Abstract
Mitochondria are the major site of adenosine triphosphate (ATP) production in mammalian cells. Moreover, mitochondria produce most of the reactive oxygen species (ROS) in nucleated cells. Redox and bioenergetic abnormalities have been seen in mitochondria during the onset and progression of neurodegenerative diseases. In that context, excitotoxicity induced by glutamate (GLU) plays an important role in mediating neurotoxicity. Several drugs have been used in the treatment of diseases involving excitotoxicity. Nonetheless, some patients (20–30%) present drug resistance. Thus, it is necessary to find chemicals able to attenuate mitochondrial dysfunction in the case of excitotoxicity. In this work, we treated the human neuroblastoma SH-SY5Y cell line with the diterpene carnosic acid (CA) at 1 μM for 12 h prior to the exposure to GLU for further 24 h. We found that CA prevented the GLU-induced mitochondrion-related redox impairment and bioenergetic decline in SH-SY5Y cells. CA also downregulated the pro-apoptotic stimulus elicited by GLU in this experimental model. CA exerted mitochondrial protection by a mechanism associated with the transcription factor nuclear factor erythroid 2–related factor 2 (Nrf2), since silencing of this protein with small interfering RNA (siRNA) suppressed the CA-induced protective effects. Future directions include investigating whether CA would be able to modulate mitochondrial function and/or dynamics in in vivo experimental models of excitotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production of adenosine triphosphate (ATP) occurs mainly in the mitochondria in mammalian cells. These organelles are specialized in the oxidation of different metabolic substrates, leading to the conservation of the energy and posterior synthesis of ATP (Brown 1992). Mitochondria utilize oxygen (O2) gas as a final acceptor of electrons in the respiratory chain, which is composed by complex I (NADH dehydrogenase), complex II (succinate dehydrogenase (SDH)), complex III (ubiquinol-cytochrome c reductase), and complex IV (cytochrome c oxidase) (Chance and Williams 1955; Korzeniewski 1996; Papa et al. 2012). The complexes I, III, and IV pump protons from the mitochondrial matrix to the intermembrane space (IMS), which is located between the inner mitochondrial membrane (IMM) and the outer mitochondrial membrane (OMM) (Alvarez-Paggi et al. 2017; Genova et al. 2005; Genova and Lenaz 2011; Gibson et al. 2005; Nohl et al. 2003). The electrochemical gradient generated by the proton pumping is used by complex V to produce ATP from adenosine diphosphate (ADP) and inorganic phosphate (Pi) (Papa et al. 2012; Solaini et al. 2007). Even though this is a crucial process regarding the maintenance of the energetic status virtually in any nucleated human cell, there is evidence that the respiratory chain is a major source of reactive oxygen species (ROS), such as superoxide anion radical (O2−•) (Naoi et al. 2005; Tsujimoto et al. 2006). Actually, mitochondria have been viewed as the major site of reactive species production (Naoi et al. 2005).
The physiological level of reactive species produced by the mitochondria may be enhanced during intoxication in pathological conditions (Blajszczak and Bonini 2017; de Oliveira 2015; de Oliveira 2016a; de Oliveira and Jardim 2016), as is the case in diseases affecting brain cells (Naoi et al. 2005; Yan et al. 1997). Abnormalities in the metabolism of the excitatory neurotransmitter glutamate are associated with epilepsy, Alzheimer’s disease (AD), and Huntington’s disease (HD), among others (Hamilton et al. 2017; Pchitskaya et al. 2018; Silva et al. 2017). Increased levels of extracellular glutamate (GLU) cause abnormal excitation of neurons and consequent hyperproduction of ROS by a mechanism related to calcium ions (Ca2+) and mitochondrial dysfunction (Pchitskaya et al. 2018). The mechanism underlying the GLU-induced excitotoxicity is not completely understood, but pharmacological strategies aiming to reduce the impact of GLU on the brain cells also focus on mitochondrial protection, since these organelles take a central role in the maintenance of bioenergetics and redox states in animal cells, as well as modulate cell fate by the intrinsic apoptotic pathway (Green et al. 2014; Lai et al. 2014; Olloquequi et al. 2018). Importantly, some individuals (20–30%) present drug resistance during the treatment, decreasing the chance of success (Schmidt and Schachter 2014). Besides, different drugs are prescribed according to the seizure type, as is the case of carbamazepine and lamotrigine, which are indicated to treat patients with partial onset seizures (Nevitt et al. 2017). On the other hand, sodium valproate is the first-line treatment for subjects with generalized tonic-clonic seizure (Nevitt et al. 2017).
The mitochondria contain a series of enzymatic and non-enzymatic defenses, such as the Mn superoxide dismutase (Mn-SOD) and glutathione peroxidase (GPx) enzymes and the reduced glutathione (GSH), the major non-enzymatic antioxidant in mammalian cells (Sies et al. 2017). Mn-SOD converts O2−• into hydrogen peroxide (H2O2), a non-radical, which generates water after reacting with GPx or catalase (CAT) (Sies et al. 2017). GPx consumes GSH in order to reduce H2O2, and this reaction is crucial in the mitochondria to avoid the diffusion of H2O2 to other cellular compartments (Lu 2013; Morris et al. 2014; Sies et al. 2017). The modulation of these antioxidant defenses depends on, at least partially, the transcription factor nuclear factor erythroid 2–related factor 2 (Nrf2), the redox master regulator in mammalian cells (Nguyen et al. 2009; Sies et al. 2017). Furthermore, Nrf2 presents a role in modulating mitochondrial function and dynamics (Dinkova-Kostova and Abramov 2015; Negrette-Guzmán et al. 2013). Nrf2 activity is controlled by different signaling pathways, according to the cell type (Nguyen et al. 2009). Some dietary factors, such as sulforaphane (de Oliveira et al. 2017a; Negrette-Guzmán et al. 2013; Tarozzi et al. 2013), resveratrol (Ahmed et al. 2017; Jardim et al. 2018), pinocembrin (de Oliveira et al. 2017b, 2018a), naringenin (de Oliveira et al. 2017c; Lou et al. 2014), and others (Chandrasekhar et al. 2018; de Oliveira et al. 2017d; Jing et al. 2016; Jo et al. 2018), may be listed as stimulators of Nrf2. In this context, the diterpene carnosic acid (CA; C20H28O4) has been reported as a potent Nrf2 inducer, causing cytoprotection at low concentrations (i.e., 1–5 μM) in several experimental models (de Oliveira 2016b, 2018). CA is isolated from Rosmarinus officinalis (known as rosemary or “alecrim”) and Salvia officinalis and exhibits antioxidant, anti-inflammatory, and antitumor actions, among others (Birtić et al. 2015; de Oliveira 2016b). In addition to CA, such vegetal species contain also rosmarinic acid (RA), among other cytoprotective agents, that exert antioxidant effects in different experimental models (Amoah et al. 2016). However, CA exhibits a more potent action when compared to RA, since CA at 1 μM can significantly attenuate loss of cell viability, for example in some cell types exposed to a myriad of chemical stressors (de Oliveira 2018). On the other hand, some research groups have demonstrated that RA at 56 μM caused cytoprotection in experimental models involving disruption in the redox environment (Lee et al. 2008).
Even though CA is a widely known inducer of Nrf2 and a mitochondrial protective agent, it was not previously investigated whether and how CA would be able to protect mitochondria of neuronal cells exposed to GLU in an experimental model of excitotoxicity. Therefore, we analyzed here whether a pretreatment with CA would be effective in preventing the mitochondrial disturbances induced by GLU-elicited excitotoxicity in the human neuroblastoma SH-SY5Y cell line.
Materials and Methods
Materials
Plastic materials used to maintain cell culture were acquired from Corning, Inc. (NY, USA) and Becton Dickson (NJ, USA). We obtained CA and the culture analytical grade reagents from Sigma (MO, USA). Other chemicals and assay kits were obtained as described here.
Cell Culture
The human dopaminergic neuroblastoma SH-SY5Y cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F-12 HAM nutrient medium (1:1 mixture) containing 10% fetal bovine serum, 2 mM l-glutamine, penicillin (1000 units/mL), streptomycin (1000 μg/mL), and amphotericin B (2.5 μg/mL) in a 5% CO2-humidified incubator at 37 °C. The SH-SY5Y cells were trypsinized after reaching an 80–90% confluence.
Chemical Treatments
We utilized glutamate at 10–80 mM in order to obtain the concentration of this neurotransmitter able to reduce the viability of the cells by 50%. After this initial step, glutamate was used at 80 mM in the other assays. The cells were incubated with glutamate for 3–24 h, depending on the assay. CA (dissolved in 0.1% DMSO) at 0.1–2 μM was administrated to the cells 12 h before the chemical challenge with GLU. Detailed information regarding the experimental design may be obtained also in the figure legends.
Analysis of Cell Viability and Cytotoxicity Assay
We analyzed the viability of SH-SY5Y cells through the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Mosmann 1983). Cytotoxicity was investigated by measuring the activity of lactate dehydrogenase (LDH) in the medium using a commercial kit, according to the instructions of the manufacturer (CytoTox 96 NonRadioactive Cytotoxicity Assay, Promega).
Measurement of Mitochondrion-Related Apoptotic Factors
We quantified the immunocontents of Bax, cytosolic cytochrome c, mitochondrial cytochrome c, and cleaved PARP by utilizing ELISA kits based on the instructions of the manufacturer (Abcam, MA, USA). Caspase-9 and caspase-3 enzyme activities were evaluated by using fluorimetric assay kits following the instructions of the manufacturer (Abcam, MA, USA). The levels of DNA fragmentation in cell lysates were quantified by using an ELISA kit following the manufacturer’s instructions (Roche, Germany) (de Oliveira et al. 2017c, 2018b).
Measurement of the Generation of Intracellular ROS
The non-polar compound 2′-7′-dichlorodihydrofluorescein diacetate (DCFH-DA) was used to quantify the intracellular production of ROS in SH-SY5Y cells (LeBel et al. 1992).
Evaluation of Nitric Oxide Production
The production of nitric oxide (NO•) was measured by using an assay kit, as indicated by the manufacturer (Abcam, MA, USA).
Quantification of MDA, Protein Carbonyl, Protein Thiol Groups, and 8-Oxo-dG Levels
The levels of MDA, protein carbonyl, protein thiol groups, and 8-oxo-dG were measured by using commercial kits (Abcam, MA, USA) (de Oliveira et al. 2015; de Oliveira et al. 2017b).
Evaluation of 3-Nitrotyrosine Levels
The immunocontent of 3-nitrotyrosine in total samples and mitochondrial membranes was measured by using a polyclonal antibody to 3-nitrotyrosine (Calbiochem, Germany), which was diluted 1:2000 in phosphate-buffered saline (PBS) (pH 7.4) with 5% albumin in an indirect ELISA assay, as previously reported (de Oliveira et al. 2015).
Isolation of Mitochondria
Mitochondria were isolated from SH-SY5Y cells by utilizing a protocol published by Wang et al. (2014). The cells were washed and resuspended in a buffer (250 mM sucrose, 10 mM KCl, 1 mM EDTA, 1 mM MgCl2, 1 mM EGTA, 1 mM DTT, 1 mM PMSF, 1 mM benzamidine, 1 mM pepstatin A, 10 mg/mL leupeptin, 2 mg/mL aprotinin, and 20 mM HEPES, pH 7.4). After differential centrifugations, we obtained purified mitochondria, which were used in specific assays.
Isolation of Submitochondrial Particles
The purified mitochondria were frozen and thawed (three times), leading to the rupture of mitochondrial membranes and leakage of mitochondrial matrix–located enzymes, such as Mn superoxide dismutase. Thus, the submitochondrial particles (SMPs) were washed (twice) with a buffer (140 mM KCl, 20 mM Tris-HCl, pH 7.4), causing the complete leakage of Mn superoxide dismutase from mitochondria. We utilized this protocol in order to measure the production of O2−• by mitochondria and to assess the redox-related effects of glutamate and/or CA in mitochondrial membranes (Poderoso et al. 1996).
Evaluation of Enzyme Activities
We analyzed the activity of the mitochondrial enzymes aconitase, α-ketoglutarate dehydrogenase (α-KGDH), SDH, complex I, and complex V by using commercial kits, as recommended by the manufacturer (Abcam, MA, USA).
Quantification of ATP Levels
In order to evaluate the levels of ATP, we utilized a commercial kit according to the instructions of the manufacturer (Abcam, MA, USA).
Determination of Mitochondrial Membrane Potential
We analyzed mitochondrial membrane potential (MMP) by utilizing a commercial kit based on tetraethylbenzimidazolylcarbocyanine iodine (JC-1), as described by the manufacturer (Abcam, MA, USA).
Isolation of Cell Nucleus
Isolation of the cell nucleus was performed by using the Nuclear Extraction Kit (Cayman Chemical, MI, USA). The cells (1 × 107; 80–90% confluence) were collected in ice-cold PBS (pH 7.4). The cells were centrifuged at 300×g for 5 min at 4 °C and resuspended in ice-cold hypotonic buffer, in order to cause cell swelling. Cell membranes were dissolved by using Nonidet P-40 reagent at 10%, which favored the access to the cytoplasmic fraction without damaging the nuclear membrane. The samples were centrifuged at 13,000×g for 30 s at 4 °C to obtain purified nuclei, whose lysis was performed by using the ice-cold extraction buffer. The nuclear extracts were obtained after a centrifugation at 14,000×g for 10 min at 4 °C. These samples were utilized to measure the activity of the transcription factor Nrf2.
Analysis of the Activity of Nrf2
Nrf2 activity was quantified by using a commercial assay kit following the instructions of the manufacturer (Active Motif, CA, USA).
Silencing of Nrf2
We have transfected the SH-SY5Y cells by using small interfering RNA (siRNA) targeting the Nrf2 sequence (5′-CCCATTGATGTTTCTGATCTA-3′) or siRNA against non-target mRNA (scrambled sequence) as a negative control (NC), as recommended by the manufacturer (Santa Cruz, CA, USA) and as previously described (de Oliveira et al. 2016; Jin et al. 2015; Quesada et al. 2011).
Statistical Analyses
The GraphPad 5.0 software was used in order to perform statistical analyses in this work. Data are exhibited here as the mean ± standard error of the mean (SEM) of three or five independent experiments, each done in triplicate. The p values were considered significant when p is < 0.05. The differences between the experimental groups were analyzed by one-way ANOVA, followed by post hoc Tukey’s test.
Results
CA Prevented the Decrease in the Viability and the Mitochondrion-Related Apoptotic Cell Death in SH-SY5Y Exposed to GLU
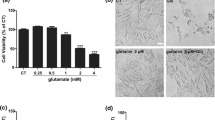
According to Fig. 1a, GLU at 80 mM induced a 50% decrease in the viability of SH-SY5Y cells (p < 0.05). In order to analyze whether CA would be able to prevent the GLU-elicited decrease in cell viability, we have treated the SH-SY5Y cells with CA at 0.1–2 μM for 12 h before a challenge with glutamate. As depicted in Fig. 1b, CA at 0.1 μM and 0.5 μM was not effective in preventing the decrease in cell viability induced by GLU. However, CA pretreatment at 1 μM and 2 μM significantly reduced the impact of GLU on the viability of SH-SY5Y cells (p < 0.05). Based on these data, we decided to utilize CA at 1 μM in the other analyzes we have performed.
a, b The effects of glutamate (GLU) and/or carnosic acid (CA) on the viability of SH-SY5Y cells. CA (0.1–2 μM) was administrated for 12 h before induction of excitotoxicity with GLU at 80 mM for additional 24 h. The results are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by post hoc Tukey’s test (*p < 0.05 vs control cells; #p < 0.05 vs GLU-treated cells)
In this regard, we next examined whether CA would be effective in preventing the mitochondrion-related apoptotic cell death induced by GLU in this experimental model. As demonstrated in Fig. S1A, CA prevented the GLU-induced upregulation in the levels of the pro-apoptotic protein Bax (p < 0.05). Also, CA blocked the release of cytochrome c from the mitochondria (p < 0.05; Fig. S1B), preventing the GLU-induced loss of cytochrome c in the organelles (p < 0.05; Fig. S1C). In this context, CA prevented the upregulation of the pro-apoptotic enzymes caspase-9 and caspase-3 in the SH-SY5Y cells exposed to GLU (p < 0.05; Fig. S1D and Fig. S1E, respectively). The cleavage of PARP and the fragmentation of DNA, two hallmarks of the apoptotic cell death, were downregulated by CA (p < 0.05), as may be observed in Fig. 2a, b, respectively.
The effects of a pretreatment with CA at 1 μM for 12 h on the levels of cleaved PARP (a) and DNA fragmentation (b) in SH-SY5Y cells exposed to glutamate (GLU) at 80 mM for further 24 h. The results are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by post hoc Tukey’s test (*p < 0.05 vs control cells; #p < 0.05 vs GLU-treated cells)
CA Induced an Antioxidant Effect in GLU-Treated SH-SY5Y Cells
We next evaluated whether a pretreatment with CA at 1 μM would prevent the GLU-induced redox impairment in SH-SY5Y cells experiencing excitotoxicity. According to Fig. 4, CA pretreatment decreased the production of O2−• (p < 0.05; Fig. 3a) in SMP obtained from SH-SY5Y cells, as well as reduced the generation of NO• (p < 0.05; Fig. 3b) and other reactive species (p < 0.05; Fig. 3c) in this experimental model. Interestingly, CA was not able to affect the auto-oxidation of epinephrine and pyrogallol (data not shown).
The effects of a pretreatment with CA at 1 μM for 12 h on the production of O2−• (a), NO• (b), and general reactive species (c) in SH-SY5Y cells exposed to glutamate (GLU) at 80 mM for further 6 h. The results are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by post hoc Tukey’s test (*p < 0.05 vs control cells; #p < 0.05 vs GLU-treated cells)
Based on these data, we investigated the effects of a pretreatment with CA on the levels of the markers of redox impairment in GLU-treated SH-SY5Y cells. As depicted in Fig. 4, CA pretreatment significantly reduced the total levels of lipid peroxidation (p < 0.05; Fig. 4a), protein carbonylation (p < 0.05; Fig. 4b), protein thiol oxidation (p < 0.05; Fig. 4c), and protein nitration (p < 0.05; Fig. 4d) in SH-SY5Y challenged with GLU. Moreover, CA pretreatment was effective in reducing the levels of 8-oxo-dG, a marker of DNA oxidative damage (p < 0.05; Fig. 4e). Next, we examined the effects of a pretreatment with CA on the levels of the markers of redox impairment in the membranes of mitochondria isolated from SH-SY5Y cells. According to Fig. 5, CA prevented mitochondrial lipid peroxidation (p < 0.05; Fig. 5a), protein carbonylation (p < 0.05; Fig. 5b), and protein nitration (p < 0.05; Fig. 5c) in GLU-treated SH-SY5Y cells.
The effects of a pretreatment with CA at 1 μM for 12 h on the total levels of lipid peroxidation (a), protein carbonylation (b), protein thiol (c), protein nitration (d), and 8-oxo-dG (e) in SH-SY5Y cells exposed to glutamate (GLU) at 80 mM for further 24 h. The results are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by post hoc Tukey’s test (*p < 0.05 vs control cells; #p < 0.05 vs GLU-treated cells)
The effects of a pretreatment with CA at 1 μM for 12 h on the levels of lipid peroxidation (a), protein carbonylation (b), and protein nitration (c) in mitochondrial membranes obtained from SH-SY5Y cells exposed to glutamate (GLU) at 80 mM for further 24 h. The results are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by post hoc Tukey’s test (*p < 0.05 vs control cells; #p < 0.05 vs GLU-treated cells)
CA Suppressed the Bioenergetics Decline Induced by Glutamate in SH-SY5Y Cells
Based on the previous data, we next examined whether CA would prevent the bioenergetic decline induced by GLU in SH-SY5Y cells. As may observed in Fig. 6, CA suppressed the GLU-induced reduction in the activity of the complex I (p < 0.05; Fig. 6a) and complex V (p < 0.05; Fig. 6b), as well as prevented the GLU-elicited decrease in the levels of ATP (p < 0.05; Fig. 6c) in SH-SY5Y cells. As expected, CA prevented the loss of MMP induced by GLU in SH-SY5Y cells (p < 0.05; Fig. 6d). CA was effective also in preventing the GLU-mediated inhibition of the tricarboxylic acid cycle aconitase (p < 0.05; Fig. 7a), α-KGDH (p < 0.05; Fig. 7b), and SDH (p < 0.05; Fig. 7c).
The effects of a pretreatment with CA at 1 μM for 12 h on the activities of the mitochondrial complexes I (a) and V (b) and on the levels of ATP (c) and MMP (d) in SH-SY5Y cells exposed to glutamate (GLU) at 80 mM for further 24 h. The results are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by post hoc Tukey’s test (*p < 0.05 vs control cells; #p < 0.05 vs GLU-treated cells)
The effects of a pretreatment with CA at 1 μM for 12 h on the activities of the mitochondrial enzymes aconitase (a), α-ketoglutarate dehydrogenase (b), and succinate dehydrogenase (c) in SH-SY5Y cells exposed to glutamate (GLU) at 80 mM for further 24 h. The results are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by post hoc Tukey’s test (*p < 0.05 vs control cells; #p < 0.05 vs GLU-treated cells)
CA Induced Cytoprotection by an Nrf2-Dependent Mechanism in SH-SY5Y Exposed to Glutamate
Aiming to investigate the mechanism underlying the cytoprotection induced by CA in GLU-treated SH-SY5Y cells, we silenced the Nrf2 transcription factor by using siRNA targeting Nrf2. The knockdown of Nrf2 abolished the preventive effect induced by CA on the activities of aconitase (p < 0.05; Fig. 8a) and complex I (p < 0.05; Fig. 8b). Moreover, Nrf2 silencing abrogated the effect of CA pretreatment on MMP in this experimental model (p < 0.05; Fig. 9a). Nrf2 knockdown also suppressed the effects induced by CA regarding the viability of SH-SY5Y cells challenged with GLU (p < 0.05; Fig. 9b). The effects of CA at 1 μM on the activity of the transcription factor Nrf2 was checked and may be viewed in Fig. S2. Moreover, data regarding the activity of Nrf2 in SH-SY5Y cells transfected with siRNA targeting Nrf2 are presented in Fig. S3.
The effects of Nrf2 siRNA (48 h) on the activities of the mitochondrial enzymes aconitase (a) and complex I (b) in SH-SY5Y cells treated or not with carnosic acid and/or glutamate. The results are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by post hoc Tukey’s test (*p < 0.05 vs the control cells; (a) p < 0.05 vs glutamate-treated cells transfected with negative control (NC) siRNA; (b) p < 0.05 vs the glutamate and CA-treated cells transfected with NC siRNA)
The effects of Nrf2 siRNA (48 h) on the mitochondrial membrane potential (MMP) (a) and on the viability (b) of the SH-SY5Y cells treated or not with carnosic acid and/or glutamate. The results are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by post hoc Tukey’s test (*p < 0.05 vs the control cells; (a) p < 0.05 vs glutamate-treated cells transfected with negative control (NC) siRNA; (b) p < 0.05 vs the glutamate and CA-treated cells transfected with NC siRNA)
Discussion
GLU-induced redox impairment has been seen in different neurological disturbances and may present a link with increased cell death rates observed in such maladies (Bondy and LeBel 1993). Indeed, the utilization of antioxidants in experimental models of excitotoxicity demonstrated that redox impairment and apoptosis are strongly correlated in cultured cells and tissue protocols and in experimental animals (Rebai et al. 2017; Zádori et al. 2018). In this scenario, mitochondria are central figures due to their role in both energetic maintenance and cell fate modulation (Jodeiri Farshbaf and Kiani-Esfahani 2017). The production of ROS by mitochondria is a natural consequence of the flux of electrons in the respiratory chain, which is part of the oxidative phosphorylation system, the main site of ATP production, in mammalian cells (Sies et al. 2017). Moreover, damaged mitochondria produce reactive species at higher rates when compared to normal organelles (Sies et al. 2017). Thus, mitochondrial damage leads to impaired redox biology, decreased ATP synthesis, and increased rates of cell death (Sies et al. 2017). Actually, these three consequences resulting from mitochondrial damage have been observed in conditions in which glutamate is mediating excitotoxicity (Lai et al. 2014; Nevitt et al. 2017; Olloquequi et al. 2018; Schmidt and Schachter 2014). Therefore, the investigation of potential mitochondrial protective agents in the case of glutamate-induced neuronal dysfunction is of pharmacological interest.
In the present work, we demonstrate that a pretreatment with CA induced mitochondrial protection in SH-SY5Y cells exposed to GLU in an experimental model of excitotoxicity. CA pretreatment suppressed the GLU-induced disturbances in mitochondrial function, causing a reduction in the generation of ROS and in the triggering of apoptosis in this experimental model. The mechanism by which CA elicited mitochondrial protection involved the Nrf2 transcription factor, since the silencing of this protein attenuated the effects induced by CA seen here. The diterpene CA is a potent activator of Nrf2, which is not only a regulator of the redox environment, but it also controls mitochondrial function (Holmström et al. 2016). CA upregulates the levels of two important mitochondrion-located antioxidants, namely Mn-SOD and GSH, by an Nrf2-dependent manner, as previously reported by our research group (de Oliveira et al. 2016) and by others (Chen et al. 2012). Importantly, it was elegantly demonstrated by Miller et al. (2013) that an in vivo treatment with CA attenuated the effects of a challenge with 4-hydroxynonenal on mitochondria in an ex vivo experimental model, indicating that CA elicited mitochondrial protection in vivo, causing a decrease in the mitochondrial vulnerability to a pro-oxidant agent. Thus, it is apparent that CA possesses the ability to modulate mitochondrion-related protective pathways, which may become useful in the case of neurodegenerative processes.
It was previously demonstrated that CA induces neuroprotection without consuming GSH, as occurs with other xenobiotics during the phase II detoxification reactions (Satoh et al. 2008). Actually, CA upregulates the synthesis of GSH by an Nrf2-dependent mechanism involving the expression of the γ-glutamate-cysteine ligase (γ-GCL) enzyme subunits (Nguyen et al. 2009). Therefore, CA contributes with the maintenance of the redox environment by promoting an increase in the levels of the major non-enzymatic antioxidant in mammalian cells. This is particularly important during excitotoxicity, since excessive extracellular concentrations of GLU affect the transport of components necessary for the synthesis of GSH, such as cystine, into the cells (Lewerenz et al. 2013). Further analyses would be necessary to confirm that CA elicits mitochondrial protection in glutamate-treated cells by a mechanism involving GSH.
Cunha et al. (2016) have reported that GLU at 80 mM induces cytotoxicity in undifferentiated SH-SY5Y cells by a mechanism associated with increased production of NO•. The same research group also demonstrated that GLU impaired the redox environment and upregulated caspase-3 activity in SH-SY5Y cells in a similar manner when compared to the present work. The administration of creatine at 10 mM, which exhibits mitochondrion-related protectant activity, attenuated the GLU-induced cytotoxicity by decreasing NO• production and general ROS generation, as well as blocking caspase-3 activation in SH-SY5Y cells in a pretreatment experimental model. Accordingly, Sun et al. (2010) have shown that tanshinone IIA, a major antioxidant agent found in the plant Salvia miltiorrhiza Bunge, reduced the production of ROS and blocked nuclear condensation, an index of apoptotic cell death, in SH-SY5Y cells undergoing redox impairment–related excitotoxicity mediated by GLU. Therefore, the administration of antioxidant agents may be useful in the prevention of toxicity in the case of GLU-induced excitotoxicity. Actually, pretreatment with CA attenuated the production of O2−• and NO•, as well as suppressed the mitochondrial dysfunction seen here in SH-SY5Y cells challenged with GLU.
In conclusion, CA prevented mitochondrial dysfunction by a mechanism associated with the transcription factor Nrf2 in SH-SY5Y cells exposed to GLU. It is particularly recommended to perform in vivo experimental models aiming to analyze whether the cytoprotective effects of CA would be observed in a similar way, since CA bioavailability may affect the levels of this diterpene in the mammalian brain.
References
Ahmed T, Javed S, Javed S, Tariq A, Šamec D, Tejada S, Nabavi SF, Braidy N, Nabavi SM (2017) Resveratrol and Alzheimer’s disease: mechanistic insights. Mol Neurobiol 54:2622–2635. https://doi.org/10.1007/s12035-016-9839-9
Alvarez-Paggi D, Hannibal L, Castro MA, Oviedo-Rouco S, Demicheli V, Tórtora V, Tomasina F, Radi R, Murgida DH (2017) Multifunctional cytochrome c: learning new tricks from an old dog. Chem Rev 117:13382–13460. https://doi.org/10.1021/acs.chemrev.7b00257
Amoah SK, Sandjo LP, Kratz JM, Biavatti MW (2016) Rosmarinic acid-pharmaceutical and clinical aspects. Planta Med 82:388–406. https://doi.org/10.1055/s-0035-1568274
Birtić S, Dussort P, Pierre FX, Bily AC, Roller M (2015) Carnosic acid. Phytochemistry 115:9–19. https://doi.org/10.1016/j.phytochem.2014.12.026
Blajszczak C, Bonini MG (2017) Mitochondria targeting by environmental stressors: implications for redox cellular signaling. Toxicology 391:84–89. https://doi.org/10.1016/j.tox.2017.07.013
Bondy SC, LeBel CP (1993) The relationship between excitotoxicity and oxidative stress in the central nervous system. Free Radic Biol Med 14:633–642
Brown GC (1992) Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J 284:1–13
Chance B, Williams GR (1955) Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 217:383–393
Chandrasekhar Y, Phani Kumar G, Ramya EM, Anilakumar KR (2018) Gallic acid protects 6-OHDA induced neurotoxicity by attenuating oxidative stress in human dopaminergic cell line. Neurochem Res 43:1150–1160. https://doi.org/10.1007/s11064-018-2530-y
Chen JH, Ou HP, Lin CY, Lin FJ, Wu CR, Chang SW, Tsai CW (2012) Carnosic acid prevents 6-hydroxydopamine-induced cell death in SH-SY5Y cells via mediation of glutathione synthesis. Chem Res Toxicol 25:1893–1901. https://doi.org/10.1021/tx300171u
Cunha MP, Lieberknecht V, Ramos-Hryb AB, Olescowicz G, Ludka FK, Tasca CI, Gabilan NH, Rodrigues AL (2016) Creatine affords protection against glutamate-induced nitrosative and oxidative stress. Neurochem Int 95:4–14. https://doi.org/10.1016/j.neuint.2016.01.002
Dinkova-Kostova AT, Abramov AY (2015) The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med 88:179–188. https://doi.org/10.1016/j.freeradbiomed.2015.04.036
Genova ML, Lenaz G (2011) New developments on the functions of coenzyme Q in mitochondria. Biofactors 37:330–354. https://doi.org/10.1002/biof.168
Genova ML, Bianchi C, Lenaz G (2005) Supercomplex organization of the mitochondrial respiratory chain and the role of the coenzyme Q pool: pathophysiological implications. Biofactors 25:5–20
Gibson GE, Blass JP, Beal MF, Bunik V (2005) The alpha-ketoglutarate-dehydrogenase complex: a mediator between mitochondria and oxidative stress in neurodegeneration. Mol Neurobiol 31:43–63
Green DR, Galluzzi L, Kroemer G (2014) Metabolic control of cell death. Science 345:1250256. https://doi.org/10.1126/science.1250256
Hamilton J, Brustovetsky T, Brustovetsky N (2017) Oxidative metabolism and Ca2+ handling in striatal mitochondria from YAC128 mice, a model of Huntington’s disease. Neurochem Int 109:24–33. https://doi.org/10.1016/j.neuint.2017.01.001
Holmström KM, Kostov RV, Dinkova-Kostova AT (2016) The multifaceted role of Nrf2 in mitochondrial function. Curr Opin Toxicol 1:80–91. https://doi.org/10.1016/j.cotox.2016.10.002
Jardim FR, de Rossi FT, Nascimento MX, da Silva Barros RG, Borges PA, Prescilio IC, de Oliveira MR (2018) Resveratrol and brain mitochondria: a review. Mol Neurobiol 55:2085–2101. https://doi.org/10.1007/s12035-017-0448-z
Jin X, Liu Q, Jia L, Li M, Wang X (2015) Pinocembrin attenuates 6-OHDA-induced neuronal cell death through Nrf2/ARE pathway in SH-SY5Y cells. Cell Mol Neurobiol 35:323–333. https://doi.org/10.1007/s10571-014-0128-8
Jing X, Wei X, Ren M, Wang L, Zhang X, Lou H (2016) Neuroprotective effects of tanshinone I against 6-OHDA-induced oxidative stress in cellular and mouse model of Parkinson’s disease through upregulating Nrf2. Neurochem Res 41:779–786. https://doi.org/10.1007/s11064-015-1751-6
Jo MG, Ikram M, Jo MH, Yoo L, Chung KC, Nah SY, Hwang H, Rhim H, Kim MO (2018) Gintonin mitigates MPTP-induced loss of nigrostriatal dopaminergic neurons and accumulation of α-synuclein via the Nrf2/HO-1 pathway. Mol Neurobiol 56:39–55. https://doi.org/10.1007/s12035-018-1020-1
Jodeiri Farshbaf M, Kiani-Esfahani A (2017) Succinate dehydrogenase: prospect for neurodegenerative diseases. Mitochondrion 42:77–83. https://doi.org/10.1016/j.mito.2017.12.002
Korzeniewski B (1996) What regulates respiration in mitochondria? Biochem Mol Biol Int 39:415–419
Lai TW, Zhang S, Wang YT (2014) Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol 115:157–188. https://doi.org/10.1016/j.pneurobio.2013.11.006
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Lee HJ, Cho HS, Park E, Kim S, Lee SY, Kim CS, Kim DK, Kim SJ, Chun HS (2008) Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology 250:109–115. https://doi.org/10.1016/j.tox.2008.06.010
Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M, Smith SB, Ganapathy V, Maher P (2013) The cystine/glutamate antiporter system x(c)(−) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal 18:522–555. https://doi.org/10.1089/ars.2011.4391
Lou H, Jing X, Wei X, Shi H, Ren D, Zhang X (2014) Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology 79:380–388. https://doi.org/10.1016/j.neuropharm.2013.11.026
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta 1830:3143–3153. https://doi.org/10.1016/j.bbagen.2012.09.008
Miller DM, Singh IN, Wang JA, Hall ED (2013) Administration of the Nrf2-ARE activators sulforaphane and carnosic acid attenuates 4-hydroxy-2-nonenal-induced mitochondrial dysfunction ex vivo. Free Radic Biol Med 57:1–9. https://doi.org/10.1016/j.freeradbiomed.2012.12.011
Morris G, Anderson G, Dean O, Berk M, Galecki P, Martin-Subero M, Maes M (2014) The glutathione system: a new drug target in neuroimmune disorders. Mol Neurobiol 50:1059–1084. https://doi.org/10.1007/s12035-014-8705-x
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Naoi M, Maruyama W, Shamoto-Nagai M, Yi H, Akao Y, Tanaka M (2005) Oxidative stress in mitochondria: decision to survival and death of neurons in neurodegenerative disorders. Mol Neurobiol 31:81–93
Negrette-Guzmán M, Huerta-Yepez S, Tapia E, Pedraza-Chaverri J (2013) Modulation of mitochondrial functions by the indirect antioxidant sulforaphane: a seemingly contradictory dual role and an integrative hypothesis. Free Radic Biol Med 65:1078–1089. https://doi.org/10.1016/j.freeradbiomed.2013.08.182
Nevitt SJ, Sudell M, Weston J, Tudur Smith C, Marson AG (2017) Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data. Cochrane Database Syst Rev (12):CD011412. https://doi.org/10.1002/14651858.CD011412.pub3
Nguyen T, Nioi P, Pickett CB (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284:13291–13295. https://doi.org/10.1074/jbc.R900010200
Nohl H, Staniek K, Kozlov AV, Gille L (2003) The biomolecule ubiquinone exerts a variety of biological functions. Biofactors 18:23–31
de Oliveira MR (2015) Vitamin A and retinoids as mitochondrial toxicants. Oxidative Med Cell Longev 2015:140267–140213. https://doi.org/10.1155/2015/140267
de Oliveira MR (2016a) Fluoxetine and the mitochondria: a review of the toxicological aspects. Toxicol Lett 258:185–191. https://doi.org/10.1016/j.toxlet.2016.07.001
de Oliveira MR (2016b) The dietary components carnosic acid and carnosol as neuroprotective agents: a mechanistic view. Mol Neurobiol 53:6155–6168. https://doi.org/10.1007/s12035-015-9519-1
de Oliveira MR (2018) Carnosic acid as a promising agent in protecting mitochondria of brain cells. Mol Neurobiol 55:6687–6699. https://doi.org/10.1007/s12035-017-0842-6
de Oliveira MR, Jardim FR (2016) Cocaine and mitochondria-related signaling in the brain: a mechanistic view and future directions. Neurochem Int 92:58–66. https://doi.org/10.1016/j.neuint.2015.12.006
de Oliveira MR, Ferreira GC, Schuck PF, Dal Bosco SM (2015) Role for the PI3K/Akt/Nrf2 signaling pathway in the protective effects of carnosic acid against methylglyoxal-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Chem Biol Interact 242:396–406. https://doi.org/10.1016/j.cbi.2015.11.003
de Oliveira MR, Ferreira GC, Schuck PF (2016) Protective effect of carnosic acid against paraquat-induced redox impairment and mitochondrial dysfunction in SH-SY5Y cells: role for PI3K/Akt/Nrf2 pathway. Toxicol in Vitro 32:41–54. https://doi.org/10.1016/j.tiv.2015.12.005
de Oliveira MR, de Bittencourt Brasil F, Fürstenau CR (2017a) Sulforaphane promotes mitochondrial protection in SH-SY5Y cells exposed to hydrogen peroxide by an Nrf2-dependent mechanism. Mol Neurobiol 55:4777–4787. https://doi.org/10.1007/s12035-017-0684-2
de Oliveira MR, Peres A, Ferreira GC (2017b) Pinocembrin attenuates mitochondrial dysfunction in human neuroblastoma SH-SY5Y cells exposed to methylglyoxal: role for the Erk1/2-Nrf2 signaling pathway. Neurochem Res 42:1057–1072. https://doi.org/10.1007/s11064-016-2140-5
de Oliveira MR, Brasil FB, Andrade CMB (2017c) Naringenin attenuates H2O2-induced mitochondrial dysfunction by an Nrf2-dependent mechanism in SH-SY5Y cells. Neurochem Res 42:3341–3350. https://doi.org/10.1007/s11064-017-2376-8
de Oliveira MR, Schuck PF, Bosco SMD (2017d) Tanshinone I induces mitochondrial protection through an Nrf2-dependent mechanism in paraquat-treated human neuroblastoma SH-SY5Y cells. Mol Neurobiol 54:4597–4608. https://doi.org/10.1007/s12035-016-0009-x
de Oliveira MR, da Costa Ferreira G, Brasil FB, Peres A (2018a) Pinocembrin suppresses H2O2-induced mitochondrial dysfunction by a mechanism dependent on the Nrf2/HO-1 axis in SH-SY5Y cells. Mol Neurobiol 55:989–1003. https://doi.org/10.1007/s12035-016-0380-7
de Oliveira MR, Brasil FB, Fürstenau CR (2018b) Evaluation of the mitochondria-related redox and bioenergetics effects of gastrodin in SH-SY5Y cells exposed to hydrogen peroxide. J Mol Neurosci 64:242–251. https://doi.org/10.1007/s12031-018-1027-0
Olloquequi J, Cornejo-Córdova E, Verdaguer E, Soriano FX, Binvignat O, Auladell C, Camins A (2018) Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: therapeutic implications. J Psychopharmacol 32:265–275. https://doi.org/10.1177/0269881118754680
Papa S, Martino PL, Capitanio G, Gaballo A, De Rasmo D, Signorile A, Petruzzella V (2012) The oxidative phosphorylation system in mammalian mitochondria. Adv Exp Med Biol 942:3–37. https://doi.org/10.1007/978-94-007-2869-1_1
Pchitskaya E, Popugaeva E, Bezprozvanny I (2018) Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 70:87–94. https://doi.org/10.1016/j.ceca.2017.06.008
Poderoso JJ, Carreras MC, Lisdero C, Riobó N, Schöpfer F, Boveris A (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys 328:85–92
Quesada A, Ogi J, Schultz J, Handforth A (2011) C-terminal mechano-growth factor induces heme oxygenase-1-mediated neuroprotection of SH-SY5Y cells via the protein kinase Cϵ/Nrf2 pathway. J Neurosci Res 89:394–405. https://doi.org/10.1002/jnr.22543
Rebai O, Belkhir M, Sanchez-Gomez MV, Matute C, Fattouch S, Amri M (2017) Differential molecular targets for neuroprotective effect of chlorogenic acid and its related compounds against glutamate induced excitotoxicity and oxidative stress in rat cortical neurons. Neurochem Res 42:3559–3572. https://doi.org/10.1007/s11064-017-2403-9
Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J, Kamins J, Okamoto S, Izumi M, Shirasawa T, Lipton SA (2008) Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem 104:1116–1131
Schmidt D, Schachter SC (2014) Drug treatment of epilepsy in adults. BMJ 348:g254. https://doi.org/10.1136/bmj.g254
Sies H, Berndt C, Jones DP (2017) Oxidative stress. Annu Rev Biochem 86:715–748. https://doi.org/10.1146/annurev-biochem-061516-045037
Silva FR, Miranda AS, Santos RPM, Olmo IG, Zamponi GW, Dobransky T, Cruz JS, Vieira LB, Ribeiro FM (2017) N-type Ca2+ channels are affected by full-length mutant huntingtin expression in a mouse model of Huntington’s disease. Neurobiol Aging 55:1–10. https://doi.org/10.1016/j.neurobiolaging.2017.03.015
Solaini G, Sgarbi G, Lenaz G, Baracca A (2007) Evaluating mitochondrial membrane potential in cells. Biosci Rep 27:11–21. https://doi.org/10.1007/s10540-007-9033-4
Sun ZW, Zhang L, Zhu SJ, Chen WC, Mei B (2010) Excitotoxicity effects of glutamate on human neuroblastoma SH-SY5Y cells via oxidative damage. Neurosci Bull 26:8–16. https://doi.org/10.1007/s12264-010-0813-7
Tarozzi A, Angeloni C, Malaguti M, Morroni F, Hrelia S, Hrelia P (2013) Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxidative Med Cell Longev 2013:415078. https://doi.org/10.1155/2013/415078
Tsujimoto Y, Nakagawa T, Shimizu S (2006) Mitochondrial membrane permeability transition and cell death. Biochim Biophys Acta 1757:1297–1300. https://doi.org/10.1016/j.bbabio.2006.03.017
Wang K, Zhu L, Zhu X, Zhang K, Huang B, Zhang J, Zhang Y, Zhu L, Zhou B, Zhou F (2014) Protective effect of paeoniflorin on Aβ25-35-induced SH-SY5Y cell injury by preventing mitochondrial dysfunction. Cell Mol Neurobiol 34:227–234. https://doi.org/10.1007/s10571-013-0006-9
Yan LJ, Levine RL, Sohal RS (1997) Oxidative damage during aging targets mitochondrial aconitase. Proc Natl Acad Sci U S A 94:11168–11172
Zádori D, Veres G, Szalárdy L, Klivényi P, Vécsei L (2018) Alzheimer’s disease: recent concepts on the relation of mitochondrial disturbances, excitotoxicity, neuroinflammation, and kynurenines. J Alzheimers Dis 62:523–547. https://doi.org/10.3233/JAD-170929
Funding
This work was supported by CNPq (Edital Universal 2016). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Brazil) Finance Code 001 (ALC receives a CAPES Fellow (Bolsa de Mestrado)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Figure S1
The effects of a pretreatment with CA at 1 μM for 12 h on Bax immunocontent (A), cytosolic cytochrome c content (B), mitochondrial cytochrome c content (C), caspase-9 activity (D), and caspase-3 activity (E) in SH-SY5Y cells exposed to glutamate (GLU) at 80 mM for further 24 h. The results are presented as the mean ± S.E.M. of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, * p < 0.05 vs control cells; # p < 0.05 vs GLU-treated cells. (PDF 90 kb)
Figure S2
The effects of CA at 1 μM for different periods on the activity of the transcription factor Nrf2. The results are presented as the mean ± S.E.M. of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, * p < 0.05 vs control cells. (PDF 4 kb)
Figure S3
The effects of Nrf2 siRNA (48 h) on the activity of the transcription factor Nrf2 in SH-SY5Y cells exposed to CA for 1 h. The results are presented as the mean ± S.E.M. of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, * p < 0.05 vs control cells; # p < 0.05 vs CA-treated cells transfected with negative control (NC) siRNA. (PDF 5 kb)
Rights and permissions
About this article
Cite this article
de Oliveira, M.R., Duarte, A.R., Chenet, A.L. et al. Carnosic Acid Pretreatment Attenuates Mitochondrial Dysfunction in SH-SY5Y Cells in an Experimental Model of Glutamate-Induced Excitotoxicity. Neurotox Res 36, 551–562 (2019). https://doi.org/10.1007/s12640-019-00044-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-019-00044-8