Abstract
Solidification of ionic liquid (SIL) has been applied in microwave-assisted liquid-liquid microextraction method for analyzing Sudan dyes in juice samples. 1-Ethy-3-methylimidazolium hexafluorophosphate ([C2MIM][PF6]) was selected as the extraction solvent due to its relatively high melting point. With the aid of microwave irradiation, the dyes could be easily extracted into ionic liquid phase without dispersive solvent. The solid microdroplet could be easily separated from liquid. Compared with traditional dispersive liquid-liquid microextraction, this method will greatly simplify the collection procedure and shorten the operation time. Several experimental parameters, including the amount of ionic liquid, microwave power and irradiation time, pH of sample solution, and ionic strength, were systematically investigated and optimized. The limits of detection for Sudan I, II, III, and IV were 1.08, 1.30, 1.14, and 1.19 μg/L, respectively. The recoveries of the analytes ranged from 83.42 to 115.56 % and relative standard deviations were lower than 11.53 %, when the present method was applied to the analysis of real samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sudan dyes are a group of lipophilic azo dyes that are widely used as coloring agents in oils, waxes, floor and shoe polishes, and printing inks (Li et al. 2014; Murty et al. 2009). However, the azo functional groups and aromatic rings in Sudan dyes may lead to adverse effects on health such as allergic and asthmatic reactions, DNA damage, hyperactivity, and liver cancer (Zhao et al. 2012). International Agency for Research on Cancer (IARC) has classified these Sudan dyes as category 3 carcinogen (Ávila et al. 2011). Consequently, the addition of Sudan dyes to foodstuff is forbidden in any national and international food regulation act (Qiao et al. 2011). Unfortunately, the illegal use of the dyes has still been found in varieties of foodstuffs due to their fresh color, colorfastness, wide availability, and low cost (Schummer et al. 2013). Therefore, there is a pressing need to develop a rapid, reliable, effective, and environmental friendly method for the identification and quantification of Sudan dyes.
Until now, high-performance liquid chromatography (HPLC; Ertas et al. 2007), liquid chromatography–mass spectrometry (LC-MS; Chen et al. 2013), gas chromatography–mass spectrometry (GC-MS; He et al. 2007), immunoaffinity chromatography (IAC; Li et al. 2010), and capillary electrophoresis (Mejia et al. 2007) have been applied to the determination of Sudan dyes from different foodstuffs. However, because the excellent dyeing property of Sudan dyes, the dosage of the dye is precious little. Considering the low concentration of the dyes and the matrix complexity of the samples, sample preparation plays an important role in the whole analytical procedure. Various methods have been applied in the pretreatment process, such as solid-phase extraction (SPE; Qi et al. 2011), liquid-liquid extraction (LLE; Long et al. 2011), solid-phase microextraction (SPME; Hu et al. 2012), liquid-phase microextraction (LPME; Yu et al. 2008), and cloud point extraction (CPE; Liu et al. 2007). SPE and LLE were initially developed in sample preparation, but they are somewhat tedious and unfriendly to both the environment and operators. SPME, LPME, and CPE overcome the disadvantages of expenditure of organic solvent, but still suffer from long extraction time, low enrichment factor, or repeatability.
Dispersive liquid-liquid microextraction (DLLME; Viñas et al. 2015a; Farajzadeh et al. 2015) was developed to reduce the extraction time and enhance enrichment factors by injecting extraction solvents and disperser solvents into aqueous sample to form cloudy state. However, the extraction solvents are usually chlorobenzene, chloroform, and carbon tetrachloride; all of them are very toxic and environmentally hazardous (Viñas et al. 2014a). As an environmentally friendly solvent, the room temperature ionic liquids (RTILs) are semiorganic salts, with important features including high viscosity, low vapor pressure, good thermal stability, a wide range of miscibility with water and other organic solvents, nonflammability, and dual natural polarity (Poole and Poole 2010; Ravelo-Pérez et al. 2009). Ionic liquid (IL) has been applied in LPME (Wang et al. 2013a), SPME (Wang et al. 2014), and aqueous two-phase systems extraction (Yu et al. 2015). Furthermore, IL could also be a good alternative to traditional organic solvents in dispersive liquid-liquid microextraction. Ionic liquid dispersive liquid-liquid microextraction (IL-DLLME) has been developed and shows superiority in respect of rapidity, low-cost, high-enrichment factor, and perfect recovery (Wen and Zhu 2014; Viñas et al. 2015b).
Based on traditional DLLME methods, many IL-DLLME methods have been developed, including ultrasonic-assisted IL-DLLME (Zeeb et al. 2014), in situ IL-DLLME (Fernández et al. 2014), and temperature control IL-DLLME (Amjadi et al. 2013). Recently, it was reported that ILs could efficiently absorb microwave energy and thus could be used as solvents and co-solvents for microwave-assisted extraction. Contrast to the conventional extraction methods, microwave-assisted IL-DLLME greatly reduces the extraction time and enhances the recoveries.
However, the main challenge of DLLME (including IL-DLLME) is the separation of the small volume of extraction solvent from the water phase. Although the liquid is immiscible with water phase, the flexible liquid is hard to be separated. Therefore, some researchers invented micropipettes or cone tip needle syringes from appropriate or specialized apparatus (i.e., conical neck centrifuge vials, narrow-neck flasks, etc.) to resolve the problem, but it still cost patience and can hardly extract perfectly from water. Besides, these apparatus are either home-designed or not readily available, especially for larger volumes (Giannoulis et al. 2013). Song etc. developed the homogeneous ionic liquid microextraction which is based on utilizing a hydrophilic IL ([C12MIM]Br) as extraction solvent, then transfered into hydrophobic solid-state ionic liquid after adding ammonium hexafluorophosphate ([NH4][PF6]) (Song et al. 2015). This method simplifies the collection process of the extraction solvents; however, we still could combine the extraction and preconcentration into one step to improve and optimize the methods. Without the additional of anion-exchange reagent, the method could be easy, cheap, green, and low-consuming.
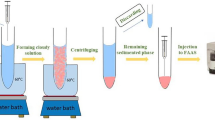
In this method, solidification of ionic liquid (SIL) has been applied in microwave-assisted liquid-liquid microextraction method for the extraction and enrichment of Sudan dyes from juice samples. 1-Ethy-3-methylimidazolium hexafluorophosphate ([C2MIM][PF6]) was chosen as the extraction solvent. It was solid at room temperature with the melt point of 62.5 °C. Then, the heating effect of microwave irradiation made the IL melt and dissolve in the sample solution. The IL containing the dyes transferred into solid IL again by freezing and separated from the sample solution after centrifugation. The whole process took a short time without any extra equipment or traditional toxic extraction solvent. Furthermore, the method was applied into real sample analysis with good performance in detection limit and recoveries. Thus, a rapid, green, effective, and sensitive method for the determination of Sudan dyes in juice samples was established.
Experimental
Chemicals and Reagents
The standards of Sudan I–IV were obtained from the National Institutes for the Control of Pharmaceutical and Biological Products (Beijing, China). Standard stock solutions for the dyes at the concentration level of 500 μg/mL were prepared in acetonitrile and stored at 4 °C. The mixed working solutions were obtained by diluting the standard stock solutions with acetonitrile. Chromatographic grade acetonitrile and methanol were purchased from Fisher Scientific Company (Pittsburgh, PA, USA). Pure water was obtained with a Milli-Q water purification system (Millipore Co., USA). Sodium chloride, sodium hydroxide, and hydrochloric acid were of analytical-reagent grade and purchased from Beijing Chemical Factory (Beijing, China). 1-Ethy-3-methylimidazolium hexafluorophosphate ([C2MIM][PF6]) was obtained from Chengjie Chemical (Shanghai, China).
Apparatus and Instruments
Chromatographic separation and determination of the Sudan dyes were performed on the 1100 series liquid chromatograph (Agilent Technologies Inc., USA) equipped with quaternary gradient pump, a degasser, photodiode-array detector (DAD), a heated column compartment, an injection value, and an LC workstation. Eclipse XDB-C18 column (3.5 μm, 4.6 mm × 150 mm, Agilent, USA) was used.
The extraction was performed on a modified household microwave oven (SANYO, China) with a maximum microwave output power of 600 W. The microwave output power can be controlled with a continuously changeable transformer. A DELTA-320 acidity meter (Mettler-Toledo Instruments, Shanghai, China) was used for pH measurement. The phase separation was performed on an LDZ4-1.2 centrifuge (Jingli centrifuge, Beijing, China).
Sample Preparation
Four juice samples were purchased from a local supermarket (Changchun, China), including pink juice (sample 1), red juice (sample 2), pale pink juice (samples 3), and purple juice (sample 4). The sample solution was filtered through 0.45-μm filters before extraction.
The samples used for recovery and precision studies were previously determined to be free of the Sudan dyes considered. The spiked samples were prepared by spiking the mixed working standard solution into samples. All results were obtained with sample 1 except for those mentioned in Sections 3.2.4.
Extraction Procedure
Two milliliters of the spiked sample and 0.14 g NaCl were placed into 5 mL glass centrifuge tube. Then, 0.18 g [C2MIM][PF6] was added into the sample solution. The tube was immediately placed in the microwave oven and irradiated under the microwave power of 180 W for 90 s. The solid IL was melted with the assistance of microwave irradiation and dispersed as very fine droplets into sample solution by shaking with hand for 30 s. After that, the analytes were extracted into the IL droplets. The tube was placed into a refrigerator for 5 min; the IL solidified because of the low melting point (62.5 °C). After centrifugation at 3,500 rpm for 5 min, the IL was deposited at the bottom of the tube. The solid IL phase was separated from the mixture, then it was purged under mild nitrogen stream to remove the remaining sample solution in the solid IL. Then, the IL was dissolved in 100 μL of acetonitrile. The resulting solution was filtrated through 0.22 μm PTFE filter membrane, and then directly analyzed by HPLC-UV.
Chromatographic Determination
A gradient elution solvent was applied which contained water as mobile phase A and acetonitrile as mobile phase B. The gradient condition was as follows: 0–5 min, 90–95 % B; 5–8 min, 95–100 % B; 8–18 min, 100 % B; 18–22 min, 100–95 % B; 22–24 min, 95–90 % B. The flow rate of the mobile phase was 0.5 mL/min and the temperature of the column was kept at 35 °C. The injection volume of analytical solution was 20 μL. The monitored wavelengths were 478 nm for Sudan I and Sudan II, 520 nm for Sudan III and Sudan IV (Sun et al. 2011).
Results and Discussion
Optimization of Extraction
Effect of Amount of IL
The influence of amount of IL on extraction efficiency was studied by adding different amounts of IL (0.12–0.24 g). As shown in Fig. 1, the extraction efficiency increased along with the amount of IL from 0.12 to 0.18 g, because the analytes’ diffusion rate from the solution into micro-droplet is directly related to the interfacial area between the two liquid phases. When the amount exceeded 0.18 g, the recoveries remained approximately constant. Hence, 0.18 g was IL was chosen as the ideal amount in the proposed method.
Effect of pH of Sample Solution
The pH of the sample solution plays an important role in the extraction of organic compounds. Therefore, in the present study, the effect of pH values ranging from 1.0 to 11.0 was investigated. The results indicated that the pH does not affect the extraction recovery significantly. The reason may be that Sudan dyes exist in neutral molecules under ordinary conditions and do not undergo noticeable dissociation or protonation in moderately acidic or basic media (Yu et al. 2012). Accordingly, the pH of the sample solution was not adjusted for simplify.
Effect of Ionic Strength
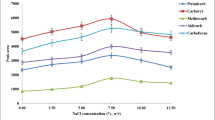
The addition of salt to the extract is usually made to improve the enrichment efficiency of the analytes because the increased ionic strength of aqueous phase could aid the partition of analytes to organic phase (Lv et al. 2010). To examine the influence of ionic strength on the extraction efficiency, we compared sodium sulfate with sodium chloride in the experiment; the effects were similar. Sodium chloride was chosen due to it dissolved in water easily (Sun et al. 2011). A series of experiments were performed by adding different amounts of sodium chloride (0–11 %, w/v). As shown in Fig. 2, the recoveries of Sudan dyes increased with the increase of NaCl concentration from 0 to 7 %, and then decreased when NaCl concentration exceeds 7 %. The addition of salt into sample solution can decrease the solubility of analytes in sample solution and contribute to phase separation. However, when the amount of NaCl was excessively large, the viscosity of sample solution increased, thereby decreased the transfer of Sudan dyes into extraction phase. Therefore, the concentration of NaCl was chosen as 7 % in all subsequent experiments.
Effect of Microwave Power
In this method, the proper temperature makes the solid IL melt absolutely and then contributes to the formation of cloudy solution. The analytes were transferred from the sample solution to the extraction solvent due to the existence of the fine IL droplets. The temperature is strongly related to the power and time of microwave (Xu et al. 2011). The effect of microwave power was studied by varying the power between 60 and 360 W when the irradiation time was 90 s. It was shown in Fig. 3 that the recoveries of dyes increased with the increase of the microwave power from 60 to 180 W, and slowly decreased thereafter. If the microwave power was too low, the IL could not well disperse into the sample solution or even keep solid phase, which will directly affect the extraction efficiency. Excessively, too high microwave power may also contribute to the degradation of the analytes. Based on the results, 180 W was selected as the appropriate microwave power.
Effect of Microwave Irradiation Time
The influence of microwave extraction time on extraction recovery was studied under the microwave power of 180 W. As shown in Fig. 4, the recoveries of Sudan dyes increased obviously with the increase of the irradiation time from 60 to 90 s and the maximum recoveries were obtained at 90 s. When the irradiation time was longer than 90 s, the recovery decreased slightly due to the increase in solubility of [C2MIM][PF6] at higher temperature (You et al. 2007). Hence, 90 s was adopted in the following experiments.
Orthogonal Experiment
In order to determine the optimum operating conditions and the probable interaction between variables, an orthogonal experiment (L9(34)) was carried out based on the previous experimental results obtained by the univariate method. The amount of IL (A1, 0.16 g; A2, 0.18 g; A3, 0.20 g), ionic strength (B1, 5 %; B2, 7 %; B3, 9 %), microwave power (C1, 150 W; C2, 180 W; C3, 240 W), and microwave irradiation time (D1, 75 s; D2, 90 s; D3 105 s) on recoveries of analytes are shown in Table 1. In the study, the other experimental parameters are the same as those selected by univariate method. The Kn, which is the mean effect of each factor at the different levels, and R value, which is the range, are calculated and listed in Table 2. Based on the experimental results, the amount of IL, ionic strength, microwave power, and microwave irradiation time were selected as 1.8 g, 7 %, 180 W, and 90 s, respectively.
Method Validation
The target analytes were identified by comparing their retention times and absorption spectra with those of the authentic standard analytes (Wang et al. 2013b). The spectral data for each chromatographic peak are helpful in the identification of species. The results are shown in Fig. 5.
Linearity
The working curves were constructed by plotting the corresponding peak areas measured versus the concentrations of Sudan dyes in a series of spiked samples (Viñas et al. 2014b). The corresponding linear regression equations and correlation coefficients are listed in Table 3. Good linearities are obtained with the correlation coefficients ranging from 0.9991 to 0.9993.
Limit of Detection and Quantification
The limit of detection (LOD) and limit of quantification (LOQ) are the lowest concentration yielding a signal/noise ratio of 3 and 10, respectively. The results obtained are given in Table 3. The LODs and LOQs for Sudan dyes are in the range of 1.08–1.30 and 3.60–4.34 μg/L, respectively.
Precision and Accuracy
The intra- and inter-day precision of the present method were obtained by analyzing the spiked sample at concentrations of 70 μg/L. The intra-day precision was performed by analyzing spiked samples five times on the same day. The inter-day precision was performed by analyzing spiked samples over five working days. As Table 4 show, acceptable RSD values, ranging from 4.77 to 5.97 % and from 3.29 to 6.16 % for intra- and inter-day, were obtained, respectively. The recoveries in the range of 88.06–95.57 % and 89.4–92.35 % for intra- and inter-day, were also obtained, respectively.
Application of the Method
The practical applicability of the present method was evaluated by determining Sudan dyes from the four kinds of spiked juice samples. The typical chromatograms of the blank and spiked samples are shown in Fig. 6. As listed in Table 5, the present method provides good recoveries (85.00–115.56 %) and acceptable precision (0.79–11.53 %). It can be considered that the present method provides good recoveries and acceptable precisions for the determination of Sudan dyes in real juice samples.
Comparison of MA-LLME-SFO with Other Methods
In order to evaluate the performances, some other methods reported in the literature, including dispersive liquid-liquid microextraction (DLLME) (Sun et al. 2011; Zhou et al. 2014), aqueous two-phase systems extraction (ATPSE) (Yu et al. 2015), magnetic solid-phase extraction (MSPE) (Li et al. 2014), were compared with the present method for Sudan dye extraction, and the results are shown in Table 6. From the table, the proposed method showed superiority over others in respect of extraction time and the amount of organic solvent with acceptable recoveries and detection limits.
Conclusion
In the present work, SIL has been applied in microwave-assisted liquid-liquid microextraction method for analyzing Sudan dyes in juice samples. This method provided satisfactory linearity, repeatability, and detection limit within short time. Moreover, the use of solid-state IL as substitute of traditional chlorinated extraction solvent makes the process easy and environmentally friendly. It is more eco-friendly with the absence of the organic dispersive solvent in the whole process. Therefore, the proposed method indicated great potential in the analysis of banned dyes in many complicated matrices.
References
Amjadi M, Manzoori JL, Hamedpour V (2013) Optimized ultrasound-assisted temperature-controlled ionic liquid microextraction coupled with FAAS for determination of tin in canned foods. Food Anal Methods 6:1657–1664
Ávila M, Zougaghb M, Escarpa A, Ríos A (2011) Determination of Sudan dyes in food samples using supercritical fluid. J Supercrit Fluid 55:977–982
Chen DM, Li XQ, Tao YF, Pan YH, Wu QH, Liu ZL, Peng DP, Wang X, Huang LL, Wang YL, Yuan ZH (2013) Development of a liquid chromatography-tandem mass spectrometry with ultrasound-assisted extraction method for the simultaneous determination of Sudan dyes and their metabolites in the edible tissues and eggs of food-producing animals. J Chromatogr B 939:45–50
Ertas E, Ozer H, Alasalvar C (2007) A rapid HPLC method for determination of Sudan dyes and Para Red in red chilli pepper. Food Chem 105:756–760
Farajzadeh MA, Abbaspour M, Mogaddam MRA, Ghorbanpour H (2015) Determination of some synthetic phenolic antioxidants and bisphenol A in honey using dispersive liquid–liquid microextraction followed by gas chromatography-flame ionization detection. Food Anal Methods 8:2035–2043
Fernández E, Vidal L, Iniesta J, Metters JP, Banks CE, Canals A (2014) Screen-printed electrode-based electrochemical detector coupled with in-situ ionic-liquid-assisted dispersive liquid-liquid microextraction for determination of 2,4,6-trinitrotoluene. Anal Bioanal Chem 406:2197–2204
Giannoulis KM, Giokas DL, Zhu Q, Tsogas GZ, Vlessidis AG, Pan QM (2013) Surfactant-enhanced liquid-liquid microextraction coupled to micro-solid phase extraction onto highly hydrophobic magnetic nanoparticles. Microchim Acta 180:775–782
He LM, Su YJ, Fang BH, Shen XG, Zeng ZL, Liu YH (2007) Determination of Sudan dye residues in eggs by liquid chromatography and gas chromatography–mass spectrometry. Anal Chim Acta 594:139–146
Hu XG, Fan YN, Zhang Y, Dai GM, Cai QL, Cao YJ, Guo CJ (2012) Molecularly imprinted polymer coated solid-phase microextraction fiber prepared by surface reversible addition-fragmentation chain transfer polymerization for monitoring of Sudan dyes in chilli tomato sauce and chilli pepper samples. Anal Chim Acta 731:40–48
Li Y, Wang YZ, Yang H, Gao Y, Zhao HH, Deng AP (2010) Establishment of an immunoaffinity chromatography for simultaneously selective extraction of Sudan I, II, III and IV from food samples. J Chromatogr A 1217:7840–7847
Li CY, Chen LG, You XX (2014) Extraction of Sudan dyes from environmental water by hemimicelles-based magnetic titanium dioxide nanoparticles. Environ Sci Pollut Res 21:12382–12389
Liu W, Zhao WJ, Chen JB, Yang MM (2007) A cloud point extraction approach using Triton X-100 for the separation and preconcentration of Sudan dyes in chilli powder. Anal Chim Acta 605:41–45
Long CY, Mai ZB, Yang XF, Zhu BH, Xu XM, Huang XD, Zou XY (2011) A new liquid-liquid extraction method for determination of 6 azo-dyes in chilli products by high-performance liquid chromatography. Food Chem 126:1324–1329
Lv LL, Xu H, Song D, Cui YF, Hu S, Zhang GB (2010) Analysis of volatile aldehyde biomarkers in human blood by derivatization and dispersive liquid-liquid microextraction based on solidification of floating organic droplet method by high performance liquid chromatography. J Chromatogr A 1217:2365–2370
Mejia E, Ding YS, Mora MF, Garcia CD (2007) Determination of banned Sudan dyes in chili powder by capillary electrophoresis. Food Chem 102:1027–1033
Murty MRVS, Sridhara Chary N, Prabhakar S, Prasada Raju N, Vairamani M (2009) Simultaneous quantitative determination of Sudan dyes using liquid chromatography-atmospheric pressure photoionization-tandem mass spectrometry. Food Chem 115:1556–1562
Poole CF, Poole SK (2010) Extraction of organic compounds with room temperature ionic liquids. J Chromatogr A 1217:2268–2286
Qi P, Zeng T, Wen ZJ, Liang XY, Zhang XW (2011) Interference-free simultaneous determination of Sudan dyes in chili foods using solid phase extraction coupled with HPLC-DAD. Food Chem 125:1462–1467
Qiao FX, Geng YR, He CQ, Wu YP, Pan PY (2011) Molecularly imprinted microspheres as SPE sorbent for selective extraction of four Sudan dyes in catsup products. J Chromatogr B 879:2891–2896
Ravelo-Pérez LM, Hernández-Borges J, Herrera-Herrera AV, Rodríguez-Delgado MA (2009) Pesticide extraction from table grapes and plums using ionic liquid based dispersive liquid-liquid microextraction. Anal Bioanal Chem 395:2387–2395
Schummer C, Sassel J, Bonenberger P, Moris G (2013) Low-level detections of Sudan I, II, III and IV in spices and chili containing foodstuffs using UPLC-ESI-MS/MS. J Agric Food Chem 61:2284–2289
Song Y, Wu LJ, Li N, Hu MZ, Wang ZM (2015) Utilization of a novel microwave-assisted homogeneous ionic liquid microextraction method for the determination of Sudan dyes in red wines. Talanta 135:163–169
Sun S, Wang Y, Yu WZ, Zhao TQ, Gao SQ, Kang MQ, Zhang YP, Zhang HQ, Yu Y (2011) Determination of sudan dyes in red wine and fruit juice using ionic liquid-based liquid-liquid microextraction and high-performance liquid chromatography. J Sep Sci 34:1730–1737
Viñas P, Campillo N, López-García I, Hernández-Córdoba M (2014a) Dispersive liquid-liquid microextraction in food analysis: a critical review. Anal Bioanal Chem 406:2067–2099
Viñas P, Bravo-Bravo M, López-García I, Pastor-Belda M, Hernández-Córdoba M (2014b) Pressurized liquid extraction and dispersive liquid–liquid microextraction for determination of tocopherols and tocotrienols in plant foods by liquid chromatography with fluorescence and atmospheric pressure chemical ionization-mass spectrometry detection. Talanta 119:98–104
Viñas P, Campillo N, Pastor-Belda M, Oller A, Hernández-Córdoba M (2015a) Determination of phthalate esters in cleaning and personal care products by dispersive liquid-liquid microextraction and liquid chromatography-tandem mass spectrometry. J Chromatogr A 1376:18–25
Viñas P, Campillo N, Andruch V (2015b) Recent achievements in solidified floating organic drop microextraction. Trends Anal Chem 68:48–77
Wang S, Liu C, Yang S, Liu F (2013a) Ionic liquid-based dispersive liquid-liquid microextraction following high-performance liquid chromatography for the determination of fungicides in fruit juices. Food Anal Methods 6:481–487
Wang ZB, Li N, Wang M, Wang Y, Du L, Ji XF, Yu AM, Zhang HQ, Qiu FP (2013b) Simultaneous determination of nucleosides and their bases in Cordyceps sinensisand its substitutes by matrix solid-phase dispersion extraction and HPLC. J Sep Sci 36:2348–2357
Wang TT, Chen YH, Ma JF, Hu MJ, Li Y, Fang JH, Gao HQ (2014) A novel ionic liquid-modified organic-polymer monolith as the sorbent for in-tube solid-phase microextraction of acidic food additives. Anal Bioanal Chem 406:4955–4963
Wen SP, Zhu SH (2014) Speciation analysis of Mn(II)/Mn(VII) in tea samples using flame atomic absorption spectrometry after room temperature ionic liquid-based dispersive liquid-liquid microextraction. Food Anal Methods 7:291–297
Xu X, Su R, Zhao X, Liu Z, Li D, Li XY, Zhang HQ, Wang ZM (2011) Determination of formaldehyde in beverages using microwave-assisted derivatization and ionic liquid-based dispersive liquid-liquid microextraction followed by high-performance liquid chromatography. Talanta 85:2632–2638
You JY, Zhang HR, Zhang HQ, Yu AM, Xiao TT, Wang YT, Song DQ (2007) Determination of triazines in infant nutrient cereal-based foods by pressurized microwave-assisted extraction coupled with high-performance liquid chromatography-mass spectrometry. J Chromatogr B 856:278–284
Yu CH, Liu Q, Lan LD, Hu B (2008) Comparison of dual solvent-stir bars microextraction and U-shaped hollow fiber-liquid phase microextraction for the analysis of Sudan dyes in food samples by high-performance liquid chromatography-ultraviolet/mass spectrometry. J Chromatogr A 1188:124–131
Yu X, Sun Y, Jiang CZ, Gao Y, Wang YP, Zhang HQ, Song DQ (2012) Magnetic solid-phase extraction and ultrafast liquid chromatographic detection of Sudan dyes in red wines, juices, and mature vinegars. J Sep Sci 35:3403–3411
Yu W, Liu ZL, Li Q, Zhang HQ, Yu Y (2015) Determination of Sudan I-IV in candy using ionic liquid/anionic surfactant aqueous two-phase extraction coupled with high-performance liquid chromatography. Food Chem 173:815–820
Zeeb M, Mirza B, Zare-Dorabei R, Farahani H (2014) Ionic liquid-based ultrasound-assisted in situ solvent formation microextraction combined with electrothermal atomic absorption spectrometry as a practical method for preconcentration and trace determination of vanadium in water and food samples. Food Anal Methods 7:1783–1790
Zhao S, Yin J, Zhang J, Ding XJ, Wu YN, Shao B (2012) Determination of 23 dyes in chili powder and paste by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Food Anal Methods 5:1018–1026
Zhou QX, Zhao KF, Xing A (2014) Dispersive liquid-liquid microextraction combined with high-performance liquid chromatography for the enrichment and sensitive determination of Sudan Red pollutants in water samples. J Sep Sci 37:3347–3353
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.20905030) and the China Postdoctoral Science Foundation (No. 20090461039).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with human participants performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Hu, M., Wu, L., Song, Y. et al. Determination of Sudan Dyes in Juice Samples via Solidification of Ionic Liquid in Microwave-Assisted Liquid-Liquid Microextraction Followed by High-Performance Liquid Chromatography. Food Anal. Methods 9, 2124–2132 (2016). https://doi.org/10.1007/s12161-015-0389-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0389-y