Abstract
During l-glutamate production, phosphoenolpyruvate carboxylase and pyruvate carboxylase (PCx) play important roles in supplying oxaloacetate to the tricarboxylic acid cycle. To explore the significance of PCx for l-glutamate overproduction, the pyc gene encoding PCx was amplified in Corynebacterium glutamicum GDK-9 triggered by biotin limitation and CN1021 triggered by a temperature shock, respectively. In the fed-batch cultures, GDK-9pXMJ19pyc exhibited 7.4 % lower l-alanine excretion and no improved l-glutamate production. In contrast, CN1021pXMJ19pyc finally exhibited 13 % lower l-alanine excretion and identical l-glutamate production, however, 8.5 % higher l-glutamate production was detected during a short period of the fermentation. It was indicated that pyc overexpression in l-glutamate producer strains, especially CN1021, increased the supply of oxaloacetate for l-glutamate synthesis and decreased byproduct excretion at the pyruvate node.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corynebacterium glutamicum is widely used for the industrial production of l-glutamate and other amino acids (Kinoshita et al. 2004; Eikmanns et al. 1993). The currently annual production of l-glutamate and monosodium glutamate is ~2,400,000 tons. There are several strategies to trigger l-glutamate overproduction by C. glutamicum, including biotin limitation, addition of detergent or lactam antibiotics, and temperature shock (Shirai et al. 2007).
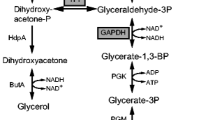
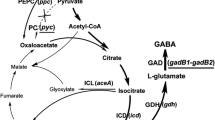
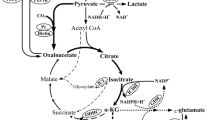
l-Glutamate is synthesized from 2-oxoglutarate which is an intermediate in the tricarboxylic acid (TCA) cycle. One of the initial components of the cycle, oxaloacetate, is not only synthesized from the TCA cycle, but is also replenished from phosphoenolpyruvate and pyruvate catalyzed by phosphoenolpyruvate carboxylase (PEPCx, encoded by ppc) and pyruvate carboxylase (PCx, a biotin-containing enzyme, encoded by pyc), respectively, and which are involved in the anaplerotic pathways (Peters-Wendisch et al. 1998). During l-glutamate production, the two anaplerotic reactions have been believed to play important roles in supplying oxaloacetate to the TCA cycle (Sauer and Eikmanns 2005).
Metabolic flux analysis of anaplerotic pathways indicated that the reaction catalyzed by PCx markedly increased in the l-glutamate production phase induced by Tween 40 addition (Shirai et al. 2007). A pyc deletion in C. glutamicum induced by Tween 60 resulted in about half the l-glutamate accumulating than in the parental strain (Peters-Wendisch et al. 2001). PEPCx amplification in a temperature-triggered l-glutamate producer strain also slightly decreased l-glutamate production, indicating that PCx was responsible for the majority of l-glutamate synthesis (Delaunay et al. 1999).
Biotin is a coenzyme of PCx. Hasegawa et al. (2008) reported that PCx activity under a biotin-limited condition was ~20 % of that under the condition with sufficient biotin in the l-glutamate production phase. Nonetheless, biotin consumption is a gradual process, and the influences of enhancing PCx activity on growth and accumulation of intracellular l-glutamate are still unclear. In contrast, temperature-triggered l-glutamate fermentation is a process requiring excess biotin, enhancing PCx activity in the l-glutamate producer C. glutamicum would draw more flux from pyruvate to oxaloacetate.
The pyruvate node comprises a series of metabolic pathways competing for carbon fluxes with l-glutamate synthesis, including l-alanine and l-lactate (Çalık et al. 2001). This makes it difficult to improve the l-glutamate production and to extract l-glutamate of high purity during the downstream process. Therefore, enhancing PCx activity would be expected to further regulate the carbon flux distribution at the pyruvate node so as to decrease the production of byproducts accordingly.
In this study, the pyc gene was overexpressed in the l-glutamate producer, C. glutamicum GDK-9, triggered by biotin limitation and C. glutamicum CN1021 triggered by a temperature shock but requiring excess biotin, to investigate the effect of pyc overexpression on the growth and l-glutamate production by these two strains. This is the first report of pyc overexpression in these two l-glutamate producer strains.
Materials and methods
Strains, plasmids and media
The bacterial strains and plasmids used in this study are listed in Table 1, and The l-glutamate producers, C. glutamicum GDK-9 and CN1021, were used as the host strains.
Luria–Bertani medium was used for harvesting the bacterial cells. The batch cultures and seeds of C. glutamicum were grown in modified CGVIII medium containing 50 g glucose/l and 2 mg biotin/l (Eikmanns et al. 1991). For the batch cultures of C. glutamicum GDK-9, either 30 μg biotin/l was added into the seed medium or 1 μg biotin/l was added to create the biotin-limited condition. In the fed-batch cultures, the seed and production media for C. glutamicum GDK-9 were described as our previous reports (Chen et al. 2009). The seed medium for C. glutamicum CN1021 contained 30 g glucose/l, 40 ml corn steep liquor/l, 30 ml soybean protein hydrolysate/l, 0.6 mg vitamin B1/l, 0.6 mg biotin/l, 3 g KH2PO4/l, 2 g MgSO4·7H2O/l, 5 mg MnSO4/l, 5 mg FeSO4/l and 5 g urea/l. The production medium for C. glutamicum CN1021 contained 50 g glucose/l, 40 ml corn steep liquor/l, 20 ml soybean protein hydrolysate/l, 0.6 mg vitamin B1/l, 0.6 mg biotin/l, 4.5 g K2HPO4/l, 2 g MgSO4·7H2O/l, 30 mg MnSO4/l and 30 mg FeSO4/l. The pH was adjusted to 7.2 with 4.0 M NaOH.
Construction of C. glutamicum harboring pXMJ19pyc
The pyc gene fragments were PCR-amplified from C. glutamicum GDK-9 and CN1021, respectively, using the following primer pair designed based on the DNA sequence of pyc (GenBank accession number: Y09548.1): pyc-S (5′-CTTGAAGCTTATCACCCTTGGCGGTCTCT-3′) and pyc-A (5′-TGCGTCTAGAATCCAACTCACCCATCTCCC-3′). The resulting PCR fragment was digested by HindIII and XbaI, subcloned into the E. coli-C. glutamicum shuttle vector pXMJ19, and then transformed into E. coli DH5αMCR. The transformants were selected on LB agar containing 25 μg chloramphenicol/ml. The resulting recombinant vector was verified by DNA sequencing, indicating that the two genes from C. glutamicum GDK-9 and CN1021 had no mutation compared with C. glutamicum ATCC 13032 (wild type). The recombinant plasmid pXMJ19pyc and pXMJ19 without insert were extracted and electroporated into C. glutamicum GDK-9 and CN1021, respectively, according to the methods described previously (Wolf et al. 1989).
RNA isolation and quantitative analysis
Total RNA isolation from C. glutamicum was performed with the RNAiso Plus kit (Takara, Japan) according to the manufacturer’s instructions. The double-strand cDNA was synthesized using the UltraSYBR two step RT-qPCR Kit (CWBIO Co., Ltd., Beijing, China) with the primer pair of RT-S (5′-CTTCAACGCTTCCAGCATTCAA-3′) and RT-A (5′-AGAAGCAAAAGAGCGGTGGAATG-3′), and then measured by RT-qPCR with an initial denaturation at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and then 60 °C for 1 min for denaturation and annealing/elongation, respectively, using a Stepone Real-Time PCR instrument (Applied Biosystems ABI, USA). In the qPCR test, the 16S rDNA was used as the internal reference (Takle et al. 2007) and the data were analyzed by the 2−ΔΔCT model.
Fermentation conditions
For the batch cultures of all strains in the minimal medium, the overnight culture in a 500 ml Erlenmeyer flask (200 rpm, 33 °C) was inoculated into 27 ml fresh CGVIII medium in a 500 ml baffled flask with a 10 % (v/v) inoculum. In the medium, 10 μg chloramphenicol/ml was used to maintain the plasmid, and the pH was adjusted to 7.2 by adding 2.5 % (w/v) CaCO3. The fermentation was continued until the concentration of residual glucose fell below 5 g/l. In the fed-batch fermentation, the seed culture was inoculated into the production medium with a 10 % (v/v) inoculum similar to the method used for the batch cultures. The glucose concentration was maintained at ~15 g/l by adding 80 % (w/v) glucose, and the pH was controlled between 7 and 7.2 by adding 25 % (v/v) NH4OH.
In all experiments, the temperature was 34 °C at the beginning of fermentation, and then was gradually raised 0.5 °C every 5 h for C. glutamicum GDK-9. For C. glutamicum CN1021, it was increased to 39 °C when the biomass reached about 3.4 g/l in the batch cultures and/or 6.5 g/l in the fed-batch fermentation.
PCx assays
For the PCx assays, the batch cultures of C. glutamicum were performed in the modified CGVIII medium. PCx activity was determined using a discontinuous glutamate–oxaloacetate-transaminase coupled assay as described previously (Peters-Wendisch et al. 1997). 1 U of PCx activity was defined as the amount of enzyme corresponding to 1 μM l-aspartate formed per min.
Analysis methods
The biomass were calculated from the OD600 values [1 U OD600 nm = 0.3 g dry cell wt (DCW)/l]. The concentrations of glucose, l-glutamate and l-lactate were measured using biosensors and l-alanine and l-aspartate were determined using HPLC.
Statistical analysis
All date were the average of triplicate experiments and presented as mean ± standard deviation. One-way analysis of variance followed by Dunnett’s multiple comparison test was used to determine significant difference, and the statistical significance was defined as p ≤ 0.05.
Results
Quantitative analysis of mRNA transcription
The mRNA levels of pyc gene from GDK-9 and CN1021 were converted to 1 unit as the control group. The pyc gene mRNA levels from GDK-9pXMJ19pyc and CN1021pXMJ19pyc were about 894 and 168 U, respectively, which were significantly higher than from the strains carrying pXMJ19 (1.07 and 1.04 U), indicating that the pyc gene was transcribed efficiently in the recombinant strains (Fig. 1).
Analysis of PCx activities in different recombinant strains
As shown in Table 2, the PCx activity in C. glutamicum GDK-9pXMJ19pyc and CN1021pXMJ19pyc was 66 and 120 % higher than in the two strains carrying pXMJ19 at 6 h, respectively (p ≤ 0.05). For GDK-9pXMJ19 and GDK-9pXMJ19pyc, the PCx activities decreased sharply (~20 and ~11 % of the activity at 6 h, respectively), in contrast, 75 and 63 % of the PCx activities of CN1021pXMJ19 and CN1021pXMJ19pyc were retained at 14 h when the amounts of l-glutamate increased significantly.
Effect of pyc overexpression on l-glutamate production by both recombinant strains in the batch fermentation
As shown in Fig. 2a, there was almost no effect on the growth of GDK-9pXMJ19pyc due to pyc amplification, but the growth of CN1021pXMJ19pyc was obviously improved compared with CN1021pXMJ19 (6.7 vs. 6.1 g DCW/l). Glucose consumption and l-glutamate accumulation were also quantified. As shown in Fig. 2b, c, identical glucose consumption and l-glutamate production were detected for GDK-9pXMJ19pyc, while higher glucose consumption and l-glutamate production were observed for CN1021pXMJ19pyc (44 vs. 40 g/l; 31 vs. 28 g/l, respectively).
Effect of pyc overexpression on l-glutamate production by both recombinant strains in the fed-batch fermentation
As shown in Fig. 3a, the effect of pyc amplification on the growth of both recombinant C. glutamicum strains in the fed-batch fermentation was similar to that observed in the batch cultures. The glucose consumption and l-glutamate accumulation of GDK-9pXMJ19pyc were not markedly improved by pyc amplification (Fig. 3b, c). Glucose consumption by CN1021pXMJ19pyc increased significantly compared with CN1021pXMJ19 (128 vs. 118 g/l) (Fig. 3b), and l-glutamate accumulation was 8.5 % higher from 10 to 16 h, but eventually reached almost the same levels as that of CN1021pXMJ19 (Fig. 3c).
Effect of pyc overexpression on the excretion of byproducts at the pyruvate node
Compared with the two strains carrying pXMJ19, GDK-9pXMJ19pyc and CN1021pXMJ19pyc excreted almost identical amounts of l-lactate, and 9 and 19.5 % lower amounts of l-alanine in the batch cultures (0.33 vs. 0.36 g/l; 0.41 vs. 0.49 g/l, respectively) (Fig. 4a). In the fed-batch fermentation, the amounts of l-lactate excreted by both recombinant strains were also identical to that by their respective parental strains, but the amounts of l-alanine were 7.4 and 13 % lower (1.22 vs. 1.31 g/l; 1.75 vs. 1.98 g/l, respectively) (Fig. 4a).
Discussion
Previous studies have shown that pyc overexpression in C. glutamicum ATCC 13032 (wild-type) resulted in an improvement of l-glutamate production triggered by Tween 60 addition (Peters-Wendisch et al. 2001). C. glutamicum GDK-9 and CN1021 were used to further analyze the effects of pyc overexpression on l-glutamate overproduction triggered by different strategies with different concentration of biotin.
The PCx activities of GDK-9pXMJ19pyc and CN1021pXMJ19pyc increased expectedly, but for the former, it decreased sharply in the l-glutamate production phase. It is conceivable that biotin, the coenzyme of PCx, was almost depleted to trigger l-glutamate overproduction, which resulted in the decreased PCx activity. This observation is in accordance with previous reports (Hasegawa et al. 2008).
As the improvement of PCx activity in both recombinant strains, increased precursor supply of oxaloacetate was expected to improve l-glutamate production proportionally. Yet the recombinant GDK-9pXMJ19pyc exhibited no improvement of growth and l-glutamate production in the fermentations. The possible reasons are that biotin is also a coenzyme of acyl-CoA carboxylase involved in cellular membrane synthesis, and thus, limited biotin supply leads to lowered acyl-CoA carboxylase activity in the growth phase of C. glutamicum (Peters-Wendisch et al. 1997). In addition, the sharply decreased PCx activity caused by biotin limitations in the l-glutamate production phase does not contribute to bacterial growth and l-glutamate production. Moreover, plasmid replication could increase the metabolic burden of recombinant C. glutamicum resulting in the decreased production of l-glutamate compared with GDK-9 in the batch cultures (data not shown).
In contrast, CN1021pXMJ19pyc exhibited increased growth and glucose consumption. Interestingly, improved PCx activity slightly increased l-glutamate production of CN1021pXMJ19pyc in the batch cultures, while in the fed-batch fermentation, l-glutamate production was higher during a short period, and reached almost identical levels finally. It was reasonable to assume that an increase in the precursor, oxaloacetate, led to greater accumulation of intracellular l-glutamate during the growth phase of CN1021pXMJ19pyc, and the extracellular l-glutamate would proportionally increase after a temperature shock during a short period of l-glutamate overproduction. This observation is in accordance with previous reports that the intracellular l-glutamate concentration decreased immediately after the temperature shock (Delaunay et al. 2004). PEPCx activity was inhibited by a high concentration of intracellular l-glutamate, while PCx was not affected by l-glutamate and its salts (Delaunay et al. 2004), which further demonstrates that PCx predominantly functions as an anaplerotic enzyme.
Although oxaloacetate supply was enhanced, there may be a metabolic flux imbalance between the pyruvate and 2-oxoglutarate nodes during the l-glutamate production phase, which resulted in no overall improvement of l-glutamate production. In this case, increased pyruvate consumption led to more glucose utilization accordingly. Additionally, some groups reported that glutamate excretion or another metabolic step other than anaplerosis, such as pyruvate being supplied from glucose, could limit the performance of l-glutamate overproduction (Börmann-El Kholy et al. 1992; Lapujade et al. 1999).
To our surprise, both recombinant strains exhibited lower l-alanine excretion compared with their parental strains in the fermentations, but the distinction was more obvious between CN1021pXMJ19 and CN1021pXMJ19pyc. It is likely that the intracellular concentration of pyruvate decreased because of the improved PCx activity, which resulted in a decrease in the conversion of pyruvate to other metabolites such as l-alanine. Differing from CN1021pXMJ19 and CN1021pXMJ19pyc, the identical and lower PCx activity in the l-glutamate production phase resulted in a small distinction between GDK-9pXMJ19 and GDK-9pXMJ19pyc. The amounts of l-lactate between the recombinant and parental strains were almost identical at the end of the cultures. It was conceivable that extracellular l-lactate could be transported back into the cell and reutilized due to the lack of pyruvate during the l-glutamate production phase, which resulted in the identical l-lactate concentration. Stansen et al. (2005) reported that the excreted l-lactate (catalyzed by a NAD-dependent l-lactate dehydrogenase encoded by ldhA) was reutilized when the specific activity of quinone-dependent l-lactate dehydrogenase (encoded by lldD) increased during the l-glutamate fermentation.
Results of this study indicated that pyc overexpression in l-glutamate producer strains, especially C. glutamicum CN1021, increased the supply of precursor oxaloacetate, however, the metabolic flux balance between the pyruvate-oxaloacetate node and the 2-oxoglutarate node might be disturbed. Therefore, except for an increase in the supply of oxaloacetate, an improvement of glutamate dehydrogenase activity or an increase in one certain mesostate supply taken from citrate to 2-oxoglutarate in the TCA cycle may be an effective way to improve l-glutamate production. In addition, metabolic flux analysis between the pyruvate node and the 2-oxoglutarate node can be further performed for l-glutamate overproduction by the temperature-triggered l-glutamate producer C. glutamicum.
References
Börmann-El Kholy ER, Eikmanns BJ, Gutmann M, Sahm H (1992) Glutamate dehydrogenase is not essential for glutamate formation by Corynebacterium glutamicum. Appl Environ Microbiol 59:2329–2331
Çalık G, Ünlütabak F, Özdamar TH (2001) Product and by-product distributions in glutamic acid fermentation by Brevibacterium flavum: effects of the oxygen transfer. Biochem Eng J 9:91–101
Chen N, Du J, Liu H, Xu Q (2009) Elementary mode analysis and metabolic flux analysis of l-glutamate biosynthesis by Corynebacterium glutamicum. Ann Microbiol 59:317–322
Delaunay S, Uy D, Baucher MF, Engasser JM, Guyonvarch A, Goergen JL (1999) Importance of phosphoenolpyruvate carboxylase of Corynebacterium glutamicum during temperature triggered glutamic acid fermentation. Metab Eng 1:334–343
Delaunay S, Daran-Lapujade P, Engasser JM, Goergen JL (2004) Glutamate as an inhibitor of phosphoenolpyruvate carboxylase activity in Corynebacterium glutamicum. J Ind Microbiol Biotechnol 31:183–188
Eikmanns BJ, Metzger M, Reinscheid DJ, Kircher M, Sahm H (1991) Amplification of three threonine biosynthesis genes in Corynebacterium glutamicum and its influence on carbon flux in different strains. Appl Microbiol Biotechnol 34:617–622
Eikmanns BJ, Eggeling L, Sahm H (1993) Molecular aspects of lysine, threonine, and isoleucine biosynthesis in Corynebacterium glutamicum. Biomed Life Sci 64:145–163
Grant SGN, Jessee J, Bloom FR, Hanahan D (1990) Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci 87:4645–4649
Hasegawa T, Hashimoto K, Kawasaki H, Nakamatsu T (2008) Changes in enzyme activities at the pyruvate node in glutamate-overproducing Corynebacterium glutamicum. J Biosci Bioeng 105:12–19
Jakoby M, Ngouoto-Nkili CE, Burkovski A (1999) Construction and application of new Corynebacterium glutamicum vectors. Biotechnol Tech 13:437–441
Kinoshita S, Udaka S, Shimono M (2004) Studies on the amino acid fermentation. Part 1. Production of glutamic acid by various microorganisms. J Gen Appl Microbiol 50:331–343
Lapujade P, Goergen JL, Engasser JM (1999) Glutamate excretion as a major kinetic bottleneck for the thermally triggered production of glutamic acid by Corynebacterium glutamicum. Metab Eng 1:255–261
Peters-Wendisch PG, Wendisch VF, Paul S, Eikmanns BJ, Sahm H (1997) Pyruvate carboxylase as an anaplerotic enzyme in Corynebacterium glutamicum. Microbiology 143:1095–1103
Peters-Wendisch PG, Kreutzer C, Kalinowski J, Patek M, Sahm H, Eikmanns BJ (1998) Pyruvate carboxylase from Corynebacterium glutamicum: characterization, expression and inactivation of the pyc gene. Microbiology 144:915–927
Peters-Wendisch PG, Schiel B, Wendisch VF, Katsoulidis E, Möckel B, Sahm H, Eikmanns BJ (2001) Pyruvate carboxylase is a major bottleneck for glutamate and lysine production by Corynebacterium glutamicum. J Mol Microbiol Biotechnol 3:295–300
Sauer U, Eikmanns BJ (2005) The PEP–pyruvate–oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol Rev 29:765–794
Shirai T, Fujimura K, Furusawa C, Nagahisa K, Shioya S, Shimizu H (2007) Study on roles of anaplerotic pathways in glutamate overproduction of Corynebacterium glutamicum by metabolic flux analysis. Microb Cell Fact. 6:19. doi:10.1186/1475-2859-6-19
Stansen C, Uy D, Delaunay S, Eggeling L, Goergen JL, Wendisch VF (2005) Characterization of a Corynebacterium glutamicum lactate utilization operon induced during temperature-triggered glutamate production. Appl Environ Microbiol 71:5920–5928
Takle GW, Toth IK, Brurberg MB (2007) Evaluation of reference genes for real-time RT-PCR expression studies in the plant pathogen Pectobacterium atrosepticum. BMC Plant Biol 7:50. doi:10.1186/1471-2229-7-50
Wolf H, Pfihler A, Neumann E (1989) Electrotransformation of intact and osmotically sensitive cells of Corynebacterium glutamicum. Appl Microbiol Biotechnol 30:283–289
Acknowledgments
We gratefully acknowledge the support from Jing Wang. This work was supported by National Key Technology Research and Development Program of the Ministry of Science and Technology (2011BAC11B03), National High Technology Research and Development Program (SS2013AA100106) and Program for Changjiang Scholars and Innovative Research Team in University (IRT 1166).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, X., Wang, J., Xie, X. et al. Enhancing the supply of oxaloacetate for l-glutamate production by pyc overexpression in different Corynebacterium glutamicum . Biotechnol Lett 35, 943–950 (2013). https://doi.org/10.1007/s10529-013-1241-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-013-1241-3