Abstract
A novel ionic liquid-modified organic-polymer monolithic capillary column was prepared and used for in-tube solid-phase microextraction (SPME) of acidic food additives. The primary amino group of 1-aminopropyl-3-methylimidazolium chloride was reacted with the epoxide group of glycidyl methacrylate. The as-prepared new monomer was then copolymerized in situ with acrylamide and N,N’-methylenebisacrylamide in the presence of polyethylene glycol (PEG)-8000 and PEG-10,000 as porogens. The extraction performance of the developed monolithic sorbent was evaluated for benzoic acid, 3-hydroxybenzoic acid, cinnamic acid, 2,4-dichlorophenoxyacetic acid, and 3-(trifluoromethyl)-cinnamic acid. Such a sorbent, bearing hydrophobic and anion-exchange groups, had high extraction efficiency towards the test compounds. The adsorption capacities for the analytes dissolved in water ranged from 0.18 to 1.74 μg cm−1. Good linear calibration curves (R 2 > 0.99) were obtained, and the limits of detection (S/N = 3) for the analytes were found to be in the range 1.2–13.5 ng mL−1. The recoveries of five acidic food additives spiked in Coca-Cola beverage samples ranged from 85.4 % to 98.3 %, with RSD less than 6.9 %. The excellent applicability of the ionic liquid (IL)-modified monolithic column was further tested by the determination of benzoic acid content in Sprite samples, further illustrating its good potential for analyzing food additives in complex samples.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antimicrobial food additives, including benzoic acid (BA), 3-hydroxybenzoic acid (HBA), cinnamic acid (CA), 2,4-dichlorophenoxyacetic acid (DPAA), and 3-(trifluoromethyl)-cinnamic acid (TFMCA), have an increasingly important function in the food industry, because they are commonly used to prevent alteration and degradation by microorganisms during storage. However, excessive use of these additives could cause adverse effects in humans, including metabolic acidosis, convulsions, and hyperpnoea [1]. Because the additives are often at low concentration in highly complicated matrices, effective and robust sample preparation, especially quantitative extraction, is indispensable for monitoring the presence and/or the amount of these compounds.
Because of its advantages of high sensitivity, solvent-free extraction, small sample volume, simplicity, and easy automation, solid-phase microextraction (SPME) is becoming recognized as a reliable method of sample pretreatment before traditional analytical processes, especially for analysis in complex sample matrices (e.g. biological, environmental, and food samples) [2, 3]. Selection of an appropriate fiber is crucial to SPME. However, the problems of fragility, limited lifetime, and sample carry-over are challenges of the many applications of fibers. As an alternative to fiber-based SPME, in-tube SPME, in which the stationary phase is coated, packed, or in-situ synthesized (e.g., as a monolith) inside capillaries or tubes [4], is straightforward and can be easily coupled on-line to HPLC. Of the three types of stationary phase, in-situ synthesized monoliths, especially the organic-polymer monoliths, have attracted great interest because of their inherent advantages, including relatively easy preparation, increased loading capacity, diverse surface chemistry, high porosity, and excellent pH stability.
Feng’s group [5] first introduced an organic-polymer monolithic capillary to in-tube SPME, and since then a variety of organic-polymer monoliths have been developed and used for in-tube SPME, including poly(methacrylic acid-co-ethylene dimethacrylate) monolith [6], poly(butyl methacrylate-co-ethylene glycol dimethacrylate) monolith [7], poly(butyl methacrylate-co-ethylene glycol dimethacrylate-co-graphene) monolith [8], poly(trimethyl-2-methacroyloxyethylammonium chloride-co-ethylene glycol dimethacrylate) monolith [9], and poly(acrylamide-vinylpyridine-N,N’-methylene bisacrylamide) monolith [10]. These varieties of polymer monolithic capillary-based in-tube SPME have been used for analysis of 28-epihomobrassinolide [6], polycyclic aromatic hydrocarbons [7], glucocorticoids [8], brominated flame retardants [9], and trace Cd, Tl, and Pb [10]. However, there are relatively few reports on the use of organic-polymer monoliths for the selective extraction of acidic antimicrobial additives directly from food samples [11].
Ionic liquid (IL), a kind of burgeoning green solvent, has particular advantages, including negligible vapor pressure, good thermal stability, tunable viscosity and miscibility with water and organic solvents, and good extractability for a variety of organic compounds and metal ions [12]. Therefore, it has been widely used in many fields of analytical chemistry, including in mobile-phase additives [13, 14] and as the extraction solvent in sample preparation [15]. Because of the combined merits of ionic liquids and a variety of supports, the development and application of ionic liquids immobilized onto different materials has recently aroused much interest. Silica-based IL particles [16], polymer-based IL particles [17, 18], and polymer-based IL coatings [19, 20] have been synthesized and used as sorbents in sample preparation. However, use of ILs as the functional monomers for preparation of the organic-polymer-based monolithic columns has not been reported.
In this study, a novel 1-aminopropyl-3-methylimidazolium chloride-modified poly(glycidyl methacrylate-acrylamide-N,N’-methylenebisacrylamide) monolith was prepared by chemical grafting of IL onto the surface of the organic-polymer monolithic column. As far as we are aware, this is the first report on using a 1-aminopropyl-3-methylimidazolium chloride-modified poly(glycidyl methacrylate-acrylamide-N,N’-methylenebisacrylamide) monolithic capillary column as sorbent for the extraction of antimicrobial food additives. The extraction conditions were thoroughly optimized, and analytical variables evaluated. Then the IL-monolith-based in-tube SPME was successfully used for the extraction of acidic additives in Coca-Cola samples and Sprite samples.
Experimental
Chemicals and materials
1-Aminopropyl-3-methylimidazolium chloride ([APMIm]Cl, 99 %) was purchased from Shanghai Chengjie Chemical Co. Ltd (Shanghai, China). Acrylamide and N,N’-methylenebisacrylamide were obtained from J&K Scientific LTD (Shanghai, China). Azobisisobutyronitrile was purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Acetonitrile (optima grade) was obtained from Fisher Scientific (Fair Lawn, NJ, USA). Ammonium formate, formic acid, and 3-methacryloxypropyltrimethoxysiliane (γ-MAPS) were purchased from Sigma (St. Louis, MO, USA). Polyethylene glycol (PEG, molecular weight 8000 and 10,000), formamide, and glycidyl methacrylate (GMA) were all purchased from Aladdin (Shanghai, China). All test analytes, including HBA, BA, CA, DPAA, and TFMCA, were purchased from Aladdin (Shanghai, China). Standard stock solutions of analytes were prepared in methanol and stored at 4 °C. Working solutions were freshly prepared by diluting the stock solutions with HCl-NH3·H2O solution (pH 6.3). All inorganic reagents were of analytical-reagent grade. Ultrapure water was supplied by a Milli-Q system (Millipore, Molsheim, France). The filter (Acrodisc GHP, 13 mm, 0.2 μm) and total recovery vials (9 μL residual) were purchased from Waters (Milford, MA, USA). The polyimide-coated fused-silica capillaries (250 μm i.d. × 375 μm o.d.) were purchased from Yongnian Optical Fiber Factory (Hebei, China). A six-ports valve was obtained from Knauer (Berlin, Germany).
Instrumentation
The scanning electron micrographic images were obtained using a Hitachi S-4800 scanning electron microscope (Tokyo, Japan). Infrared spectra (4000–400 cm−1) in KBr were recorded on a Bruker MPA Fourier transform infrared (FT-IR) spectrometer (Bremen, Germany). All experiments for quantitative analysis were performed on an Ultimate 3000 high-pressure-gradient LC system equipped with a UV detector, a vacuum degasser, a column oven, and a quaternary pump (Dionex, Sunnyvale, CA, USA). RPLC analysis after in-tube SPME extraction was performed using a Dionex AcclaimTM RSLC 120 C18 (2.1 × 150 mm, 2.2 μm). The centrifuge was purchased from Xiangyi Company (H1650-W, Hunan, China).
Preparation of the IL-modified monolithic column
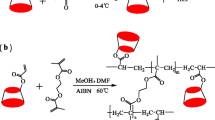
A fused-silica capillary was coated using γ-MAPS according to our previous report [21]. The preparation procedure for the IL-modified monolithic column was as follows (Fig. 1a). In brief, 21.2 mg glycidyl methacrylate and 31.1 mg [APMIm]Cl were dissolved in 699.2 mg formamide. After being mixed for a few minutes, the mixture was adjusted to pH 8.0 using solid NaOH, and then put in a water bath at 40 °C for 1 h. Subsequently, 10.3 mg acrylamide, 20.5 mg N,N’-methylenebisacrylamide, 45.9 mg PEG-8000, and 25.6 mg PEG-10,000 were added into the mixture. After the addition of 1.0 mg azobisisobutyronitrile, the solution was mixed thoroughly and degassed with N2 for 5 min. Then the polymerization solution was injected into a γ-MAPS-coated capillary. The capillary was sealed at both ends with silicone rubber, and then kept at 75 °C for 20 h. Finally, the IL-modified monolithic column was conditioned by rinsing with methanol for 2 h and then water for 1 h.
In-tube SPME procedure
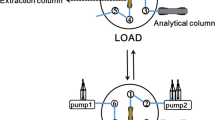
The configuration of the in-tube SPME system used for the study is shown in Fig. 1b. Before extraction, the solution (HCl-NH3 · H2O solution, pH 6.3) was pumped through the capillary for conditioning at 20 μL min−1. Meanwhile, the sample loop was filled with the sample solution by use of a syringe. Then the valve was switched from the LOAD to the INJECT position. After 40 min pumping of the solution (HCl-NH3 · H2O solution, pH 6.3) through the monolithic column at 20 μL min−1, the solution in the syringe pump was changed to elution solvent (10 % formic acid and 40 % ACN). The analytes were desorbed from the monolithic column by the elution solvent at a flow of 10 μL min−1 for 4 min. The pH of the extracted analytes was adjusted to 7.0 using sodium bicarbonate, followed by centrifugation for 10 min at 12,000 rpm before analysis by LC. Coca-Cola samples were adjusted to pH 6.3 by use of aqueous ammonia solution, and then filtered to remove potential particulates before in-tube SPME.
Chromatographic conditions
The mobile phase for target analytes was 10 mmol L−1 ammonium formate and 0.1 % formic acid and ACN. The gradient profile was 15 % ACN initially for 2 min, which was increased to 60 % ACN over 4 min. The mobile phase was then returned to the initial conditions (15 % ACN) over 6 min. UV detection was at 230 nm for all the analytes. The flow was 0.3 mL min−1, and the temperature of the column oven was set at 30 ºC. The injection volume was 10 μL.
Results and discussion
Characterization of the IL-modified monolithic column
Figure 2 shows the scanning electron microscopic image of the internal morphology of the IL-modified monolithic column, which is tightly attached to the inner wall of capillary via the covalent interaction between the polymer and the vinylized surface. The uniform and small sizes of the particles could provide a high surface area for the adsorption of analytes. From the images, the largest pore diameters are estimated to be ~0.5 μm. Abnormally large pores are excluded, presumably because of the shrinkage of monoliths under vacuum.
The FT-IR spectrogram of the IL-modified monolithic column is shown in Fig. 3. A broad band at approximately 3337 cm−1 is attributed to the overlapping of the strong absorption of the N–H stretching vibrations of amine groups and that of the O–H bonds which formed from the epoxide-ring-opening reaction. The band at 1680 cm−1 can be assigned to the C = O stretching vibration. The spectrum of the IL-modified monolithic column has a characteristic peak at 1455 cm−1, which is attributed to vibration of the imidazole ring [22]. These results reveal the successful immobilization of IL on the monolithic materials.
Optimization
To further investigate the performance of IL-modified monolithic material as in-tube SPME sorbent, food additives including HBA (pK a 4.08, log p 1.50), BA (pK a 4.20, log p 1.89), CA (pK a 3.88, log p 2.41), DPAA (pK a 2.98, log p 2.58), and TFMCA (pK a 3.67, log p 3.27) were used as model compounds. To maximize retention and minimize elution of analytes, several conditions affecting the extraction efficiency were optimized and investigated in detail.
The selected analytes were acidic compounds of food additives with pK a 2.98–4.2. The loading step was fixed at pH 6.3 to ensure deprotonation of the acidic analytes and electrostatic capture by the imidazole group. Under this condition, all analytes were adsorbed on the IL-modified monolithic column. To confirm the effect of the imidazole group on the adsorption of acidic compounds, a monolithic column without ionic liquid immobilized was used as in-tube SPME sorbent. The adsorption rates of HBA, BA, CA, DPAA, and TFMCA were 7.2 %, 9.2 %, 14.3 %, 50.1 %, and 31.3 %, respectively. Therefore, the imidazole group has an important function in the adsorption of acidic compounds, further indicating successful immobilization of the ionic liquid onto the monolith.
To elute the most-acidic compounds, the percentage of HCOOH (2 %, 10 %, and 15 %) in the solution was optimized. As shown in Fig. 4a, the percentage of HCOOH significantly affected the recovery of the IL-modified monolithic column for acidic analytes. A significantly improved recovery was achieved with an increase of HCOOH from 2 % (pH 2.11) to 10 % (pH 1.63), with no obvious changes resulting from a further increase to 15 % (pH 1.29). This may be explained by the protonation of the IL-modified monolithic column and analytes. As the percentage of HCOOH increased, the neutral forms of analytes were increased, leading to reduced anion-exchange interaction between the positive charge of the IL-modified monolithic column and the ionized analytes. Therefore, 10 % HCOOH was included in the elution solution in the following experiments.
Because of the hydrophobic action of the imidazole groups [23], the effect of including organic solvent in the elution solution was also examined. Methanol was investigated first, as an organic modifier in an elution solution containing 10 % HCOOH. However, the matrix was co-eluted with analytes, affecting the quantification of HBA. Thus, methanol was not regarded as an optimum organic modifier. Acetonitrile (ACN), another typical RPLC mobile phase component, was also tested as elution solvent. As shown in Fig. 4b, 40 % ACN provided the highest recoveries for all analytes. The recoveries of target compounds increased with increased percentage of ACN until the optimum percentage of 40 % ACN was exceeded, after which the recoveries of five analytes decreased dramatically. Therefore, 40 % ACN was selected as an organic modifier in the subsequent study.
To obtain a high enrichment factor, it is very important to achieve the maximum sample loading volume with satisfactory recoveries for selected analytes. Four different volumes (20, 200, 800, and 1600 μL) of analytes were optimized; each one was spiked with 0.4 μg HBA, 0.4 μg BA, 0.8 μg CA, 0.4 μg DPAA, and 0.8 μg TFMCA. Following the experiment procedure, the recoveries of the analytes at different sample loading volumes were obtained (Fig. 5), and acceptable recoveries were achieved at sample loading volumes of 20–800 μL. However, when the sample loading volume exceeded 1600 μL, the recovery of BA was reduced to 73.9 %. Therefore, an 800 μL sample loading volume was used for the preconcentration of analytes.
Elution volume was also identified as a major condition affecting the preconcentration factor. To this end, elution volume was optimized in the range 40–90 μL. In the investigated range, no obvious change in the recoveries of all analytes was observed (data not shown). Therefore, a 40 μL elution volume was used in this work. When using the optimum sample loading volume and elution volume, an enrichment factor of 20 was obtained.
In in-tube SPME, the flow rates of sample loading and elution not only affect the recovery of analytes, but also control the analysis time. Thus, under the optimum sample-loading conditions, the effect of sample-loading flow rate was investigated by passing 800 μL sample solution through the monolithic column with a syringe pump. The flow rate was adjusted in the range 5.0–30.0 μL min−1. It was found that all the analytes were adsorbed on the IL-modified monolithic column. However, 5.0 μL min−1 was too slow to load the sample, and flow rates higher than 30.0 μL min−1 were unsuitable because of the increase in flow resistance. Thus, a flow rate of 20 μL min−1 was adopted for sample loading.
Elution flow rate was also investigated, in the range 5–20 μL min−1. No obvious change to the recovery of HBA, BA, CA, and DPAA was found in the investigated range (data not shown). However, the recovery of TFMCA was reduced at the flow rate of 20 μL min−1. Therefore, 10 μL min−1 was selected for subsequent extraction.
The above-mentioned optimization used water as the matrix. To assess the effect of a complex matrix on the adsorption capacity, the beverage Coca-Cola was chosen as a complex matrix. Although the Coca-Cola sample did not contain the tested compounds (Fig. 6a), it had a relatively high concentration of salts. Thus, the effect of ionic strength on extraction efficiency was also investigated. In this study, the concentration of Na2SO4 in the sample solution (pH 6.3) was increased from 0 to 250 mmol L−1. As shown in Fig. 7, the recoveries of most tested compounds slightly reduced as the Na2SO4 concentration increased from 0 to 100 mmol L−1, with the lowest recoveries obtained at 100 mmol L−1 Na2SO4. The decreasing recoveries can be attributed to ion-exchange interaction. However, recovery of the compounds gradually increased with the further increase of Na2SO4 concentration (Fig. 7). The salting-out effect may be responsible for this [24]. To simplify sample preparation for the analysis of Coca-Cola samples, the spiked target compounds were directly loaded onto the monolithic column.
Matrix effect
During the method development, the calibration curves were constructed for water or for Coca-Cola samples by plotting the peak area (mAU) against the concentration of the target analytes (ng mL−1) to estimate the matrix effect. The slope of the calibration curve for standards in water was greater than that obtained for Coca-Cola samples, indicating that the signals of target analytes were suppressed in the real-sample matrix. However, the signal suppression can be considered negligible, with values of ~5 % for all compounds tested [25]. To obtain more reliable results, the calibration curves for Coca-Cola samples were used for quantification of real samples.
Adsorption capacity
The adsorption capacity of a monolithic column is an important factor because it determines how long a monolithic column is required to retain selected analytes. To measure the adsorption capacity, a 5 cm-length monolithic column was equilibrated with 100 μL of different concentrations of analytes (0.002–0.104 mg.mL−1 for HBA, BA, and DPAA, and 0.004–0.208 mg mL−1 for CA and TFMCA) dissolved in HCl-NH3.H2O at pH 6.3. The column effluents were collected in tubes and the analytes were detected by LC. It was found that, as loading concentration increased, the adsorbed amounts of all analytes first increased and then reached a plateau under higher concentrations. The adsorption capacity of the IL-modified monolithic column for DPAA, TFMCA, CA, HBA, and BA was calculated to be 1.74, 1.37, 0.41, 0.23, and 0.18 μg cm−1, respectively. To assess the effect of the matrix on the adsorption capacity, the target analytes were dissolved in the Coca-Cola sample (pH 6.3) and the absorption amounts were determined by a similar method. The adsorption capacity of the IL-modified monolithic column for DPAA, TFMCA, CA, HBA, and BA was 0.45, 0.86, 0.37, 0.17, and 0.12 μg cm−1, respectively. These results suggest that the IL-modified monolithic column has a high adsorption capacity for the target analytes even in such complex matrices as Coca-Cola, although slightly lower than that for analytes in water.
Method validation
Under the optimized sample loading and elution conditions for the IL-modified monolithic column, the linearity, limits of detection (LODs) and limits of quantification (LOQs) were determined for all analytes in Coca-Cola samples. The linearity was studied in the concentration range 40–625 ng mL−1 for HBA, CA, and TFMCA and 20–625 ng mL−1 for BA and DPAA. Good correlations, with R 2 ranging from 0.9960 to 0.9999, were achieved (Table 1). LODs and LOQs, determined on the basis of signal-to-noise ratios of 3 and 10, respectively, varied in the range 1.2–13.5 ng mL−1 and 4.0–45 ng mL−1, respectively. In addition, the different concentration levels of target analytes spiked in Coca-Cola samples were evaluated. As listed in Table 2, the mean recoveries of the five analytes were in the range 85.4–98.3 %, with RSDs in the range 1.6–6.9 %. Of note, the LODs, recovery, and repeatability for four acidic food additives are comparable to those obtained from sorbents in previous studies [26–28]. However, the direct analysis of acidic compounds in complex samples using the newly-developed IL-modified monolithic column will establish its unique applicability to multiple additives in other matrices.
Application to real samples
Although Coca-Cola does not contain the test compounds, the spike-ins were successfully used to establish the performance of the IL-modified monolithic column, as discussed above. Sprite, another product which contains a high concentration of BA, was used to further test the applicability of the newly prepared column. The matrix effect of the Sprite sample was evaluated, with values ranging from 4.6–6.6 % for all compounds except BA. It can be concluded that the matrix effect of Coca-Cola and Sprite samples is not a serious problem, and thus the difference resulting from the matrix effect can be ignored. Before analysis, Sprite samples were diluted 200-fold by Coca-Cola samples (pH 6.3), and the calibration curves for Coca-Cola samples were also used for the analysis of Sprite samples. As a result, BA was detectable at concentrations of 91.86 μg mL−1, further illustrating excellent applicability of the IL-modified monolithic column for analyzing food additives in complex samples.
Conclusion
A novel IL-modified organic-polymer monolithic column was synthesized via a two-step copolymerization and used as a sorbent for an in-tube SPME system. The developed material provides a mixed mode of hydrophobicity and anion-exchange capability. The utility of the in-tube SPME sorbent was tested with five acidic food additives (HBA, BA, CA, DPAA, and TFMCA). Under the optimized extraction conditions, excellent extraction capacity, extraction efficiency, and reproducibility were obtained. Moreover, the IL-modified organic-polymer monolithic capillary has revealed robust applicability for the analysis of trace additives in real samples. The online coupling of in-tube SPME with capillary HPLC, which would be very useful for accurate and reliable analysis of chemical additives in multiple complex samples, is in progress in our lab.
References
Tfouni SAV, Toledo MCF (2002) Food Control 13:117–123
Spietelun A, Marcinkowski L, de la Guardia M, Namieśnik J (2013) J Chromatogr A 1321:1–13
Zheng MM, Lin B, Feng YQ (2007) J Chromatogr A 1164:48–55
Zhang W, Chen Z (2013) J Chromatogr A 1278:29–36
Zhang M, Wei F, Zhang YF, Nie J, Feng YQ (2006) J Chromatogr A 1102:294–301
Wang X, Ma Q, Li M, Chang C, Bai Y, Feng Y, Liu H (2013) J Chromatogr A 1317:121–128
Liu W, Qi J, Yan L, Jia Q, Yu C (2011) J Chromatogr B 879:3012–3016
Tong S, Liu Q, Li Y, Zhou W, Jia Q, Duan T (2012) J Chromatogr A 1253:22–31
Yang T, Zhou L, Qiao J, Lian H, Ge X, Chen H (2013) J Chromatogr A 1291:1–9
Yin J, Hu B, He M, Zheng MM, Feng YQ (2009) J Anal At Spectrom 24:76–82
Wen Y, Wang Y, Feng YQ (2007) Anal Bioanal Chem 388:1779–1787
Han D, Row KH (2010) Molecules 15:2405–2426
Tian M, Liu J, Row KH (2009) Molecules 14:2127–2134
Xiao XH, Zhao L, Liu X, Jiang SX (2004) Anal Chim Acta 519:207–211
Zhou Q, Zhang X, Xiao J (2009) J Chromatogr A 1216:4361–4365
Vidal L, Parshintsev J, Hartonen K, Canals A, Riekkola M (2012) J Chromatogr A 1226:2–10
Fontanals N, Ronka S, Borrull F, Trochimczuk AW, Marcé RM (2009) Talanta 80:250–256
Yan H, Sun N, Han Y, Yang C, Wang M, Wu R (2013) J Chromatogr A 1307:21–26
Meng Y, Pino V, Anderson JL (2011) Anal Chim Acta 687:141–149
Zhao F, Meng Y, Anderson JL (2008) J Chromatogr A 1208:1–9
Wang TT, Ma JF, Zhu GJ, Shan YC, Liang Z, Zhang LH, Zhang YK (2010) J Sep Sci 33:3194–31200
Guo L, Deng Q, Fang G, Gao W, Shuo W (2011) J Chromatogr A 1218:6271–6277
Tian M, Bi W, Row KH (2009) J Sep Sci 32:4033–4039
Zheng MM, Ruan GD, Feng YQ (2009) J Chromatogr A 1216:7739–7746
Su R, Wang X, Xu X, Wang Z, Li D, Zhao X, Li X, Zhang H, Yu A (2011) J Chromatogr A 1218:5047–5054
Wang TT, Chen YH, Ma JF, Chen ML, Nie CG, Hu MJ, Li Y, Jia ZJ, Fang JH, Gao HQ (2013) J Chromatogr A 1308:63–72
Lin F, Nong S, Huang X, Yuan D (2013) Anal Bioanal Chem 405:2077–2081
Aprea C, Sciarra G, Bozzi N (1997) J Anal Toxicol 21:262–267
Acknowledgements
The Project is supported by Zhejiang Provincial Natural Science Foundation of China (LQ12B05001), Ningbo Natural Science Foundation of China (2012A610091), and Ningbo Science and Technology Innovation Team (2011B82002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, TT., Chen, YH., Ma, JF. et al. A novel ionic liquid-modified organic-polymer monolith as the sorbent for in-tube solid-phase microextraction of acidic food additives. Anal Bioanal Chem 406, 4955–4963 (2014). https://doi.org/10.1007/s00216-014-7923-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-7923-4