Abstract
In this study, we investigated whether tanshinone I (T-I) has therapeutic effects in cellular and animal model of Parkinson’s disease (PD), and explore its possible mechanism. For this purpose, human neuroblastoma SH-SY5Y cells were cultured and exposed to 100 μM 6-hydroxydopamine (6-OHDA) in the absence or presence of T-I (1, 2.5 and 5 μM). The results revealed that 6-OHDA-induced cell death was reduced by T-I pretreatment as measured by MTT assay, lactate dehydrogenase release and flow cytomety analysis of cell apoptosis. The increase in the reactive oxygen species caused by 6-OHDA treatment was also attenuated by T-I in SH-SY5Y cells. T-I pretreatment was also shown to result in an increase in nuclear factor erythroid-2-related factor 2 (Nrf2) protein levels and its transcriptional activity as well as the upregulation of Nrf2-dependent genes encoding the antioxidant enzymes heme oxygenase-1, glutathione cysteine ligase regulatory subunit and glutathione cysteine ligase modulatory subunit in SH-SY5Y cells. Moreover, in the in vivo experiment, T-I treatment significantly attenuated 6-OHDA-induced striatal oxidative stress and ameliorated dopaminergic neurotoxicity in 6-OHDA-lesioned mice, as evidenced by western blot analysis of tyrosine hydroxylase (TH) and TH immunostaining of dopaminergic neurons in the substantia nigra and the striatum. Taken together, the results suggest that T-I may be beneficial for the treatment of neurodegenerative diseases like PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by a loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) for which no effective neuroprotective treatment is currently available [1]. Although the exact mechanisms underlying PD are largely unknown, oxidative stress is thought to be one of the critical factors that induce the onset of the disease [2].
The transcription factor nuclear factor erythroid-2 related factor 2 (Nrf2) is a key regulator in the coordinated induction of a battery of cytoprotective genes, including those encoding for endogenous antioxidant such as heme oxygenase-1 (HO-1), glutathione cysteine ligase regulatory subunit (GCLC) and glutathione cysteine ligase modulatory subunit (GLCM). Loss of Nrf2-mediated transcription exacerbates the vulnerability of dopaminergic neurons to oxidative stress [3–5], whereas Nrf2 activation provides a neuroprotective response [6–9]. Due to this, the Nrf2/antioxidant response element (ARE) pathway is considered important and has great promise to be explored for neurotherapeutics in neurodegenerative diseases such as PD.
Danshen (Radix Salvia miltiorrhiza root) extract was widely used in oriental medicine for the treatment of various microcirculatory disturbance-related conditions, including cardiovascular and cerebrovascular diseases. Tanshinone I (T-I) was one the main lipophilic diterpenoid quinines in Danshen. Tanshinone I has the potential to penetrate the blood–brain barrier because of its molecule with high lipophilic property and small molecular weight, and has been reported to exert antioxidant and anti-inflammatory effects in the prevention of ischemic injury in animal models [10]. Recent studies have shown that T-I was able to activate the Nrf2 pathway and induce the expression of antioxidant proteins, which could protect against As(III)-induced lung inflammation in vitro and in vivo via the Nrf2 pathway [11]. However, the potential neuroprotective effects of T-I on PD have not been studied. Based on the previous study, we hypothesize that T-I may have therapeutic effects in 6-hydroxydopamine (6-OHDA)-induced cellular and animal model of PD via upregulating Nrf2 activity.
Materials and Methods
Reagents
T-I and 6-OHDA were purchased from Sigma Chemical Company (St. Louis, MO, USA). Primary antibodies to Nrf2, HO-1, GCLC, GCLM, Lamin A and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Primary antibody to tyrosine hydroxylase (TH) was purchased from Chemicon (Bill card city, MA, USA).
Neuroblastoma SH-SY5Y Cell Culture
Human neuroblastoma SH-SY5Y cells were obtained from American Type Culture Collection (Rockville, MD, USA), and the cells were maintained in DMEM-F12 supplemented with 10 % FBS at 37 °C in a humidified atmosphere of 5 % CO2.
Cell Death/Viability Assessment
Cells seeded in 96-well plate were pretreated with T-I at the indicated concentrations (1, 2.5 and 5 μM) for 2 h before being exposed to 100 μM 6-OHDA for an additional 24 h. Cell viability was quantified using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay 24 h after 6-OHDA exposure.
6-OHDA-induced cell death was quantified by measuring lactate dehydrogenase (LDH) release from damaged cells into the culture medium using a kit according to the manufacturer’s instructions (Cat. No. 04744926001, Roche Diagnostic, Indianapolis, IN). All data were expressed as percentage of LDH release compared with the control group.
Apoptosis Assay
Apoptosis was detected with an Annexin V-FITC/PI double staining Kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instruction. Briefly, cells were treated with T-I (1 and 2.5 μM) for 2 h before being exposed to 6-OHDA for an additional 24 h, and then 1 × 106 cells were harvested, washed twice with ice-cold PBS, and evaluated for apoptosis by double staining with annexin V-FITC and PI in binding buffer using a Cytoflex flow cytometer.
GSH and ROS Determination
Cells were treated with different concentrations of T-I for 24 h. Glutathione (GSH) level was measured using a fluorometric method as previously described [12].
The level of oxidative stress was determined by measuring intracellular reactive oxygen species (ROS) generation. The production of cellular ROS was detected using the 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) fluorescence assay. The level of ROS generated was measured based on the fluorescence intensity of DCF at 525 nm after excitation at 485 nm on a fluorescence microplate reader (Thermo Scientific Varioskan Flash).
Cellular Extraction, Western Blot and Nrf2 Activity Assay
Nuclear extracts and whole cell extracts from SH-SY5Y cells and mice brain tissues were prepared as described previously [13]. Protein concentrations were determined by the bicinchonininc acid (BCA) method (Thermo/Pierce). The following antibodies were utilized for the western blot: Nrf2, HO-1, GCLC, GCLM, TH, Lamin A and β-actin. TransAM Nrf2 assay (Active Motif, Carlsbad, CA) was used to measure Nrf2 DNA binding activity according to the manufacturer’s instruction.
Animal Surgery
All animal experiments were approved by Institutional Animal care and Use committee of Shandong University. Eight-week-old male C57BL/6 mice were treated with 10 mg/kg T-I or vehicle by intraperitoneal injection for 3 days prior to lesioning. On the 3th day of treatment, 1 h after final dosing, mice were placed in a stereotaxic device under 30 mg/kg pentobarbital sodium anesthesia. 6 μg of 6-OHDA (in 2 μl of normal saline with 0.02 % ascorbic acid) or saline alone was injected into the 2 different sites of the striatum on the right side of the brain separately. Mice were sacrificed at different time points following 6-OHDA for biochemical or histological assessment. The stereotaxic coordinates, measured in millimeters, from bregma were: anterior/posterior +1.0, medial/lateral ±2.1, and dorsal/ventral −2.9 as well as anterior/posterior +0.3, medial/lateral ±2.3, and dorsal/ventral −2.9.
TH Immunostaining
Animals were anesthetized with sodium pentobarbital at 3 weeks after 6-OHDA administration, transcardially perfused with 0.9 % normal saline, followed by 4 % paraformaldehyde in 0.1 M PBS (pH 7.4). Brains were dissected out, postfixed in 4 % paraformaldehyde for 24 h, and cryopreserved in 30 % sucrose for 48 h. Frozen brains were then coronally sectioned at 25 μM thickness on a cryomicrotome and sections were mounted on slides. Using an antibody against TH, DA neurons were identified with biotinylated secondary antibody and streptavidin ABC solution (Vector Laboratories). Immunostaining was visualized after diaminobenzidine staining (Vector Laboratories) using bright field microscopy (Olympus).
Statistical Analysis
All data are expressed as the mean ± SEM. The statistical significance between means was assessed by Student’s t test (single comparisons) or by ANOVA followed by Duncan’s multiple range tests (for multiple comparisons). p < 0.05 was considered statistically significant.
Results
T-I Enhances Nuclear Nrf2 Expression and Its Transcriptional Activity in SH-SY5Y Cells
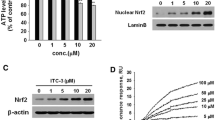
In this study, we tested the ability of T-I to activate Nrf2 signaling in SH-SY5Y cells. When cells were exposed to T-I for 8 h, endogenous Nrf2 protein levels increased significantly after T-I treatment (Fig. 1a). Next, the time dependent induction of Nrf2 by T-I was conducted using 2.5 μM dose. A significant increase in Nrf2 protein level was observed as early as 2 h and persisted up to 24 h (Fig. 1b).
T-I enhances nuclear Nrf2 expression and its transcriptional activity in SH-SY5Y cells. a–d T-I caused nuclear Nrf2 upregulation in SH-SY5Y cells. Cells were either treated with different concentrations of T-I for 8 h or treated with 2.5 μM of T-I for different time points, after which nuclear extracts were prepared to measure the protein levels of Nrf2. e, f T-I treatment resulted in a significant increase in Nrf2 transcriptional activity. Cells were either treated with indicated doses of T-I for 8 h or treated with 2.5 μM of T-I for different time points, after which nuclear extracts were prepared to measure the Nrf2 transcriptional activity. Data represented as mean ± SEM of triplicate independent experiments. *p < 0.05; **p < 0.01 versus control
To detect if T-I enhance Nrf2 transcriptional activity in SH-SY5Y cells, we incubated cells either with different concentrations of T-I for 8 h or with 2.5 μM T-I for different time points and then extracted nuclear fractions. Consistent with the result demonstrating upregulation of Nrf2 at the protein level in response to T-I treatment, the transcriptional activity of Nrf2 was also induced by T-I treatment (Fig. 1e, f).
T-1 Upregulates ARE-Regulated Genes in SH-SY5Y Cells
We next determined whether T-I induces the expression of endogenous ARE-regulated genes. Consistent with the upregulation of nuclear Nrf2 protein levels, exposure of cells to T-I strongly induced a sustained upregulation of HO-1, GCLC and GCLM (Fig. 2a, b) in SH-SY5Y cells.
T-I activates the downstream genes of Nrf2 in SHSY-5Y cells. Total cell lysates from SH-SY5Y cells treated with different concentrations of T-I for 24 h (a, c) or with 2.5 μM T-I for different time points (b, d) were subjected to immunoblot analysis for HO-1, GCLC, GCLM and β-actin. Data represented as mean ± SEM of triplicate independent experiments. **p < 0.01 versus control
T-I Attenuates 6-OHDA-Induced Neuronal Death in SH-SY5Y Cells
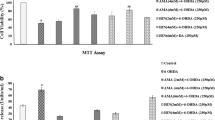
Activation of the Nrf2/ARE pathway is known to confer resistance of cells to oxidative stress-induced cell death [14]. To test this in SH-SY5Y cells, we first performed the MTT and LDH release assay after challenging cells with 100 μM 6-OHDA for 24 h. Compared to vehicle treated control cells, pretreatment of cells with T-I significantly reduced 6-OHDA -associated cell death as well as LDH release (Fig. 3a, b). This was further confirmed by Annexin V-FITC/PI double staining (Fig. 3c, d).
T-I protects against 6-OHDA neurotoxicity in SH-SY5Y cells. SH-SY5Ycells were pretreated with T-I at the indicated concentrations and exposed to 100 μM 6-OHDA. The protective effect of T-I was determined at 24 h after 6-OHDA exposure by a MTT assay and b LDH release. c Cell apoptosis was measured by Annexin-V/PI staining. The apoptotic rate calculated from the flow cytometry data were shown in (d). All data are presented as the mean ± SEM of triplicate independent experiments. ## p < 0.01 versus control, *p < 0.05, **p < 0.01 versus 6-OHDA group
T-I Preventes 6-OHDA-Induced Oxidative Damage in SH-SY5Y Cells
To examine whether T-I also prevented the production of ROS from 6-OHDA exposure, the accumulation of ROS was measured by the fluorescent probe DCFH-DA in SH-SY5Y cells. As shown in Fig. 4a, T-I effectively reduced 6-OHDA-induced intracellular ROS level.
T-1 attenuates 6-OHDA-induced oxidative stress in SH-SY5Y cells. a SH-SY5Y cells were pretreated with T-I at the indicated concentrations for 2 h and exposed to 100 μM 6-OHDA, cells were collected 24 h later for intracellular ROS level. b Total cell lysates from SH-SY5Y cells treated with T-I for 24 h were subjected to GSH measurement. Data represented as mean ± SEM of triplicate independent experiments. # p < 0.05, ## p < 0.01 versus control, *p < 0.05; **p < 0.01 versus 6-OHDA group
Since T-I significantly changed levels of key proteins that regulate GSH metabolism, we also measured cellular levels of GSH at 24 h after T-I treatment. We noted that T-I produced a significant increase in GSH levels in SH-SY5Y cells (Fig. 4b). Together, our in vitro data suggest that T-I induces nuclear accumulation of Nrf2 to upregulate downstream ARE genes, leading to increase in cellular antioxidants such as GSH.
T-I Reduces 6-OHDA-Induced Oxidative Stress in Mice Striatum
To substantiate the in vitro findings regarding the neuroprotective effects of T-I, we next measured the levels of ROS and GSH in the mice striatum at 1 and 7 days after 6-OHDA lesioning. As an index of ROS formation, we first measured the oxidation of the fluorescent probe DCFH-DA in the striatum of 6-OHDA-treated animals. As shown in Fig. 5a, 6-OHDA induced significant increases in ROS formation at 1 and 7 days after the toxin injection. In turn, T-I pretreatment significantly decreased the 6-OHDA-induced ROS formation. We also measured the levels of GSH at the same time; T-I pretreatment also exhibited a consistent protection against the 6-OHDA-induced oxidative stress by increasing GSH levels at 1 and 7 days after 6-OHDA injection (Fig. 5b). Altogether, these results indicate that the pretreatment with T-I protect mice against 6-OHDA-induced oxidant damage in the striatum.
T-I reduces 6-OHDA-induced striatal oxidative stress in mice striatum. Mice were given 10 mg/kg T-I by intraperitoneal injection for 3 days, and then received striatal infusion of 6-OHDA. At 1 and 7 days after 6-OHDA injection, striatum was collected for measurement of ROS (a) and GSH levels (b). Data represented as mean ± SEM of triplicate independent experiments. ## p < 0.01 versus control, *p < 0.05; **p < 0.01 versus 6-OHDA group
T-I Prevents 6-OHDA-Induced Dopaminergic Neuronal Loss in Mice Striatum and SNc
To further determine if the protection exerted by T-I against the 6-OHDA-induced oxidative stress also accounts to preserve the integrity of the nigrostriatal tract, we analyzed TH immunohistochemistry as a phenotypic marker for fibers of dopaminergic neurons in the SNc and striatum. To do this, brains were collected at 21 days after 6-OHDA lesioning. Immunohistochemical staining demonstrated that the 6-OHDA-induced loss of TH-positive neurons in the striatum and SNc were remarkably attenuated by T-I treatment (Fig. 6a). These results were also supported by immunoblots of striatal extracts evaluated using an anti-TH antibody, which show higher TH protein levels in T-I treated mice (Fig. 6b).
T-I protects the nigrostriatal dopaminergic system from 6-OHDA -induced degeneration in mice. Brain sections from the striatum and SNc showing TH immunoreactivity (a) and TH immunoblot (b) 21 days after striatal infusion of 6-OHDA. All data are presented as the mean ± SEM. ## p < 0.01 versus control, **p < 0.01 versus 6-OHDA group. n = 8–10 per group for all experiments
Discussion
In this paper, we evaluated whether T-I may represent a possible pharmacological intervention in PD. Our in vitro results demonstrated that T-I activate the Nrf2 pathway and protect SH-SY5Y cells against 6-OHDA-induced neurotoxicity. While in vivo studies using 6-OHDA-lesioned PD model showed that T-I could also ameliorate dopaminergic neurodegeneration in mice. This in vivo data provides further confirmative evidence supporting the observed neuroprotective effects of T-I in vitro. Taken together, the result of this study support our hypothesis that T-I protects against 6-OHDA-induced neurotoxicity and this protective effect involves the Nrf2 signaling pathway.
The pathogenesis of neuronal degeneration in PD is yet to be fully understood; however, oxidative stress appears to be a prominent pathogenic component in PD. Among potential radical-induced antioxidant pathways, the Nrf2/ARE signaling pathway has emerged as a promising therapeutic target for neurodegenerative disorders; there is an increasing clinical interest in using Nrf2 activators for therapeutic purposes. One way to achieve this is using drugs that are already used in clinic that can activate the Nrf2 pathway. Unlike many experimental Nrf2 activators with undefined or unfavorable pharmacokinetic profiles, tanshinones are investigational drugs currently in advanced stages of clinical development in human patients with cardiovascular diseases and other indications. T-I has been reported to be neuroprotective for PD [16]. However, the mechanism that underlies its neuroprotection against PD remains poorly understood. Therefore, we investigated the therapeutical potential of T-I in protecting against 6-OHDA model both in vitro and in vivo. Our results indicate that T-I can induce the expression and activate the transcriptional activity of Nrf2; the induction of Nrf2 can trigger the downstream regulation of antioxidant enzymes, as evidenced by the subsequent change in the cellular redox status through the upregulation of HO-1, GCLC and GCLM protein expression, indicating a protective role of Nrf2 signaling in this process.
Neuroprotective effects of Tanshinones have been shown in several neurodegenerative disease models. Chong et al. [15] revealed that Dan shensu (β-3,4-dihydroxyphenyl-lactic acid), a compound extracted from Radix Salviae Miltiorrhizae (known as ‘Danshen’ in Chinese), protected against 6-hydroxydopamine-induced damage of PC12 cells in vitro and dopaminergic neurons in zebrafish. During the preparation of the manuscript, a recent article demonstrated that T-I could selectively suppress pro-inflammatory genes expression in activated microglia and prevent nigrostriatal dopaminergic neurodegeneration in a MPTP-mouse model of PD [16]. In consistent with the previous reports, we also observed that T-I can elicit robust neuroprotection against 6-OHDA PD model possibly through activating the Nrf2 pathway.
Our study indicates that the neuroprotective effect of T-I is associated with its ability to activate Nrf2. A recent study showed that T-I induced Nrf2-dependent response primarily by hindering its ubiquitination and degradation of Nrf2, and stabilizing Nrf2 protein level in a Keap1-dependent manner [11]. While the current study focuses on Nrf2, it does not exclude the possibility that T-I may exhibit its neuroprotective effects through other mechanisms. Further studies are still needed to elucidate the precise protective mechanisms exerted by T-I in several toxic models.
In conclusion, the results of this study demonstrate a neuroprotective role for T-I against experimental PD models. We also observed that T-I increase the expression of the nuclear level of Nrf2 and its transcriptional activity. Together with the previous reports, T-I may provide therapeutic benefit in the treatment of PD.
References
Savitt JM, Dawson VL, Dawson TM (2006) Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest 116:1744–1754
Hwang O (2013) Role of oxidative stress in Parkinson’s disease. Exp Neurobiol 22:11–17
Burton NC, Kensler TW, Guilarte TR (2006) In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology 27:1094–1100
Jakel RJ, Townsend JA, Kraft AD, Johnson JA (2007) Nrf2-mediated protection against 6-hydroxydopamine. Brain Res 1144:192–201
Rojo AI, Innamorato NG, Martin-Moreno AM, De Ceballos ML, Yamamoto M, Cuadrado A (2010) Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson’s disease. Glia 58:588–598
Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA (2009) Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: critical role for the astrocyte. Proc Natl Acad Sci USA 106:2933–2938
Jazwa A, Rojo AI, Innamorato NG, Hesse M, Fernandez-Ruiz J, Cuadrado A (2011) Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid Redox Signal 14:2347–2360
Kaidery NA, Banerjee R, Yang L, Smirnova NA, Hushpulian DM, Liby KT, Williams CR, Yamamoto M, Kensler TW, Ratan RR, Sporn MB, Beal MF, Gazaryan IG, Thomas B (2013) Targeting Nrf2-mediated gene transcription by extremely potent synthetic triterpenoids attenuate dopaminergic neurotoxicity in the MPTP mouse model of Parkinson’s disease. Antioxid Redox Signal 18:139–157
Lou H, Jing X, Wei X, Shi H, Ren D, Zhang X (2014) Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology 79:380–388
Lee JC, Park JH, Park OK, Kim IH, Yan BC, Ahn JH, Kwon SH, Choi JH, Kim JD, Won MH (2013) Neuroprotective effects of tanshinone I from Danshen extract in a mouse model of hypoxia-ischemia. Anat Cell Biol 46:183–190
Tao S, Zheng Y, Lau A, Jaramillo MC, Chau BT, Lantz RC, Wong PK, Wondrak GT, Zhang DD (2013) Tanshinone I activates the Nrf2-dependent antioxidant response and protects against As(III)-induced lung inflammation in vitro and in vivo. Antioxid Redox Signal 19:1647–1661
Rahman I, Kode A, Biswas SK (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1:3159–3165
Jing X, Ren D, Wei X, Shi H, Zhang X, Perez RG, Lou H (2013) Eriodictyol-7-O-glucoside activates Nrf2 and protects against cerebral ischemic injury. Toxicol Appl Pharmacol 273:672–679
Cuadrado A, Moreno-Murciano P, Pedraza-Chaverri J (2009) The transcription factor Nrf2 as a new therapeutic target in Parkinson’s disease. Expert Opin Ther Targets 13:319–329
Chong CM, Zhou ZY, Razmovski-Naumovski V, Cui GZ, Zhang LQ, Sa F, Hoi PM, Chan K, Lee SM (2013) Danshensu protects against 6-hydroxydopamine-induced damage of PC12 cells in vitro and dopaminergic neurons in zebrafish. Neurosci Lett 543:121–125
Wang S, Jing H, Yang H, Liu Z, Guo H, Chai L, Hu L (2015) Tanshinone I selectively suppresses pro-inflammatory genes expression in activated microglia and prevents nigrostriatal dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. J Ethnopharmacol 164:247–255
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (Nos. 81274124, 81200982) and Shandong Province Science and Technology Program (No. 2014GSF118038).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Jing, X., Wei, X., Ren, M. et al. Neuroprotective Effects of Tanshinone I Against 6-OHDA-Induced Oxidative Stress in Cellular and Mouse Model of Parkinson’s Disease Through Upregulating Nrf2. Neurochem Res 41, 779–786 (2016). https://doi.org/10.1007/s11064-015-1751-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1751-6