Abstract
Mitochondria are the major site of ATP production in mammalian cells. Furthermore, these organelles are a source and a target of reactive oxygen species (ROS), such as radical anion superoxide (O2 −·) and hydrogen peroxide (H2O2). The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) is the master regulator of the mammalian redox biology and controls the expression of antioxidant and phase II detoxifying enzymes in several cell types. Naringenin (NGN, 5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one), a flavanone, exhibits cytoprotective effects by acting as an antioxidant and anti-inflammatory agent. NGN is a potent activator of Nrf2. Nonetheless, it was not examine yet whether NGN would induce mitochondrial protection in cells under redox stress. Therefore, we investigate here whether Nrf2 would be involved in the mitochondrial protection elicited by NGN in SH-SY5Y cells exposed to H2O2. We observed that a pretreatment with NGN at 80 µM for 2 h reduced the levels of lipid peroxidation, protein carbonylation, and protein nitration in the membranes of mitochondria obtained from H2O2-treated SH-SY5Y cells. Additionally, NGN prevented the H2O2-induced impairment in the function of the enzymes aconitase, α-ketoglutarate dehydrogenase, and succinate dehydrogenase. The activites of the complexes I and V, as well as the production of ATP, were restored by NGN. NGN also suppressed the H2O2-induced mitochondria-related apoptosis. Interestingly, NGN promoted an increase in the levels of both total and mitochondrial glutathione (GSH). Silencing of Nrf2 abolished the protective effects induced by NGN. Overall, NGN induced mitochondrial protection by an Nrf2-dependent mechanism in H2O2-treated SH-SY5Y cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mitochondria are the major organelles responsible for the synthesis of ATP in mammalian cells [1,2,3]. Furthermore, mitochondria are an important source of reactive oxygen species (ROS), such as radical anion superoxide (O2 −·) and hydrogen peroxide (H2O2) [4]. Mitochondrial damage may lead to bioenergetics impairment and cell death due to the release of cytochrome c to the cytosol [5]. Indeed, mitochondrial dysfunction has been viewed in several human disorders, such as neurodegeneration and cardiovascular diseases [6,7,8]. Moreover, mitochondria are a target of several toxicants that increase the production of reactive species by the organelles [9,10,11,12,13]. Therefore, mitochondrial protection is of pharmacological interest. In this context, the number of publications involving the mitochondrial medicine field has increased, as recently reported [14].
The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) is the master regulator of the redox environment in mammalian cells [15,16,17]. Under physiological conditions, Nrf2 is maintained in the cytosol through the binding with the Kelch-like ECH-associated protein 1 (Keap1) [18]. Increased levels of pro-oxidant agents and/or xenobiotics cause the release of Nrf2 from Keap1 and its translocation to the cell nucleus, where this transcription factor upregulates the expression of several enzymes by binding to the antioxidant responsive element (ARE) present in the genes of these proteins [19]. Nrf2 controls the expression of the catalytic (GCLC) and regulatory (GCLM) subunits of γ-glutamate-cysteine ligase (γ-GCL), glutathione peroxidase (GPx), glutathione reductase (GR), and glutathione-S-transferase (GST), among others [20]. In this context, Nrf2 is an important regulator of both synthesis and metabolism of reduced glutathione (GSH) in mammalian cells [15].
Naringenin (NGN; 5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one; MW 272.25 g/mol) is a flavanone found in citrus fruits and has been viewed as an potent inducer of Nrf2 presenting cytoprotective and antioxidant capacities in several cell types [21, 22]. Furthermore, NGN induces anti-inflammatory effects in both in vitro and in vivo experimental models [23, 24]. Recently, Lou et al. [25] have published that NGN suppressed the 6-hydroxydopamine-induced neurotoxicity by an Nrf2-dependent mechanism in SH-SY5Y cells. Nonetheless, the role of NGN as a possible protective agent regarding mitochondrial function has not been studied yet.
Therefore, we investigated in the present work whether and how NGN would protect mitochondria in an experimental model using human neuroblastoma SH-SY5Y cells exposed to H2O2, a pro-oxidant agent widely utilized to induce redox impairment experimentally.
Materials and Methods
Materials
Plastic materials used to perform cell culture were acquired from Corning, Inc (NY, USA) and Beckton Dickson (NJ, USA). Reagents necessary to culture cell have been obtained from Sigma (MO, USA). Other chemicals and assay kits utilized here were obtained from different manufacturers, as described below.
Cell Culture and Treatments
The human dopaminergic neuroblastoma SH-SY5Y cells have been acquired from the American Type Culture Collection (Manassas, VA, USA) and were maintained in Dulbecco’s modified Eagle’s medium (DMEM)/F-12 HAM nutrient medium (1:1 mixture) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 1000 units/mL penicillin, 1000 µg/mL streptomycin, and 2.5 µg/mL amphotericin B in a 5% CO2 humidified incubator at 37 °C.
In order to induce mitochondrial dysfunction, redox impairment, and cell death in SH-SY5Y cells, we utilized H2O2 at 300 µM for different periods of incubation according to each specific assay, as previously reported by us [26,27,28]. NGN at 20–80 µM was administrated to the cells 2 h before exposure to H2O2 for additional 3 or 24 h, according to each assay.
Evaluation of Cell Viability
Cell viability was studied by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, as previously reported [29].
Quantification of Mitochondria-Related Apoptotic Factors
We quantified the levels of Bcl-2, Bax, cytochrome c (mitochondrial and cytosolic), and cleaved PARP by using commercial ELISA assay kits based on the instructions of the manufacturer (Abcam, MA, USA). We evaluated the activities of the apoptotic enzymes caspase-9 and caspase-3 through the utilization of fluorimetric assay kits according to the instructions of the manufacturer (Abcam, MA, USA). DNA fragmentation determination in cell lysates was performed by using a commercial ELISA kit based on the instructions of the manufacturer (Roche, Germany).
Quantification of the Production of Intracellular Reactive Oxygen Species (ROS)
In order to quantify the production of intracellular ROS, we utilized the nonpolar compound 2′-7′-dichlorodihydrofluorescein diacetate (DCFH-DA) assay, as previously described [30].
Evaluation of NO· Production
The production of NO· was quantified by using a commercial kit according to the instructions of the manufacturer (Abcam, MA, USA).
Quantification of Malondialdehyde (MDA), Protein Carbonyl, and 8-Oxo-dG Levels
In order to evaluate the levels of both total and mitochondrial MDA and protein carbonyl, and nuclear 8-oxo-dG content, we used commercial kits (Abcam, MA, USA) following the instructions of the manufacturer.
Examination of Mitochondrial 3-Nitrotyrosine Levels
The levels of 3-nitrotyrosine in mitochondrial membranes were evaluated by using a polyclonal antibody to 3-nitrotyrosine (Calbiochem, Germany) in an indirect ELISA assay, as previously reported [28, 31].
Determination of GSH Levels
The levels of total and mitochondrial GSH were determined according to the protocol of a commercial kit based on the instructions of the manufacturer (Abcam, MA, USA).
Isolation of Mitochondria
Mitochondria were isolated from SH-SY5Y cells by washing and re-suspending the cells in a buffer (250 mM sucrose, 10 mM KCl, 1 mM EGTA, 1 mM EDTA, 1 mM MgCl2, 1 mM dithiothreitol, 1 mM phenylmethylsulphonyl floride, 1 mM benzamidine, 1 mM pepstatin A, 10 mg/mL leupeptin, 2 mg/mL aprotonin, and 20 mM HEPES, pH 7.4). After several differential centrifugations, samples containing purified mitochondria were collected [32].
Extraction of Submitochondrial Particles (SMP)
After mitochondrial isolation, the organelles were frozen and thawed (three times) rendering superoxide dismutase-free SMP. This solution presenting SMP was washed (twice) by using a buffer (140 mM KCl, 20 mM Tris-HCl, pH 7.4), causing Mn-superoxide dismutase leakage from the organelles. We applied this protocol to verify the quantification of O2 −· production and to study the effects of H2O2 and/or NGN on the levels of markers of lipid peroxidation, protein carbonylation, and protein nitration in mitochondrial membranes [33].
Evaluation of Enzyme Activities
We analyzed the enzyme activities of aconitase, α-ketoglutarate dehydrogenase (α-KGDH), succinate dehydrogenase (SDH), complex I, and complex V by using commercial kits according to the instructions of the manufacturer (Abcam, MA, USA).
Quantification of the ATP Levels
We examined the levels of ATP by the utilization of commercial kit following the instruction of the manufacturer (Abcam, MA, USA).
Determination of MMP
We analyzed MMP by using a commercial kit applying the tetraethylbenzimidazolylcarbocyanide iodine (JC-1) following the instructions of the manufacturer (Abcam, MA, USA).
siRNA Transfection
In order to knockdown the transcription factor Nrf2, we performed transient transfection of SH-SY5Y cells by using Nrf2 siRNA based on the recommendations of the manufacturer (Santa Cruz, CA, USA).
Statistical Analyses
Statistical analyses were performed in the present work by utilizing the GraphPad 5.0 software. Data are shown as the mean ± standard error of the mean (S.E.M) of three or five independent experiments each done in triplicate; p values were considered significant when p < 0.05. Differences between the experimental groups were checked by one-way ANOVA followed by the post hoc Tukey’s test.
Results
NGN Prevented Cell Death in SH-SY5Y Cells Exposed to H2O2
According to Fig. S1, NGN at different concentrations (20–80 µM) decreased the effect of H2O2 on the viability of SH-SY5Y cells. NGN at 80 µM efficiently prevented the decrease in the levels of Bcl2 (Fig. S2A), as well as suppressed the increase in the levels of Bax in cells exposed to H2O2 (Fig. S2B). NGN blocked cytochrome c release to the cytosol (Fig. S2C), consequently maintaining the levels of this protein in the mitochondria of SH-SY5Y cells treated with H2O2 (Fig. S2D). In this regard, NGN blocked the activation of caspase-9 (Fig. S3A) and caspase-3 (Fig. S3B), leading to decreased cleavage of PARP (Fig. S3C) and fragmentation of DNA (Fig. S3D).
NGN Induced Antioxidant Effects in SH-SY5Y Cells Treated with H2O2
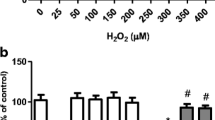
As depicted in Fig. 1a, pretreatment with NGN at 80 µM decreased the production of reactive species in SH-SY5Y cells administrated with H2O2. NGN reduced lipid peroxidation (Fig. 1b) and protein carbonylation (Fig. 1c) in H2O2-treated SH-SY5Y cells. Additionally, NGN enhanced the levels of GSH, as well as prevented the H2O2-induced decrease in the levels of this antioxidant in SH-SY5Y cells (Fig. 1d). NGN also decreased the effect of H2O2 regarding oxidative damage in DNA, as shown in Fig. 2. NGN pretreatment also reduced the production of O2 −· (Fig. 3a) and NO• (Fig. 3b) in stressed SH-SY5Y cells.
The effects of NGN on the intracellular production of ROS (a) and on the levels of lipid peroxidation (b), protein carbonylation (c), and cellular GSH (d) in SH-SY5Y cells exposed to H2O2. The cells were treated with NGN at 80 µM for 2 h prior administration of H2O2 at 300 µM for additional 24 h. ROS production was examined 3 h after exposure to H2O2 due to the high reactivity of these chemical species. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from H2O2-treated group; a different from the control group
The effects of NGN on the levels of 8-oxo-dG in SH-SY5Y cells exposed to H2O2. The cells were treated with NGN at 80 µM for 2 h prior administration of H2O2 at 300 µM for additional 24 h. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from H2O2-treated group
The effects of NGN on the production of O2 −· (a) and NO· (b) in SH-SY5Y cells exposed to H2O2. The cells were treated with NGN at 80 µM for 2 h prior administration of H2O2 at 300 µM for additional 3 h due to the high reactivity of these chemical species. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from H2O2-treated group
NGN Induced Mitochondrial Protection in SH-SY5Y Cells Administrated with H2O2
As shown in Fig. 4a, NGN prevented the H2O2-induced loss of MMP in SH-SY5Y cells. Moreover, NGN decreased lipid peroxidation (Fig. 4b), protein carbonylation (Fig. 4c), and protein nitration (Fig. 4d) in mitochondrial membranes obtained from SH-SY5Y cells treated with H2O2. NGN also upregulated the levels of GSH in the mitochondria of SH-SY5Y cells, as well as efficiently prevented the H2O2-induced decrease in mitochondrial GSH in this experimental model (Fig. 5).
The effects of NGN on the MMP (a) and on the levels of lipid peroxidation (b), protein carbonylation (c), and protein nitration (d) in mitochondrial membranes obtained from SH-SY5Y cells exposed to H2O2. The cells were treated with NGN at 80 µM for 2 h prior administration of H2O2 at 300 µM for additional 24 h. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from H2O2-treated group
The effects of NGN on the levels of GSH in mitochondria obtained from SH-SY5Y cells exposed to H2O2. The cells were treated with NGN at 80 µM for 2 h prior administration of H2O2 at 300 µM for additional 24 h. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from H2O2-treated group; a different from the control group
On the other hand, NGN prevented bioenergetics deficits induced by H2O2 in SH-SY5Y, as demonstrated in Fig. 9. NGN prevented the H2O2-mediated inhibition of the TCA cycle enzymes aconitase (Fig. 6a), α-ketoglutarate dehydrogenase (Fig. 6b), and succinate dehydrogenase (Fig. 6c). Furthermore, NGN reduced the impact of the treatment with H2O2 on the activity of complex I (Fig. 7a) and complex V (Fig. 7b), as well as on the levels of ATP (Fig. 7c) in SH-SY5Y cells.
The effects of NGN on the activities of aconitase (a), α-ketoglutarate dehydrogenase (b), and succinate dehydrogenase (c) in SH-SY5Y cells exposed to H2O2. The cells were treated with NGN at 80 µM for 2 h prior administration of H2O2 at 300 µM for additional 24 h. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from H2O2-treated group
The effects of NGN on the activities of the complexes I (a) and V (b) and on the levels of ATP in SH-SY5Y cells exposed to H2O2. The cells were treated with NGN at 80 µM for 2 h prior administration of H2O2 at 300 µM for additional 24 h. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from H2O2-treated group
NGN Induced Mitochondrial Protection by a Mechanism Dependent on Nrf2 in SH-SY5Y cells
As depicted in Fig. 8, silencing of Nrf2 by using siRNA strategy suppressed the effect of NGN on the levels of GSH in the mitochondria of SH-SY5Y cells exposed to H2O2. In a similar way, Nrf2 knockdown abrogated the effects of NGN on the activity of aconitase (Fig. 9a), complex I (Fig. 9b), and complex V (Fig. 9c). NGN also failed to prevent the H2O2-induced loss of MMP in SH-SY5Y cells treated with siRNA against Nrf2 (Fig. 10).
The effects of Nrf2 silencing (48 h) on the levels of GSH in mitochondria obtained from SH-SY5Y cells exposed to NGN and/or H2O2. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the cells transfected with scrambled control (NC) siRNA and treated with NGN and H2O2
The effects of Nrf2 silencing (48 h) on the activities of aconitase (a), complex I (b), and complex V (c) in SH-SY5Y cells exposed to NGN and/or H2O2. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, * p < 0.05 different from the cells transfected with scrambled control (NC) siRNA and treated with NGN and H2O2
The effects of Nrf2 silencing (48 h) on MMP in SH-SY5Y cells exposed to NGN and/or H2O2. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the cells transfected with scrambled control (NC) siRNA and treated with NGN and H2O2
NGN Induced Cytoprotection by an Nrf2-Dependent Mechanism in SH-SY5Y cells
Silencing of Nrf2 suppressed the NGN-induced cytoprotection in SH-SY5Y cells exposed to H2O2 (Fig. 11).
The effects of Nrf2 silencing (48 h) on the viability of SH-SY5Y cells exposed to NGN and/or H2O2. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the cells transfected with scrambled control (NC) siRNA and treated with NGN and H2O2
Discussion
In the herein presented work, we found that NGN pretreatment prevented mitochondrial dysfunction in SH-SY5Y cells exposed to H2O2. NGN suppressed the H2O2-induced redox impairment in mitochondrial membranes and attenuated the effects of this pro-oxidant regarding mitochondrial function, as assessed through the quantification of the activities of enzymes involved in the TCA cycle and in the oxidative phosphorylation system. Furthermore, NGN abrogated the pro-apoptotic signaling promoted by H2O2 in this experimental model. The mitochondria-related effects, as well as the cytoprotective action of NGN, were abolished by siRNA targeting Nrf2, demonstrating that this transcription factor may take a central role in mediating the beneficial actions elicited by NGN.
In fact, Nrf2, in addition to its role in the maintenance of the redox environment, has been linked to mitochondrial function and dynamics in different cell types, as reported by other research groups [34, 35]. Nrf2 is involved in the regulation of the expression of mitochondria-located antioxidant enzymes, such as Mn-superoxide dismutase (Mn-SOD) and GPx [36,37,38,39,40], and also takes a role in the control of mitochondria-related bioenergetics functions [41,42,43]. Recently, Nrf2 has been associated with the modulation of mitochondrial biogenesis in mammalian cells [44, 45].
In this regard, there is increasing interest in natural compounds that may regulate mitochondrial function and redox biology due to the central role these organelles present in the control of cellular homeostasis [46,47,48,49,50,51,52,53,54,55,56,57]. We have demonstrated that other bioactive molecules exhibit the ability to upregulate Nrf2, causing mitochondrial protection in SH-SY5Y cells exposed to different chemical stressors [26,27,28, 58,59,60,61,62]. These bioactive molecules share a common mechanism involving upregulation of the production of GSH in both total and mitochondrial samples. GSH is the major non-enzymatic antioxidant in mammalian cells, and is also utilized in the phase II detoxification reactions by the enzyme GST [63, 64]. GSH is utilized by GPx in the conversion of H2O2 into water, decreasing the risk this pro-oxidant agent generates hydroxyl radical (•OH) through either Fenton or Haber–Weiss reactions [15, 65]. Thus, GSH may take an important role in the NGN-induced cytoprotection in H2O2-treated SH-SY5Y cells. This effect of NGN would be particularly interesting in the case of both prevention and treatment of neurodegenerative diseases, such as Parkinson’s disease and Alzheimer’s disease, in which it has been observed decreased levels of both enzymatic and non-enzymatic antioxidant defenses [66,67,68,69]. Moreover, mitochondrial dysfunction and redox disturbances have been described as taking a pivotal role in the progression of those conditions [70]. Attenuation of mitochondrial dysfunction may lead to decreased production of reactive species and accumulation of markers of redox imbalance, which also present toxic effects, such as MDA, acrolein, and 4-hydroxynonenal [71]. Actually, we found that NGN pretreatment reduced the levels of MDA in both total and mitochondrial samples obtained from the cells exposed to H2O2. Thus, NGN is very likely to block the vicious cycle involving the production of reactive species and the accumulation of toxic agents in cells undergoing redox impairment. However, it remains to be fully understood exactly how this polyphenol interferes in cellular redox biology causing cytoprotection.
Overall, NGN induced mitochondrial protection in SH-SY5Y cells exposed to H2O2 by a mechanism dependent on the Nrf2 transcription factor. Future research should be done in in vivo experimental model focusing on different mammalian brain areas.
References
Arnold S (2012) The power of life-cytochrome c oxidase takes center stage in metabolic control, cell signalling and survival. Mitochondrion 12:46–56. doi:10.1016/j.mito.2011.05.003
Jonckheere AI, Smeitink JA, Rodenburg RJ (2012) Mitochondrial ATP synthase: architecture, function and pathology. J Inherit Metab Dis 35:211–225. doi:10.1007/s10545-011-9382-9
Flippo KH, Strack S (2017) Mitochondrial dynamics in neuronal injury, development and plasticity. J Cell Sci 130:671–681. doi:10.1242/jcs.171017
Chong SJ, Low IC, Pervaiz S (2014) Mitochondrial ROS and involvement of Bcl-2 as a mitochondrial ROS regulator. Mitochondrion 19 Pt A:39–48. doi:10.1016/j.mito.2014.06.002
Green DR, Galluzzi L, Kroemer G (2014) Metabolic control of cell death. Science 345:1250256. doi:10.1126/science.1250256
Nicholls DG, Budd SL (1998) Neuronal excitotoxicity: the role of mitochondria. Biofactors 8:287–299
Brown GC, Bal-Price A (2003) Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol 27:325–355
Bek T (2016) Mitochondrial dysfunction and diabetic retinopathy. Mitochondrion. doi:10.1016/j.mito.2016.07.011
Tillement L, Lecanu L, Papadopoulos V (2011) Alzheimer’s disease: effects of β-amyloid on mitochondria. Mitochondrion 11:13–21. doi:10.1016/j.mito.2010.08.009
Leuner K, Müller WE, Reichert AS (2012) From mitochondrial dysfunction to amyloid beta formation: novel insights into the pathogenesis of Alzheimer’s disease. Mol Neurobiol 46:186–193. doi:10.1007/s12035-012-8307-4
de Oliveira MR (2015) Vitamin A and retinoids as mitochondrial toxicants. Oxid Med Cell Longev 2015:140267. doi:10.1155/2015/140267
de Oliveira MR (2016) Fluoxetine and the mitochondria: a review of the toxicological aspects. Toxicol Lett 258:185–191. doi:10.1016/j.toxlet.2016.07.001
de Oliveira MR, Jardim FR (2016) Cocaine and mitochondria-related signaling in the brain: a mechanistic view and future directions. Neurochem Int 92:58–66. doi:10.1016/j.neuint.2015.12.006
Picard M, Wallace DC, Burelle Y (2016) The rise of mitochondria in medicine. Mitochondrion 30:105–116. doi:10.1016/j.mito.2016.07.003
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta 1830:3143–3153. doi:10.1016/j.bbagen.2012.09.008
Costa SL, Silva VD, Dos Santos Souza C, Santos CC, Paris I, Muñoz P, Segura-Aguilar J (2016) Impact of plant-derived flavonoids on neurodegenerative diseases. Neurotox Res 30:41–52. doi:10.1007/s12640-016-9600-1
Kim J, Keum YS (2016) NRF2, a key regulator of antioxidants with two faces towards cancer. Oxid Med Cell Longev 2016:2746457. doi:10.1155/2016/2746457
Suzuki T, Yamamoto M (2015) Molecular basis of the Keap1-Nrf2 system. Free Radic Biol Med 88:93–100. doi:10.1016/j.freeradbiomed.2015.06.006
Nguyen T, Sherratt PJ, Nioi P, Yang CS, Pickett CB (2005) Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. J Biol Chem 280:32485–32492
Nguyen T, Nioi P, Pickett CB (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284:13291–13295. doi:10.1074/jbc.R900010200
Podder B, Song HY, Kim YS (2014) Naringenin exerts cytoprotective effect against paraquat-induced toxicity in human bronchial epithelial BEAS-2B cells through NRF2 activation. J Microbiol Biotechnol 24:605–613
Ramprasath T, Senthamizharasi M, Vasudevan V, Sasikumar S, Yuvaraj S, Selvam GS (2014) Naringenin confers protection against oxidative stress through upregulation of Nrf2 target genes in cardiomyoblast cells. J Physiol Biochem 70:407–415. doi:10.1007/s13105-014-0318-3
Manchope MF, Calixto-Campos C, Coelho-Silva L, Zarpelon AC, Pinho-Ribeiro FA, Georgetti SR, Baracat MM, Casagrande R, Verri WA Jr (2016) Naringenin inhibits superoxide anion-induced inflammatory pain: role of oxidative stress, cytokines, Nrf-2 and the NO-cGMP-PKG-KATP Channel signaling pathway. PLoS ONE 11:e0153015. doi:10.1371/journal.pone.0153015
Martinez RM, Pinho-Ribeiro FA, Steffen VS, Silva TC, Caviglione CV, Bottura C, Fonseca MJ, Vicentini FT, Vignoli JA, Baracat MM, Georgetti SR, Verri WA Jr, Casagrande R (2016) Topical formulation containing naringenin: efficacy against ultraviolet b irradiation-induced skin inflammation and oxidative stress in mice. PLoS ONE 11:e0146296. doi:10.1371/journal.pone.0146296
Lou H, Jing X, Wei X, Shi H, Ren D, Zhang X (2014) Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology 79:380–388. doi:10.1016/j.neuropharm.2013.11.026
de Oliveira MR, Fürstenau CR, de Souza IC, da Costa Ferreira G (2016) Tanshinone I attenuates the effects of a challenge with H2O2 on the functions of tricarboxylic acid cycle and respiratory chain in sh-sy5y cells. Mol Neurobiol. doi:10.1007/s12035-016-0267-7
de Oliveira MR, da Costa Ferreira G, Brasil FB, Peres A (2017) Pinocembrin suppresses H2O2-induced mitochondrial dysfunction by a mechanism dependent on the Nrf2/HO-1 axis in SH-SY5Y cells. Mol Neurobiol. doi:10.1007/s12035-016-0380-7
de Oliveira MR, da Costa Ferreira G, Peres A, Bosco SM (2017) Carnosic acid suppresses the H2O2-induced mitochondria-related bioenergetics disturbances and redox impairment in SH-SY5Y cells: role for Nrf2. Mol Neurobiol. doi:10.1007/s12035-016-0372-7
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
de Oliveira MR, Lorenzi R, Schnorr CE, Morrone M, Moreira JC (2011) Increased 3-nitrotyrosine levels in mitochondrial membranes and impaired respiratory chain activity in brain regions of adult female rats submitted to daily vitamin A supplementation for 2 months. Brain Res Bull 86:246–253. doi:10.1016/j.brainresbull.2011.08.006
Wang K, Zhu L, Zhu X, Zhang K, Huang B, Zhang J, Zhang Y, Zhu L, Zhou B, Zhou F (2014) Protective effect of paeoniflorin on Aβ25-35-induced SH-SY5Y cell injury by preventing mitochondrial dysfunction. Cell Mol Neurobiol 34:227–234. doi:10.1007/s10571-013-0006-9
Poderoso JJ, Carreras MC, Lisdero C, Riobó N, Schöpfer F, Boveris A (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys 328:85–92
Dinkova-Kostova AT, Baird L, Holmström KM, Meyer CJ, Abramov AY (2015) The spatiotemporal regulation of the Keap1-Nrf2 pathway and its importance in cellular bioenergetics. Biochem Soc Trans 43:602–610. doi:10.1042/BST20150003
Dinkova-Kostova AT, Abramov AY (2015) The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med 88:179–188. doi:10.1016/j.freeradbiomed.2015.04.036
Holley AK, Dhar SK, St Clair DK (2010) Manganese superoxide dismutase vs. p53: regulation of mitochondrial ROS. Mitochondrion 10:649–661. doi:10.1016/j.mito.2010.06.003
Schmitt CA, Heiss EH, Dirsch VM (2010) Effect of resveratrol on endothelial cell function: Molecular mechanisms. Biofactors 36:342–349
Das J, Ramani R, Suraju MO (2016) Polyphenol compounds and PKC signaling. Biochim Biophys Acta 1860:2107–2121. doi:10.1016/j.bbagen.2016.06.022
Zhang R, Xu M, Wang Y, Xie F, Zhang G, Qin X (2016) Nrf2-a promising therapeutic target for defensing against oxidative stress in stroke. Mol Neurobiol. doi:10.1007/s12035-016-0111-0
Zhang L, Wang H (2017) Targeting the NF-E2-related factor 2 pathway: a novel strategy for traumatic brain injury. Mol Neurobiol. doi:10.1007/s12035-017-0456-z
Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H (2012) Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 22:66–79. doi:10.1016/j.ccr.2012.05.016
Armah CN, Traka MH, Dainty JR, Defernez M, Janssens A, Leung W, Doleman JF, Potter JF, Mithen RF (2013) A diet rich in high-glucoraphanin broccoli interacts with genotype to reduce discordance in plasma metabolite profiles by modulating mitochondrial function. Am J Clin Nutr 98:712–722. doi:10.3945/ajcn.113.065235
Holmström KM, Baird L, Zhang Y, Hargreaves I, Chalasani A, Land JM, Stanyer L, Yamamoto M, Dinkova-Kostova AT, Abramov AY (2013) Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open 2:761–770. doi:10.1242/bio.20134853
Piantadosi CA, Suliman HB (2012) Redox regulation of mitochondrial biogenesis. Free Radic Biol Med 53:2043–2053. doi:10.1016/j.freeradbiomed.2012.09.014
Wu KL, Wu CW, Chao YM, Hung CY, Chan JY (2016) Impaired Nrf2 regulation of mitochondrial biogenesis in rostral ventrolateral medulla on hypertension induced by systemic inflammation. Free Radic Biol Med 97:58–74. doi:10.1016/j.freeradbiomed.2016.05.012
Atamna H, Mackey J, Dhahbi JM (2012) Mitochondrial pharmacology: electron transport chain bypass as strategies to treat mitochondrial dysfunction. Biofactors 38:158–166
Gruber J, Fong S, Chen CB, Yoong S, Pastorin G, Schaffer S, Cheah I, Halliwell B (2013) Mitochondria-targeted antioxidants and metabolic modulators as pharmacological interventions to slow ageing. Biotechnol Adv 31:563–592. doi:10.1016/j.biotechadv.2012.09.005
Marí M, Morales A, Colell A, García-Ruiz C, Kaplowitz N, Fernández-Checa JC (2013) Mitochondrial glutathione: features, regulation and role in disease. Biochim Biophys Acta 1830:3317–3328. doi:10.1016/j.bbagen.2012.10.018
Valero T (2014) Mitochondrial biogenesis: pharmacological approaches. Curr Pharm Des 20:5507–5509
de Oliveira MR, Nabavi SF, Habtemariam S, Orhan IE, Daglia M, Nabavi SM (2015) The effects of baicalein and baicalin on mitochondrial function and dynamics: a review. Pharmacol Res 100:296–308. doi:10.1016/j.phrs.2015.08.021
de Oliveira MR (2016) Evidence for genistein as a mitochondriotropic molecule. Mitochondrion 29:35–44. doi:10.1016/j.mito.2016.05.005
de Oliveira MR, Nabavi SF, Manayi A, Daglia M, Hajheydari Z, Nabavi SM (2016) Resveratrol and the mitochondria: From triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim Biophys Acta 1860:727–745. doi:10.1016/j.bbagen.2016.01.017
de Oliveira MR, Jardim FR, Setzer WN, Nabavi SM, Nabavi SF (2016) Curcumin, mitochondrial biogenesis, and mitophagy: Exploring recent data and indicating future needs. Biotechnol Adv 34:813–826. doi:10.1016/j.biotechadv.2016.04.004
de Oliveira MR, Nabavi SM, Braidy N, Setzer WN, Ahmed T, Nabavi SF (2016) Quercetin and the mitochondria: a mechanistic view. Biotechnol Adv 34:532–549. doi:10.1016/j.biotechadv.2015.12.014
Oliveira MR, Nabavi SF, Daglia M, Rastrelli L, Nabavi SM (2016) Epigallocatechin gallate and mitochondria-A story of life and death. Pharmacol Res 104:70–85. doi:10.1016/j.phrs.2015.12.027
Jardim FR, de Rossi FT, Nascimento MX, da Silva Barros RG, Borges PA, Prescilio IC, de Oliveira MR (2017) Resveratrol and brain mitochondria: a review. Mol Neurobiol. doi:10.1007/s12035-017-0448-z
Asghari MH, Abdollahi M, de Oliveira MR, Nabavi SM (2017) A review of the protective role of melatonin during phosphine-induced cardiotoxicity: focus on mitochondrial dysfunction, oxidative stress and apoptosis. J Pharm Pharmacol 69:236–243. doi:10.1111/jphp.12682
de Oliveira MR, Ferreira GC, Schuck PF, Dal Bosco SM (2015) Role for the PI3K/Akt/Nrf2 signaling pathway in the protective effects of carnosic acid against methylglyoxal-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Chem Biol Interact 242:396–406. doi:10.1016/j.cbi.2015.11.003
de Oliveira MR, Ferreira GC, Schuck PF (2016) Protective effect of carnosic acid against paraquat-induced redox impairment and mitochondrial dysfunction in SH-SY5Y cells: role for PI3K/Akt/Nrf2 pathway. Toxicol In Vitro 32:41–54. doi:10.1016/j.tiv.2015.12.005
de Oliveira MR, Peres A, Gama CS, Bosco SM (2016) Pinocembrin provides mitochondrial protection by the activation of the Erk1/2-Nrf2 signaling pathway in SH-SY5Y neuroblastoma cells exposed to paraquat. Mol Neurobiol. doi:10.1007/s12035-016-0135-5
de Oliveira MR, Peres A, Ferreira GC (2016) Pinocembrin attenuates mitochondrial dysfunction in human neuroblastoma SH-SY5Y cells exposed to methylglyoxal: role for the Erk1/2-Nrf2 signaling pathway. Neurochem Res. doi:10.1007/s11064-016-2140-5
de Oliveira MR, Peres A, Ferreira GC, Schuck PF, Bosco SM (2016) Carnosic Acid affords mitochondrial protection in chlorpyrifos-treated sh-sy5y cells. Neurotox Res 30:367–379. doi:10.1007/s12640-016-9620-x
Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88
Aquilano K, Baldelli S, Ciriolo MR (2014) Glutathione: new roles in redox signaling for an old antioxidant. Front Pharmacol 5:196. doi:10.3389/fphar.2014.00196
Morris G, Anderson G, Dean O, Berk M, Galecki P, Martin-Subero M, Maes M (2014) The glutathione system: a new drug target in neuroimmune disorders. Mol Neurobiol 50:1059–1084. doi:10.1007/s12035-014-8705-x
Sofic E, Lange KW, Jellinger K, Riederer P (1992) Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson’s disease. Neurosci Lett 142:128–130
Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD (1994) Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol 36:348–355
Chinta SJ, Andersen JK (2008) Redox imbalance in Parkinson’s disease. Biochim Biophys Acta 1780:1362–1367. doi:10.1016/j.bbagen.2008.02.005
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Henchcliffe C, Beal MF (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4:600–609. doi:10.1038/ncpneuro0924
Williams TI, Lynn BC, Markesbery WR, Lovell MA (2006) Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol Aging 27:1094–1099
Acknowledgements
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Mato Grosso (FAPEMAT). FBB receives financial support from the FOPESQ/UFF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11064_2017_2376_MOESM1_ESM.pdf
Supplementary Figure S1. The effects of NGN at different concentrations on the cell viability of SH-SY5Y cells exposed to H2O2. The cells were treated with NGN at 20 - 80 µM for 2 h prior administration of H2O2 at 300 µM for additional 24 h. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from H2O2-treated group. (PDF 96 KB)
11064_2017_2376_MOESM2_ESM.pdf
Supplementary Figure S2. The effects of NGN on the levels of Bcl-2 (A), Bax (B), cytosolic cytochrome c (C), and mitochondrial cytochrome c (D) in SH-SY5Y cells exposed to H2O2. The cells were treated with NGN at 80 µM for 2 h prior administration of H2O2 at 300 µM for additional 24 h. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from H2O2-treated group. (PDF 87 KB)
11064_2017_2376_MOESM3_ESM.pdf
Supplementary Figure S3. The effects of NGN on the activities of caspase-9 (A) and caspase-3 (B) and on the levels of cleaved PARP (C) and DNA fragmentation (D) in SH-SY5Y cells exposed to H2O2. The cells were treated with NGN at 80 µM for 2 h prior administration of H2O2 at 300 µM for additional 24 h. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from H2O2-treated group. (PDF 87 KB)
Rights and permissions
About this article
Cite this article
de Oliveira, M.R., Brasil, F.B. & Andrade, C.M.B. Naringenin Attenuates H2O2-Induced Mitochondrial Dysfunction by an Nrf2-Dependent Mechanism in SH-SY5Y Cells. Neurochem Res 42, 3341–3350 (2017). https://doi.org/10.1007/s11064-017-2376-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2376-8