Abstract
In this study, we developed recombinant Escherichia coli strains expressing Lactococcus lactis subsp. lactis Il1403 glutamate decarboxylase (GadB) for the production of GABA from glutamate monosodium salt (MSG). Syntheses of GABA from MSG were examined by employing recombinant E. coli XL1-Blue as a whole cell biocatalyst in buffer solution. By increasing the concentration of E. coli XL1-Blue expressing GadB from the OD600 of 2–10, the concentration and conversion yield of GABA produced from 10 g/L of MSG could be increased from 4.3 to 4.8 g/L and from 70 to 78 %, respectively. Furthermore, E. coli XL1-Blue expressing GadB highly concentrated to the OD600 of 100 produced 76.2 g/L of GABA from 200 g/L of MSG with 62.4 % of GABA yield. Finally, nylon 4 could be synthesized by the bulk polymerization using 2-pyrrolidone that was prepared from microbially synthesized GABA by the reaction with Al2O3 as catalyst in toluene with the yield of 96 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gamma aminobutyric acid (GABA) is a 4-carbon non-essential amino acid abundantly expressed in the human brain and eyes [1–3]. It serves as a major inhibitory neurotransmitter with hypertensive and diuretic effects in animals. Due to its potential for bioactive component in foods and pharmaceuticals, GABA has been utilized as an ingredient in functional foods to promote relaxation and relieve nervous tension [1, 2]. Because direct application of chemically synthesized GABA to food may potentially be harmful, mass production of biologically produced GABA has gained much attention for food application.
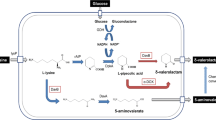
Biological production of GABA can be achieved by three independent processes. The first one is the direct synthesis of GABA from renewable carbon sources such as glucose by fermentation of microorganisms that possess an inherent or recombinant instilled GABA synthesis pathway. However, high-level production of GABA has not been successful by this strategy. Secondly, purified glutamate decarboxylase can be employed for the enzymatic conversion of glutamate monosodium salt (MSG) or glutamic acid to GABA [4]. Enzymatic synthesis of GABA from MSG using glutamate decarboxylase has several advantages, such as high conversion yield of GABA and low contamination of GABA in reaction buffer.
Thirdly, instead of glutamate decarboxylase, natural and recombinant microorganisms capable of converting MSG into GABA can be used as biocatalysts for the production of GABA using MSG as a starting material. Recent studies have suggested that GABA can efficiently be produced from l-glutamate by some lactic acid bacteria (LAB) [5, 6].
Recently, bio-based fuels and polymers from renewable biomass have drawn much attention due to the increasing environmental concerns and concern over the limited nature of fossil resources. For example, polylactic acid (PLA) made from corn has been considered as a good alternative to petroleum-based plastic and has widely been employed for general purpose plastics because of several desirable properties such as biodegradability, biocompatibility, compostability, and low toxicity to humans [7]. However, most biopolymers cannot be applied in high-value-added areas such as automobile manufacture and electronic devices because the mechanical and thermal properties of biopolymers are not able to meet high performance requirements of such applications.
Nylon 4 is four-carbon polyamide suitable for application as an engineering plastic due to its superior thermal and mechanical properties [8]. Contrary to other nylon polymers, nylon 4 is heat-resistant, biodegradable, biocompatible and compostable [9]. Nylon 4 is synthesized by ring-opening polymerization of petroleum-derived 2-pyrrolidone. Because 2-pyrrolidone can be prepared from GABA, GABA produced from renewable resources such as l-glutamate (MSG), which is eventually used as the monomer for the synthesis of nylon 4, could broaden the industrial applications of GABA.
In this study, we have demonstrated the hybrid process composed of biological and chemical processes, in which biomass-derived 2-pyrrolidone was used for the synthesis of nylon 4. GABA was synthesized to high concentration from MSG by recombinant Escherichia coli expressing glutamate decarboxylase and then GABA was converted into 2-pyrrolidone that was finally used for the synthesis of nylon 4.
Materials and methods
Bacterial strains and plasmids
All bacterial strains and plasmids used in this study are listed in Table 1. E. coli XL1-Blue (Stratagene Cloning Systems, La Jolla, CA, USA) was used for general gene cloning studies. Recombinant E. coli XL1-Blue strains were used as host strains for the production of GABA. Plasmid pKE12-MCS is the expression vector containing PLlacO-1 promoter [10].
Construction of plasmids and culture condition
All DNA manipulations were performed following standard procedures. Polymerase chain reaction (PCR) was performed with a C1000 Thermal Cycler (Bio-Rad, USA). Primers used in this study (Table 2) were synthesized at Bioneer (Daejeon, Korea). Plasmid pKE13-MCS was constructed by replacing the PLlacO-1 promoter in pKE12-MCS with the gntT104 promoter [11] amplified from the E. coli W3110 chromosomal DNA at XhoI/EcoRI sites. Plasmid pKE12-GadB and pKE13-GadB were constructed by cloning the Lactobaccillus lactis subsp. lactis Il1403 gadB gene into pKE12-MCS and pKE13-MCS at EcoRI and KpnI sites, respectively.
Culture condition
Escherichia coli XL1-Blue for gene cloning was cultured at 37 °C in Luria–Bertani (LB) medium containing 10 g/L tryptone, 5 g/L yeast extract and 5 g/L NaCl. Recombinant E. coli XL1-Blue for the production of GABA was cultured in LB and MR media supplemented with 20 g/L of glucose at 30 °C in a rotary shaker at 250 rpm for 72 h. MR medium (pH 7.0) contained 6.67 g/L KH2PO4, 4 g/L (NH4)2HPO4, 0.8 g/L MgSO4·7H2O, 0.8 g/L citric acid, and 5 mL/L trace metal solution. The trace metal solution prepared in 0.5 M HCl contained 10 g/L FeSO4·7H2O, 2 g/L CaCl2, 2.2 g/L ZnSO4·7H2O, 0.5 g/L MnSO4·4H2O, 1 g/L CuSO4·5H2O, 0.1 g/L (NH4)6Mo7O24·4H2O, and 0.02 g/L Na2B4O7·10H2O. Glucose, MgSO4·7H2O, and thiamine were sterilized separately. Recombinant E. coli XL1-Blue harboring pKE12-GadB was grown to an optical density at 600 nm (OD600) of 0.5 before induction with 1 mM of isopropylthio-β-galactoside (IPTG) for the expression of the gadB gene. Ampicillin (Ap, 50 μg/mL) was added to the medium.
Fed-batch cultures were carried out at 30 °C in a 5-L jar fermentor (Sartorius, USA) containing 1.5 L of MR medium plus 20 g/L of glucose and 10 mg/L of thiamine. Seed cultures (200 mL) were prepared in the same medium. The culture pH was controlled at 6.9 by the automatic addition of 28 % (v/v) NH4OH. The level of dissolved oxygen concentration (DOC) was controlled by automatically increasing the agitation speed up to 1,000 rpm and varying pure oxygen percentage. Nutrient feeding solution was added by the pH–stat feeding strategy. Feeding solution contained 700 g/L of glucose, 15 g/L of MgSO4·7H2O and 250 mg/L of thiamine. When the pH rose to a value greater than its set point (6.9) by 0.1 due to the depletion of glucose, an appropriate volume of feeding solution was automatically added to increase the glucose concentration in the culture broth. Foam formation was suppressed by adding Antifoam 289 (Sigma-Aldrich, USA) during the initial stage of fed-batch cultures. During fed-batch culture, cell growth was monitored by measuring OD600.
Whole cell reactions for GABA synthesis using flask cultures
Recombinant E. coli XL1-Blue harboring pKE12-GadB were taken at 6 h after 0.5 mM IPTG induction and were harvested by centrifugation for 5 min at 10,000×g at 4 °C. Recombinant E. coli XL1-Blue harboring pKE13-GadB was harvested after 24 h cultivation by centrifugation for 5 min at 10,000×g at 4 °C. After centrifugation, the supernatant was removed and the cell pellet was resuspended in Tris–HCl buffer (pH 7.0) and 50 mM sodium acetate buffer (pH 4.6) for the reaction to synthesize GABA. Conversion of sodium glutamate (MSG) into GABA was mainly carried out in 50 mM sodium acetate buffer (pH 4.6) containing 10 g/L (59 mM) and 30 g/L (177 mM) of MSG by recombinant E. coli XL1-blue expressing GadB resuspended to an OD600 of 2 and 10. The reaction was carried out at 30 °C with shaking at 200 rpm. Concentrations of GABA and MSG were measured as previously described [12].
GABA synthesis by fed-batch culture of recombinant E. coli
Recombinant E. coli XL1-Blue harboring pKE13-GadB was cultured to an OD600 of 60 and 100 by fed-batch cultures. The culture pH was reduced to 4.6 before adding MSG to the culture medium at 200 g/L (1.18 M). The reaction was carried out at 30 °C with an agitation speed of 250 rpm.
Synthesis of nylon 4 from purified GABA
After reaction for GABA production, cells were removed by centrifugation for 5 min at 10,000×g at 4 °C and 1 mL of 5 N NaOH was added to 100 mL of reaction buffer to increase the pH to 10. The solution was passed through an ion exchange column to adsorb GABA. GABA was eluted with a solution consisting of 50 % methanol and 0.1 N HCl. The eluted solution was concentrated by vacuum evaporation and GABA was further purified by crystallization. 2-Pyrrolidone was prepared using purified GABA by reaction with Al2O3 as catalyst and then used for the synthesis of nylon 4 by the bulk polymerization for which KOH was used as a catalyst and CO2 as initiator.
Polymer analysis
To determine the molecular weight of the polymer prepared, a viscosity measurement was carried out using an Ostwald viscometer at 30 °C: 0.03 g of polymer was dissolved in 6 mL of 95 % formic acid and its viscosity was measured. The viscosity molecular weight of the polymer was then calculated by using the Eq. [13]:
The thermal properties of nylon 4 such as glass transition temperature (T g), melting point (T m), and degradation temperatures were measured using differential scanning calorimetry (DSC) and a thermogravimetric analyzer (TGA). For DSC measurement, an aluminum sample pan with 5 mg of polymer was heated under a nitrogen atmosphere from −20 to 250 °C at a heating speed of 10 °C/min for the first heating, and then cooled to −20 °C. For the second heating, it was heated up to 300 °C under the same condition as the first heating. From the second heating curves, T g and T m of the polymer were determined. For the measurement of TGA, the polymer sample in an aluminum sample pan was heated up to 800 °C at a heating rate of 10 °C/min and the degradation point was determined by the weight loss of the polymer according to temperature.
Results
Synthesis of GABA from MSG by whole cell reaction
Because MSG was not efficiently converted into GABA using wild-type E. coli XL1-Blue as whole cell biocatalyst in 50 mM acetate buffer (pH 4.6), recombinant E. coli XL1-Blue strains expressing glutamate decarboxylase were used as host strains for whole cell reaction to produce GABA from MSG. As previously reported, the pH of the reaction buffer was important to achieve efficient production of GABA from MSG [12, 14, 15]. MSG was not converted to GABA in Tris–HCl buffer (pH 7.0), thus, all the reactions for the conversion of MSG into GABA were carried out in 50 mM acetate buffer (pH 4.6). GABA was produced up to 4.3 g/L from 10 g/L of MSG in 24 h, which corresponded to the 70 % of GABA yield, by employing recombinant E. coli XL1-Blue (pKE12-GadB) resuspended to an OD600 of 2 in 50 mM acetate buffer. However, when the initial MSG concentration was increased to 30 g/L, 6.1 g/L of GABA was produced, representing a decreased GABA yield to 33 %. To increase GABA yield in a whole cell reaction, recombinant E. coli XL1-Blue (pKE12-GadB) was resuspended to an OD600 of 10 in 50 mM acetate buffer. The GABA yield was increased to 78 % by the production of 4.8 g/L of GABA from 10 g/L of MSG. Also, 10 g/L of GABA could be produced from 30 g/L of MSG by employing recombinant E. coli XL1-Blue (pKE12-GadB) at the same condition, which corresponded to the 54.6 % of GABA yield.
When recombinant E. coli XL1-Blue (pKE13-GadB) was employed for the conversion of MSG into GABA, similar result was obtained (Table 3). A GABA yield of 60 % resulted (11 g/L obtained from 30 g/L of MSG) by employing recombinant E. coli XL1-Blue (pKE13-GadB) resuspended to an OD600 of 10 in 50 mM acetate buffer.
Synthesis of GABA from MSG by fed-batch culture of recombinant E. coli
Because it was necessary to employ highly concentrated cells for the efficient conversion of high concentration of MSG into GABA, fed-batch culture of recombinant E. coli was carried out to obtain a high cell concentration of E. coli expressing glutamate decarboxylase. Since E. coli XL1-Blue harboring pKE12-GadB needs IPTG for protein induction (which is an expensive compound), E. coli XL1-Blue (pKE13-GadB) that constitutively expresses glutamate decarboxylase was selected as a host strain for the fed-batch cultivation. Cells were first cultivated to an OD600 of 60 and 100 and MSG was added to the culture medium to 200 g/L after the decrease of the pH of the culture medium to 4.6 by adding HCl, which is the optimal pH for the activity of glutamate decarboxylase. A low GABA yield of 25 % (30 g/L of GABA obtained from 200 g/L MSG) was obtained by employing recombinant E. coli XL1-Blue (pKE13-GadB) concentrated up to OD600 of 60 (Fig. 1a). However, GABA yield of 62.4 % (76.2 g/L of GABA obtained from 200 g/L MSG) could be obtained in 2 h by employing recombinant E. coli XL1-Blue (pKE13-GadB) highly concentrated up to OD600 of 100. Further reaction resulted in the production of 94.8 g/L of GABA with a GABA yield of 77.7 % of GABA in 48 h (Fig. 1b).
Time profiles of conversion of MSG into GABA by E. coli XL1-Blue (pKE13-GadB) prepared by fed-batch cultures. MSG was added to the concentration of 200 g/L in the fermentation medium when the cell concentration reached to OD600 of 60 (a) and to OD600 of 100 (b) (filled rectangle MSG, open rectangle GABA)
Synthesis and characterization of nylon 4 from purified GABA
Using purified GABA, the 2-pyrrolidone monomer of nylon 4 was prepared by the reaction with Al2O3 as catalyst. For this preparation, 5.2 g of GABA was dissolved in 250 mL of toluene with 15 g of Al2O3 and the solution was refluxed for 10 h. The solution was cooled and filtered using a glass filter, washing several times with a 1:1 solution of methanol and chloroform. After removing the solvents included in the filtrate by the rotary evaporation under vacuum, 4.1 g of 2-pyrrolidone was obtained as a yellowish oily liquid with the yield of 96 %.
To examine the possibility for the synthesis of nylon 4 from 2-pyrrolidone prepared from GABA, nylon 4 was synthesized by the bulk polymerization using KOH as a catalyst and CO2 as initiator using the prepared 2-pyrrolidone. To 100 mL of 2-pyrrolidone was added KOH 17 mol %, which was dispensed by stirring. After some time a vacuum distillation set was attached to the flask and the reaction mixture was vacuum distilled for 3–4 h to remove the water formed by the reaction of 2-pyrrolidone with KOH. The reaction mixture was cooled down to room temperature, and 10 mL of the reaction mixture was transferred into a small vial and then sealed. CO2 gas was injected into the vial to 0.8 mol % against the KOH with shaking, and the vial was kept at 80 °C for 1–5 days for the polymerization. The white solid powder obtained represented nylon 4. The powder was washed with a large volume of acetone (pH 4.5) containing 10 vol % distilled water to remove any remaining monomers from the polymer. After washing, the polymer was dried under vacuum to yield a white powder of nylon 4.
Synthesis of nylon 4 was confirmed by 1H nuclear magnetic resonance, which showed the characteristic peak of nylon 4 at 2.0, 2.6, and 3.4 ppm (Fig. 2). The molecular weight of nylon 4 ranged from 200,000 to 330,000, which varied depending on polymerization conditions, such as the concentrations of CO2 and KOH used as initiator and catalyst, respectively. DSC analysis showed that the glass transition temperature was 110 °C and the melting temperature was ranged from 244.3 to 269.5 °C. Degradation temperature was 235.6 °C.
Discussion
Biomass-driven chemicals such as ethanol, lactate, and 1,3-propanediol have successfully been employed for polymer synthesis and value-added chemical production in commercial markets [16]. Also, several chemicals derived from microbial fermentation have been intensively examined for the possibility of meeting market requirements and opportunities. These include succinate [17], 3-hydroxypropionate [18], and 1,4-butanediol [19]. Since the aim with bio-based chemicals is to substitute for existing chemical precursors and use in established chemical processes, the most important factor for industrial application is that bio-based chemicals should be cost-effectively synthesized and purified to be suitable for the existing chemical process, as has been shown in some successful cases of fermentation-derived chemicals such as lactate [7] and 1,3-propanediol [20] for the development of bio- and chemical hybrid processes.
GABA has been produced in ‘generally regarded as safe’ microorganisms and by purified enzymes involved in the synthesis of GABA for the safe application as food additives to promote relaxation and relieve nervous tension. Requirement of small amounts of GABA as a higher-value-added food additive marginalizes the importance of production cost determinants such as product concentration and yield for process development. This is also the main reason why recombinant bacteria have not been intensively examined for the production of GABA.
In this study, we developed recombinant E. coli expressing glutamate decarboxylase for the efficient production of GABA from MSG by employing GABA as a precursor for the synthesis of 2-pyrrolidone, the chemical solvent that can be used as an intermediate in synthesis of other organic compounds such as pharmaceuticals and agrochemicals and as a possible monomer of nylon 4. Since MSG is one of the major amino acids produced by microbial fermentation with an annual production of more than 1.5 million tons [21], application of MSG as a precursor for the production of chemical solvent and monomer of engineering plastic, nylon 4, can significantly extend the bio-based industrial market.
There have been several reports of the biological production of GABA employing purified enzymes and microorganisms [4–6, 22], which suggest different advantages and disadvantages regarding the factors affecting the efficiency of GABA production. Enzymatic synthesis of GABA from MSG using glutamate decarboxylase has several advantages that include high conversion yield of GABA and low contamination of GABA in the reaction buffer. However, it was reasoned that enzymatic GABA synthesis is not suitable for the production of GABA to be used for chemical process due to some general characteristics of enzymatic GABA synthesis, such as the high production cost of glutamate decarboxylase, requirement of the expensive reaction cofactor pyridoxal phosphate, and the difficulty of scale-up to the production scale. We decided to develop a microbial system for the production of GABA to address these deficiencies.
Lactobacillus sp. have been employed for the production of GABA using MSG added to the medium by growth-associated manner [5, 6]. However, this system was also not suitable for the production of chemical-grade GABA because the complex medium used for the cultivation of Lactobacillus sp. makes purification process difficult and only small amount of GABA is often produced. To take advantage of enzymatic and microbial synthesis of GABA, conversion of MSG into GABA by whole cell reaction employing recombinant E. coli expressing glutamate decarboxylase was developed.
As summarized in Table 3, GABA synthesis and yield were highly dependent on the concentration of MSG and E. coli strain expressing glutamate decarboxylase employed for the reaction. As MSG concentration was increased, GABA concentration was increased with the decrease of GABA yield. GABA yield could be increased by employing E. coli concentrated up to OD600 of 10.
Based on these results, an E. coli strain expressing glutamate decarboxylase was cultivated to a high concentration, up to OD600 of 100, by fed-batch culture and then was used for the conversion of 200 g/L of MSG to GABA to achieve a high concentration of GABA with a high GABA yield. By using this system, 76.2 g/L of GABA was obtained from 200 g/L of MSG within 2 h, representing a yield of 62.4 %, by employing recombinant E. coli XL1-Blue (pKE13-GadB). Further reaction up to 48 h resulted in the production of 94.8 g/L of GABA, representing a 77.7 % GABA yield.
Recently, the effects of co-expression of the gadB gene with the gadC gene that encodes glutamate/GABA anti-porter were examined for the production of GABA from MSG during the cultivation of wild-type E. coli and the gabT mutant E. coli strains in LB medium supplemented with MSG [12]. Since GabT is involved in the conversion of GABA into succinic semialdehyde that is further metabolized into succinic acid by GabD and YneI, deletion of the gabT gene in the chromosome of E. coli increased the production of GABA from MSG [12]. Also, it was demonstrated that amplification of GadC with GadB in the gabT mutant E. coli strain increased GABA yield up to 89.5 % [12]. Therefore, E. coli mutant strains deficient in gabT, gabD, and/or yneI genes in the chromosome have been constructed and examined for their activities to convert MSG into GABA by whole cell reaction in acetate buffer (pH 4.6). However, enhanced production of GABA could not be achieved employing these E. coli mutant strains that expressed the gadB gene (data not shown). Also, the co-expression of the gadC gene with the gadB gene did not result in the increase of GABA production employing wild-type E. coli and mutant E. coli strains in whole cell reactions (data not shown). This may have been because further assimilation of GABA into other metabolites was inefficient, since E. coli strains used in this study were whole cell biocatalysts in buffer solution of pH 4.6, which was not suitable for efficient cell metabolism.
In this study, it was demonstrated that microbially synthesized GABA is suitable for the synthesis of 2-pyrrolidone by chemical conversion resulting in the conversion yield of 96 %, and can be successfully used for the synthesis of nylon 4. This suggests that fermentation-derived chemicals have the potential to be employed for the development of chemical processes for the production of polymers and value-added chemicals. Nylon 4 synthesized from 2-pyrrolidone derived from fermentation-derived GABA had similar molecular weight and thermal properties to those of nylon 4 derived from petroleum-derived 2-pyrrolidone.
Conclusions
We report the biosynthesis of GABA from MSG by recombinant E. coli strains. GABA can be produced by whole cell reaction employing recombinant E. coli strains expressing glutamate decarboxylase, which can be easily prepared by high cell density cultivation of host strains. With the increase of concentrations of initial MSG and E. coli cells expressing glutamate decarboxylase used in the reaction for GABA synthesis, GABA can be efficiently prepared from MSG with increased conversion yield. GABA synthesized by microbial cells was suitable for the synthesis of 2-pyrrolidone that is used for nylon 4 synthesis.
The strategies described here should be useful for the development of biological and chemical hybrid processes for the production of biomass-derived thermo-resistant polymers from renewable resources.
References
Roberts E, Frankel S (1951) Glutamic acid decarboxylase in brain. J Biol Chem 188:789–795
Stanton HC (1963) Mode of action of gamma aminobutyric acid on the cardiovascular system. Arch Int Pharmacodyn Ther 143:195–204
Kim SH, Shin BH, Kim YH, Nam SW, Jeon SY (2007) Cloning and expression of a full-length glutamate decarboxylase gene from Lactobacillus brevis BH2. Biotechnol Bioprocess Eng 12:707–712
Wang Q, Xin Y, Zhang F, Feng Z, Fu J, Luo L, Yin Z (2011) Enhanced γ-aminobutyric acid-forming activity of recombinant glutamate decarboxylase (gadA) from Escherichia coli. World J Microbiol Biotechnol 27:693–700
Li H, Qiu T, Huang G, Cao Y (2010) Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microb Cell Fact 9:85–92
Huang J, Mei L-H, Wu H, Lin D (2007) Biosynthesis of γ-aminobutyric acid-(GABA) using immobilized whole cells of Lactobacillus brevis. World J Microbiol Biotechnol 23:865–871
Vink ETH, Rabago KR, Glassner DA, Springs B, O’Conner RP, Kolstad J, Gruber PR (2004) The sustainability of NatureWorks™ polylactide polymers and Ingeo™ polylactide fibers: an update of the future. Macromol Biosci 4:551–564
Cho HH, Jeon JW, Lee MH, Lee SH, Kwon ST (2011) Structure and physical properties of variously drawn nylon 6- ran-nylon 4 copolymer fibers. Text Sci Eng 48:150–155
Tokiwa Y, Calabia BP, Ugwu CU, Aiba S (2009) Biodegradability of plastics. Mol Sci 10:3722–3742
Park SJ, Lee TW, Lim S-C, Kim TW, Lee H, Kim MK, Lee SH, Song BK, Lee SY (2012) Biosynthesis of polyhydroxyalkanoates containing 2-hydroxybutyrate from unrelated carbon source by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 93:273–283
Park SJ, Park JP, Lee SY (2002) Metabolic engineering of Escherichia coli for the production of medium-chain-length polyhydroxyalkanoates rich in specific monomers. FEMS Microbiol Lett 214:217–222
Le Vo TD, Kim TW, Hong SH (2012) Effects of glutamate decarboxylase and gamma-aminobutyric acid (GABA) transporter on the bioconversion of GABA in engineered Escherichia coli. Bioprocess Biosyst Eng 35:645–650
Roda J, Králiček J, Boušková Z (1977) On distribution of molecular weight of polypyrrolidone. Eur Polym J 13:119–121
Higara K, Ueno Y, Oda K (2008) Glutamate decarboxylase from Lactobacillus brevis: activation by ammonium sulfate. Biosci Biotechnol Biochem 72:1299–1306
Pennacchietti E, Lammens TM, Capitani G, Franssen MC, John RA, Bossa F, De Biase D (2009) Mutation of His465 alters the pH-dependent spectroscopic properties of Escherichia coli glutamate decarboxylase and broadens the range of its activity toward more alkaline pH. J Biol Chem 284:31587–31596
Lee JW, Kim HU, Choi S, Yi J, Lee SY (2011) Microbial production of building block chemicals and polymers. Curr Opin Biotechnol 22:758–767
Thakker C, Martínez I, San KY, Bennett GN (2012) Succinate production in Escherichia coli. Biotechnol J 7:213–224
Rathnasingh C, Raj SM, Jo JE, Park S (2009) Development and evaluation of efficient recombinant Escherichia coli strains for the production of 3-hydroxypropionic acid from glycerol. Biotechnol Bioeng 104:729–739
Yim H, Haselbeck R, Niu W, Pujol-Baxley C, Burgard A, Boldt J, Khandurina J, Trawick JD, Osterhout RE, Stephen R, Estadilla J, Teisan S, Schreyer HB, Andrae S, Yang TH, Lee SY, Burk MJ, Van Dien S (2011) Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat Chem Biol 7:445–452
Nakamura CE, Whited GM (2003) Metabolic engineering for the microbial production of 1,3-propanediol. Curr Opin Biotechnol 14:454–459
Hermann T (2003) Industrial production of amino acids by coryneform bacteria. J Biotechnol 104:155–172
Saskiawan I (2008) Biosynthesis of polyamide 4, a biobased and biodegradable polymer. Microbiol Indones 2:119–123
Acknowledgments
This work was supported by the R&D Program of MKE/KEIT (10033199 and 10033386) and a grant from the Next-Generation BioGreen 21 Program (SSAC, grant number: PJ008057), Rural Development Administration, Republic of Korea. Further support from Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the MEST (2012-0006693) is appreciated.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Park, S.J., Kim, E.Y., Noh, W. et al. Synthesis of nylon 4 from gamma-aminobutyrate (GABA) produced by recombinant Escherichia coli . Bioprocess Biosyst Eng 36, 885–892 (2013). https://doi.org/10.1007/s00449-012-0821-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-012-0821-2