine, l-ornithine and l-arginine from arabinose by recombinant Corynebacterium glutamicum. J Biotechnol. 2011;154:191–8.
Klatt S, Brammananth R, O’Callaghan S, Kouremenos KA, Tull D, Crellin PK, Coppel RL, McConville MJ. Identification of novel lipid modifications and intermembrane dynamics in Corynebacterium glutamicum using high-resolution mass spectrometry. J Lipid Res. 2018;59:1190–204.
Gutmann M, Hoischen C, Kramer R. Carrier-mediated glutamate secretion by Corynebacterium glutamicum under biotin limitation. Biochim Biophys Acta. 1992;1112:115–23.
Hirasawa T, Saito M, Yoshikawa K, Furusawa C, Shmizu H. Integrated analysis of the transcriptome and metabolome of Corynebacterium glutamicum during penicillin-induced glutamic acid production. Biotechnol J. 2018;13: e1700612.
Duperray F, Jezequel D, Ghazi A, Letellier L, Shechter E. Excretion of glutamate from Corynebacterium glutamicum triggered by amine surfactants. Biochim Biophys Acta. 1992;1103:250–8.
Gao Y, Hu X, Wang J, Li H, Wang X. Impact of mycolic acid deficiency on cells of Corynebacterium glutamicum ATCC13869. Biotechnol Appl Biochem. 2018;65:435–45.
Lea-Smith DJ, Pyke JS, Tull D, McConville MJ, Coppel RL, Crellin PK. The reductase that catalyzes mycolic motif synthesis is required for efficient attachment of mycolic acids to arabinogalactan. J Biol Chem. 2007;282:11000–8.
Xu JZ, Ruan HZ, Yu HB, Liu LM, Zhang W. Metabolic engineering of carbohydrate metabolism systems in Corynebacterium glutamicum for improving the efficiency of l-lysine production from mixed sugar. Microb Cell Fact. 2020;19:39.
Xu J, Han M, Ren X, Zhang W. Modification of aspartokinase III and dihydrodipicolinate synthetase increases the production of l-lysine in Escherichia coli. Biochem Eng J. 2016;114:79–86.
Sahm H, Eggeling L, Eikmanns B, Kramer R. Construction of l-lysine-, l-threonine-, and l-isoleucine-overproducing strains of Corynebacterium glutamicum. Ann N Y Acad Sci. 1996;782:25–39.
de Graaf AA, Eggeling L, Sahm H. Metabolic engineering for l-lysine production by Corynebacterium glutamicum. Adv Biochem Eng Biotechnol. 2001;73:9–29.
Becker J, Zelder O, Hafner S, Schroder H, Wittmann C. From zero to hero-design-based systems metabolic engineering of Corynebacterium glutamicum for l-lysine production. Metab Eng. 2011;13:159–68.
Xu JZ, Wu ZH, Gao SJ, Zhang W. Rational modification of tricarboxylic acid cycle for improving l-lysine production in Corynebacterium glutamicum. Microb Cell Fact. 2018;17:105.
Liu N, Zhang TT, Rao ZM, Zhang WG, Xu JZ. Reconstruction of the diaminopimelic acid pathway to promote l-lysine production in Corynebacterium glutamicum. Int J Mol Sci. 2021;22:9065.
Wang L, Yu H, Xu J, Ruan H, Zhang W. Deciphering the crucial roles of AraC-type transcriptional regulator Cgl2680 on NADPH metabolism and l-lysine production in Corynebacterium glutamicum. World J Microbiol Biotechnol. 2020;36:82.
Xu JZ, Ruan HZ, Chen XL, Zhang F, Zhang W. Equilibrium of the intracellular redox state for improving cell growth and L-lysine yield of Corynebacterium glutamicum by optimal cofactor swapping. Microb Cell Fact. 2019;18:65.
Hoffmann SL, Kohlstedt M, Jungmann L, Hutter M, Poblete-Castro I, Becker J, Wittmann C. Cascaded valorization of brown seaweed to produce l-lysine and value-added products using Corynebacterium glutamicum streamlined by systems metabolic engineering. Metab Eng. 2021;67:293–307.
Lei M, Peng X, Sun W, Zhang D, Wang Z, Yang Z, Zhang C, Yu B, Niu H, Ying H, et al. Nonsterile l-lysine fermentation using engineered phosphite-grown Corynebacterium glutamicum. ACS Omega. 2021;6:10160–7.
Li CL, Ruan HZ, Liu LM, Zhang WG, Xu JZ. Rational reformation of Corynebacterium glutamicum for producing l-lysine by one-step fermentation from raw corn starch. Appl Microbiol Biotechnol. 2022;106:145–60.
Lindner SN, Seibold GM, Kramer R, Wendisch VF. Impact of a new glucose utilization pathway in amino acid-producing Corynebacterium glutamicum. Bioeng Bugs. 2011;2:291–5.
Lindner SN, Seibold GM, Henrich A, Kramer R, Wendisch VF. Phosphotransferase system-independent glucose utilization in Corynebacterium glutamicum by inositol permeases and glucokinases. Appl Environ Microbiol. 2011;77:3571–81.
Blombach B, Seibold GM. Carbohydrate metabolism in Corynebacterium glutamicum and applications for the metabolic engineering of l-lysine production strains. Appl Microbiol Biotechnol. 2010;86:1313–22.
Moon MW, Kim HJ, Oh TK, Shin CS, Lee JS, Kim SJ, Lee JK. Analyses of enzyme II gene mutants for sugar transport and heterologous expression of fructokinase gene in Corynebacterium glutamicum ATCC 13032. FEMS Microbiol Lett. 2005;244:259–66.
Engels V, Georgi T, Wendisch VF. ScrB (Cg2927) is a sucrose-6-phosphate hydrolase essential for sucrose utilization by Corynebacterium glutamicum. FEMS Microbiol Lett. 2008;289:80–9.
Baumchen C, Krings E, Bringer S, Eggeling L, Sahm H. Myo-inositol facilitators IolT1 and IolT2 enhance d-mannitol formation from d-fructose in Corynebacterium glutamicum. FEMS Microbiol Lett. 2009;290:227–35.
Xu JZ, Yu HB, Han M, Liu LM, Zhang WG. Metabolic engineering of glucose uptake systems in Corynebacterium glutamicum for improving the efficiency of l-lysine production. J Ind Microbiol Biotechnol. 2019;46:937–49.
Xiao J, Wang D, Wang L, Jiang Y, Xue L, Sui S, Wang J, Guo C, Wang R, Wang J, et al. Increasing l-lysine production in Corynebacterium glutamicum by engineering amino acid transporters. Amino Acids. 2020;52:1363–74.
Malla S, van der Helm E, Darbani B, Wieschalka S, Forster J, Borodina I, Sommer MOA. A novel efficient l-lysine exporter identified by functional metagenomics. Front Microbiol. 2022;13: 855736.
Ye C, Luo QL, Guo L, Gao C, Xu N, Zhang L, Liu LM, Chen XL. Improving lysine production through construction of an Escherichia coli enzyme-constrained model. Biotechnol Bioeng. 2020;117:3533–44.
Wang Y, Li QG, Zheng P, Guo YM, Wang LX, Zhang TC, Sun JB, Ma YH. Evolving the l-lysine high-producing strain of Escherichia coli using a newly developed high-throughput screening method. J Ind Microbiol Biotechnol. 2016;43:1227–35.
Monk JM, Lloyd CJ, Brunk E, Mih N, Sastry A, King Z, Takeuchi R, Nomura W, Zhang Z, Mori H, et al. iML1515, a knowledgebase that computes Escherichia coli traits. Nat Biotechnol. 2017;35:904–8.
Dong X, Quinn PJ, Wang X. Metabolic engineering of Escherichia coli and Corynebacterium glutamicum for the production of l-threonine. Biotechnol Adv. 2011;29:11–23.
Sauer U, Eikmanns BJ. The PEP-pyruvate-oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol Rev. 2005;29:765–94.
Lee SY, Kim HU. Systems strategies for developing industrial microbial strains. Nat Biotechnol. 2015;33:1061–72.
Zhang YF, Meng QL, Ma HW, Liu YF, Cao GQ, Zhang XR, Zheng P, Sun JB, Zhang DW, Jiang WX, Ma YH. Determination of key enzymes for threonine synthesis through in vitro metabolic pathway analysis. Microb Cell Fact. 2015;14:86.
Yuzbashev TV, Vybornaya TV, Larina AS, Gvilava IT, Voyushina NE, Mokrova SS, Yuzbasheva EY, Manukhov IV, Sineoky SP, Debabov VG. Directed modification of Escherichia coli metabolism for the design of threonine-producing strains. Appl Biochem Microbiol. 2013;49:723–42.
Lee SY, Park JH. Integration of systems biology with bioprocess engineering: l-threonine production by systems metabolic engineering of Escherichia coli. Adv Biochem Eng Biotechnol. 2010;120:1–19.
Zhu L, Fang Y, Ding Z, Zhang S, Wang X. Developing an l-threonine-producing strain from wild-type Escherichia coli by modifying the glucose uptake, glyoxylate shunt, and l-threonine biosynthetic pathway. Biotechnol Appl Biochem. 2019;66:962–76.
Lee KH, Park JH, Kim TY, Kim HU, Lee SY. Systems metabolic engineering of Escherichia coli for l-threonine production. Mol Syst Biol. 2007;3:149.
Wang S, Fang Y, Wang Z, Zhang S, Wang L, Guo Y, Wang X. Improving L-threonine production in Escherichia coli by elimination of transporters ProP and ProVWX. Microb Cell Fact. 2021;20:58.
Su Y, Guo QQ, Wang S, Zhang X, Wang J. Effects of betaine supplementation on l-threonine fed-batch fermentation by Escherichia coli. Bioprocess Biosyst Eng. 2018;41:1509–18.
Smanski MJ, Zhou H, Claesen J, Shen B, Fischbach MA, Voigt CA. Synthetic biology to access and expand nature’s chemical diversity. Nat Rev Microbiol. 2016;14:135–49.
Jones JA, Toparlak OD, Koffas MA. Metabolic pathway balancing and its role in the production of biofuels and chemicals. Curr Opin Biotechnol. 2015;33:52–9.
Holtz WJ, Keasling JD. Engineering static and dynamic control of synthetic pathways. Cell. 2010;140:19–23.
Wang M, Chen B, Fang Y, Tan T. Cofactor engineering for more efficient production of chemicals and biofuels. Biotechnol Adv. 2017;35:1032–9.
Dong X, Zhao Y, Hu J, Li Y, Wang X. Attenuating l-lysine production by deletion of ddh and lysE and their effect on l-threonine and l-isoleucine production in Corynebacterium glutamicum. Enzyme Microb Technol. 2016;93–94:70–8.
Colon GE, Jetten MS, Nguyen TT, Gubler ME, Follettie MT, Sinskey AJ, Stephanopoulos G. Effect of inducible thrB expression on amino acid production in Corynebacterium lactofermentum ATCC 21799. Appl Environ Microbiol. 1995;61:74–8.
Simic P, Willuhn J, Sahm H, Eggeling L. Identification of glyA (encoding serine hydroxymethyltransferase) and its use together with the exporter ThrE to increase l-threonine accumulation by Corynebacterium glutamicum. Appl Environ Microbiol. 2002;68:3321–7.
Liu S, Liang Y, Liu Q, Tao T, Lai S, Chen N, Wen T. Development of a two-stage feeding strategy based on the kind and level of feeding nutrients for improving fed-batch production of l-threonine by Escherichia coli. Appl Microbiol Biotechnol. 2013;97:573–83.
Wang J, Cheng LK, Chen N. High-level production of l-threonine by recombinant Escherichia coli with combined feeding strategies. Biotechnol Biotechnol Equip. 2014;28:495–501.
Liu J, Li H, Xiong H, Xie X, Chen N, Zhao G, Caiyin Q, Zhu H, Qiao J. Two-stage carbon distribution and cofactor generation for improving l-threonine production of Escherichia coli. Biotechnol Bioeng. 2019;116:110–20.
Audit C, Anagnostopoulos C. Genetic studies relating to the production of transformed clones diploid in the tryptophan region of the Bacillus subtilis genome. J Bacteriol. 1973;114:18–27.
Gottardi M, Reifenrath M, Boles E, Tripp J. Pathway engineering for the production of heterologous aromatic chemicals and their derivatives in Saccharomyces cerevisiae: bioconversion from glucose. FEMS Yeast Res. 2017. https://doi.org/10.1093/femsyr/fox035.
Liu S, Xu JZ, Zhang WG. Advances and prospects in metabolic engineering of Escherichia coli for l-tryptophan production. World J Microbiol Biotechnol. 2022;38:22.
Ikeda M, Katsumata R. Hyperproduction of tryptophan by Corynebacterium glutamicum with the modified pentose phosphate pathway. Appl Environ Microbiol. 1999;65:2497–502.
Trondle J, Schoppel K, Bleidt A, Trachtmann N, Sprenger GA, Weuster-Botz D. Metabolic control analysis of l-tryptophan production with Escherichia coli based on data from short-term perturbation experiments. J Biotechnol. 2020;307:15–28.
Niu H, Li R, Liang Q, Qi Q, Li Q, Gu P. Metabolic engineering for improving l-tryptophan production in Escherichia coli. J Ind Microbiol Biotechnol. 2019;46:55–65.
Trondle J, Trachtmann N, Sprenger GA, Weuster-Botz D. Fed-batch production of l-tryptophan from glycerol using recombinant Escherichia coli. Biotechnol Bioeng. 2018;115:2881–92.
Xiong B, Zhu Y, Tian D, Jiang S, Fan X, Ma Q, Wu H, Xie X. Flux redistribution of central carbon metabolism for efficient production of l-tryptophan in Escherichia coli. Biotechnol Bioeng. 2021;118:1393–404.
Gu P, Yang F, Kang J, Wang Q, Qi Q. One-step of tryptophan attenuator inactivation and promoter swapping to improve the production of l-tryptophan in Escherichia coli. Microb Cell Fact. 2012;11:30.
Du L, Zhang Z, Xu Q, Chen N. Central metabolic pathway modification to improve l-tryptophan production in Escherichia coli. Bioengineered. 2019;10:59–70.
Liu L, Duan X, Wu J. l-Tryptophan production in Escherichia coli Improved by weakening the Pta-AckA pathway. PLoS ONE. 2016;11: e0158200.
Xu Q, Bai F, Chen N, Bai G. Gene modification of the acetate biosynthesis pathway in Escherichia coli and implementation of the cell recycling technology to increase l-tryptophan production. PLoS ONE. 2017;12: e0179240.
Gu P, Yang F, Li F, Liang Q, Qi Q. Knocking out analysis of tryptophan permeases in Escherichia coli for improving l-tryptophan production. Appl Microbiol Biotechnol. 2013;97:6677–83.
Wang J, Cheng LK, Wang J, Liu Q, Shen T, Chen N. Genetic engineering of Escherichia coli to enhance production of l-tryptophan. Appl Microbiol Biotechnol. 2013;97:7587–96.
Liu L, Chen S, Wu J. Phosphoenolpyruvate:glucose phosphotransferase system modification increases the conversion rate during l-tryptophan production in Escherichia coli. J Ind Microbiol Biotechnol. 2017;44:1385–95.
Wang J, Ma W, Wang X. Insights into the structure of Escherichia coli outer membrane as the target for engineering microbial cell factories. Microb Cell Fact. 2021;20:73.
Guo L, Diao W, Gao C, Hu G, Ding Q, Ye C, Chen X, Liu J, Liu L. lifespan for enhancing chemical production. Nat Catal. 2020;3:307–18.
Yuan SF, Nair PH, Borbon D, Coleman SM, Fan PH, Lin WL, Alper HS. Metabolic engineering of E. coli for beta-alanine production using a multi-biosensor enabled approach. Metab Eng. 2022;74:24–35.
Buschke N, Schafer R, Becker J, Wittmann C. Metabolic engineering of industrial platform microorganisms for biorefinery applications—optimization of substrate spectrum and process robustness by rational and evolutive strategies. Bioresour Technol. 2013;135:544–54.
Wang J, Ma W, Fang Y, Liang H, Yang H, Wang Y, Dong X, Zhan Y, Wang X. Core oligosaccharide portion of lipopolysaccharide plays important roles in multiple antibiotic resistance in Escherichia coli. Antimicrob Agents Chemother. 2021;65: e0034121.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32100023), the Provincial Natural Science Foundation of Jiangsu Province (BK20210466), the National Natural Science Foundation of China (32000020), the Provincial Natural Science Foundation of Jiangsu Province (BK20200615), and the Youth Fund for Basic Research Program of Jiangnan University (JUSRP122009).
Author information
Authors and Affiliations
Contributions
Authors JW and WM summarized and drafted this manuscript. JW and XW designed and examined this manuscript. JW, WM, JZ and XW approved this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Ma, W., Zhou, J. et al. Microbial chassis design and engineering for production of amino acids used in food industry. Syst Microbiol and Biomanuf 3, 28–48 (2023). https://doi.org/10.1007/s43393-022-00137-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-022-00137-0