Abstract
A novel method for the extraction of Sudan dyes including Sudan I, II, III, and IV from environmental water by magnetic titanium dioxide nanoparticles (Fe3O4@TiO2) coated with sodium dodecylsulfate (SDS) as adsorbent was reported. Fe3O4@TiO2 was synthesized by a simple method and was characterized by transmission electron microscopy, Fourier-transform infrared spectrometry, and vibrating sample magnetometer. The magnetic separation was quite efficient for the adsorption and desorption of Sudan dyes. The effect of the amount of SDS, extraction time, pH, desorption condition, maximal extraction volume, and humic acid on the extraction process were investigated. This method was employed to analyze three environmental water samples. The results demonstrated that our proposed method had wide linear range (25–5,000 ng L−1) with a good linearity (R 2 > 0.999) and low detection limits (2.9–7.3 ng L−1). An enrichment factor of 1,000 was achieved. In all three spiked levels (25, 250, and 2,500 ng L−1), the recoveries of Sudan dyes were in the range of 86.9–93.6 %. The relative standard deviations obtained were ranging from 2.5 to 9.3 %. That is to say, the new method was fast and effective for the extraction of Sudan dye from environmental water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The colored dye effluents are considered to be highly toxic to the aquatic biota, affect the symbiotic process, and disturb the natural equilibrium by reducing photosynthetic activity and primary production due to the colorized water. Effluents contain significant level of organic contaminants, which are toxic as they create odor, bad taste, unsightly color, foaming, etc. (Ravikumar et al. 2007). Sudan dyes, a group of synthetic colorants, belong to the family of lipophilic azo dyes and are traditionally used in waxes, printing inks, polishes, plastics, oils, etc. (Yu et al. 2012). International Agency for Research on Cancer (IARC) has classified these Sudan dyes as category 3 carcinogen because the dyes and their metabolites may cause human diseases, such as bladder and liver cancer (Noguerol-Cal et al. 2008). Thus Sudan dyes are banned in most countries as additives in food products destined for human consumption at any level. Owing to their low cost and the enhancement of products appearance, Sudan dyes are still used in specific food stuffs illegally, which is a severe danger for public health (Jiang et al. 2012).
The method of extraction, separation, and removal of Sudan dyes in food samples are reported commonly, including solid phase extraction (SPE) (Qi et al. 2011; Yan et al. 2012; Qiao et al. 2011; Hu et al. 2012; Puoci et al. 2005), ionic liquids extraction (Zhao et al. 2010), supercritical fluid extraction (Sun et al. 2009), liquid–liquid extraction (López-Jiménez et al. 2005), cloud point extraction (Ghaedi et al. 2009), followed by high-performance liquid chromatography (HPLC) (Puoci et al. 2005; Zhao et al. 2010; Sun et al. 2009; López-Jiménez et al. 2005; Ghaedi et al. 2009). Despite all this, there are very few research about Sudan dyes in environment water. Zhang et al. synthesized a molecularly imprinted polymer for simultaneous extraction and determination of Sudan dyes by online SPE (Zhao et al. 2010). In the above method, a SPE column packed with molecularly imprinted polymers was prepared and placed in a six-port valve loop, the online system of which was very complex. Thus, a fast and simple method to extract and determine Sudan dyes should be developed.
It was proposed that SPE based on mixed hemimicelles (hemimicelles and admicelles) was used for the preconcentration of a variety of organic pollutants from complex environmental matrices (Sun et al. 2009; López-Jiménez et al. 2005; Ghaedi et al. 2009; Afkhami et al. 2010; Zargar et al. 2009). In the method, the sorbents used are produced by adsorbing ionic surfactants onto the surface of metal oxides. During adsorption of ionic surfactants on metal oxides surface, electrostatic interactions between ionic head groups and the surface result in neutralization of surface charge at low concentration of surfactants (Parida et al. 2006). However, due to the limit of the loading flow rate and time cost, a new SPE mode was developed, namely, magnetic solid-phase extraction (MSPE) (Zhang et al. 2009; Ji et al. 2009; Kim et al. 2011).
In this work, the aim of study was to develop a novel and rapid method for determination of trace level of Sudan dyes in large volume water samples. This method was based on magnetic titanium dioxide (Fe3O4@TiO2) modified with sodium dodecylsulfate (SDS) extraction followed by HPLC analysis. Transmission electron microscopy (TEM), Fourier-transform infrared spectrometry (FTIR), and vibrating sample magnetometer (VSM) were applied to the characterization of Fe3O4@TiO2. The optimization of pretreatment procedure was conducted to obtain satisfactory recoveries of Sudan dyes including Sudan I, II, III, and IV.
Materials and methods
Chemicals and water samples
The standards of Sudan I, II, III, IV, iron (II) chloride tetrahydrate (FeCl2 · 4H2O), iron (III) chloride hexahydrate (FeCl3 · 6H2O), and humic acid were purchased from Aladdin (Shanghai, China). Chromatographic grade methanol was obtained from Fisher (Pittsburgh, PA, USA). Ammonium hydroxide (NH3 · H2O, 25 %), acetic acid (CH3COOH), and hydrochloric acid (HCl) were purchased from Guangfu (Tianjin, China). SDS, butyl titanate (TBT), methanol, and ethanol were obtained from Kermel (Tianjin, China). High purity water was obtained from a Milli-Q Water System (Millipore, Billerica, MA, USA).
Appropriate amount of Sudan dyes were dissolved in methanol to prepare the mixed stock solution (50 μg mL−1), which was stored in dark condition at 4 °C. The working solutions were prepared daily by diluting the stock solution.
Three river water samples were collected from Harbin (China). All water samples were stored at 4 °C. The spiked water samples were made by adding certain amounts of mixed stock solution of Sudan to the real water samples.
Preparation of Fe3O4@TiO2
The Fe3O4 nanoparticles (NPs) were prepared firstly by a co-precipitation method. Briefly, 0.01 mol FeCl2 · 4H2O and 0.02 mol FeCl3 · 6H2O were dissolved in water (100 mL). Then, 10 mL ammonium hydroxide was added at 80 °C, and the mixture was stirred for 1 h at 500 rpm under N2 stream. After synthesis, the precipitation was separated by Nd-Fe-B permanent magnet and washed with purity water to pH 7.0 and then washed by ethanol repeatedly.
Magnetic Fe3O4 (1.0 g) was dispersed into 100 mL ethanol, which was placed in 30 °C water bath. Afterward, 0.4 mL of ammonium hydroxide was added with stirring. Then, TBT (1 mL) was added rapidly with vigorous stir. After TBT dissolving, the system was stirred at 400 rpm for 12 h. Then, the precipitation was washed by water for several times and separated by Nd-Fe-B permanent magnet. Finally, the solid was dried at 60 °C for 12 h and annealed at 180 °C for 3 h.
Characterization of Fe3O4@TiO2
Fe3O4@TiO2 was characterized by TEM (H7650, Hitachi, Japan), FTIR (Nicolet 360, Nicolet, Madison, WI, USA). The magnetic property of the prepared Fe3O4@TiO2 was investigated with VSM (Quantum Design Instrument, San Diego, CA, USA).
Batch extraction experiment
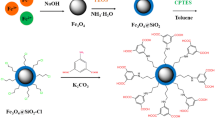
First, the water sample was adjusted to pH 2.0 by HCl solution (1 mol L−1), and then, Fe3O4@TiO2 (100 mg) and SDS (80 mg) were added into a 500 mL water sample. Subsequently, the solution was stirred for 20 min at ambient temperature. Afterward, the solid was isolated with Nd-Fe-B permanent magnet at the bottom of the beaker, and the supernatant was poured away. Then, the adsorbent was eluted by 3 × 1 mL acetonitrile assisted with sonication for 10 s each time. The eluate was combined and evaporated to near dryness under nitrogen stream and was dissolved by chromatographic mobile phase, methanol (0.5 mL). The eluate was filtered through 0.22 μm nylon membranes and injected into the HPLC system for analysis. Illustration of the whole procedure of the preparation of SDS-coated Fe3O4@TiO2 and its application as MSPE sorbent for enriching Sudan dyes can be followed in Fig. 1.
Optimization of extraction conditions were conducted by analyzing spiked river water samples. All batch experiments were performed under stirring rate of 500 rpm which led to disperse Fe3O4@TiO2 in water. Other parameters affecting the performance of the extraction, such as the amount of SDS, extraction time, pH, desorption condition, maximal extraction volume, and humic acid were investigated. When one parameter was changed, the other parameters were fixed at their optimal values. All the extraction conditions were optimized by correlating with the amount of Sudan dyes adsorbed.
Chromatographic conditions
Chromatographic analysis of Sudan dyes was performed on a LC-15C HPLC with UV detector (Shimadzu, Kyoto, Japan). A Zorbax SB-C18 column (150 mm × 4.6 mm I.D., 5 μm) (Palo Alto, CA, USA) was used as an analytical column. The column temperature was kept at ambient temperature, and the injection volume was 20 μL. Chromatographic mobile phase was methanol. The detection wavelength was 505 nm, and the flow rate of mobile phase was 1.0 mL min−1. The chromatograms of Sudan I, II, III, and IV in blank and spiked water sample were shown in Fig. 2.
Results and discussion
Characterization of Fe3O4@TiO2 composites
The microstructures of Fe3O4 particles and Fe3O4@TiO2 composites were characterized by TEM (Fig. 3). It is clear that Fe3O4 and Fe3O4@TiO2 are spherical particle. Figure 4 exhibits the FTIR spectra of Fe3O4 and Fe3O4@TiO2 NPs. The absorption peak at 584 cm−1 is assigned to Fe–O–Fe vibration of magnetite (Jiang et al. 2012). Moreover, the shoulder-like band at 640 cm−1 was attributed to the Ti–O stretching modes of the anatase TiO2 (Fan et al. 2012). The absorption peak at 1,637 and 1,639 cm−1 can be assigned to the absorbed water (Ojamäe et al. 2006), and the peak at 3,423 and 3,428 cm−1 was also attributed to OH group. The smooth curve of hysteresis loop of Fe3O4 and Fe3O4@TiO2 at room temperature was shown in Fig. 5. As can be seen, the saturation magnetization of the synthesized Fe3O4 and Fe3O4@TiO2 was 51.2 and 29.1 emu g−1, respectively, which illustrates that the synthesized Fe3O4@TiO2 could be separated with additional magnetic field efficiently.
Optimization of extraction conditions
Effect of the amount of surfactant
The outer surface of hemimicelles is hydrophobic whereas that of admicelles is ionic, which provides different mechanisms for retention of organic compounds and are both suitable for the MSPE method. In the work, different amount of SDS (0–100 mg) for 100 mg Fe3O4@TiO2 was added into water samples (500 mL). In the absence of SDS, the proportion of Sudan dyes adsorbed was lower with recoveries in the range of 15.6–19.8 %. As can be seen in Fig. 6a, with the augmenting amount of surfactant, the recoveries of Sudan dyes increased gradually; however, the adsorption decreased when surfactant amount was above 80 mg. After the mixed micelles on the surface of Fe3O4@TiO2 reached saturation, if more surfactant was used, the concentration of micelles in aqueous solution increased which resulted in partition of analytes into aqueous phase (López-Jiménez et al. 2005). Finally, 80 mg of SDS was selected for further experiments.
Effect of extraction time
The extraction time has an obvious effect on the recoveries of target analytes. In this experiment, we discussed extraction time ranging from 5 to 40 min. As shown in Fig. 6b, the recoveries of four Sudan dyes increased from 49.2–67.0 to 87.4–96.4 % with the extraction time increasing from 5 to 20 min. MSPE process of Sudan dyes from water samples should reach equilibrium to obtain satisfactory recoveries (Jiang et al. 2012). When the extraction time was more than 20 min, the recoveries of Sudan dyes had no significant change. The optimized recoveries of Sudan dyes are obtained when the extraction time was 20 min, which is greatly short compared with the traditional time-consuming column passing SPE.
Effect of pH
The charge density of mineral oxide surface is a main factor affecting the adsolubilization of analytes, and it varies strongly with pH. When pH of the solution is below its point of zero charge (PZC), the surface of Fe3O4@TiO2 is positively charged; oppositely, its surface is negatively charged. It was reported that the PZC of TiO2 was about 5.8 (Kosmulski 2002). In other words, SDS, a kind of anionic surfactant, can have a combination on the surface of TiO2 in neutralization when pH of the solution was below 5.8. In the present study, the effect of pH was studied by varying the pH in the range of 1–5. As can be seen from Fig. 6c, SDS-coated Fe3O4@TiO2 did not exhibit good adsorption for Sudan dyes when pH is above 3, that is due to the positive charge on the surface of TiO2 was little and the electrostatic attract between TiO2 and SDS was weak. When pH of solution was 1, the adsorbent based on Fe3O4 and TiO2 may be damaged, thus to impair the adsorption and decrease the recovery. The highest recoveries (87.4–96.4 %) of Sudan dyes were obtained at pH 2.
Effect of humic acid
Humic substances are the most abundant dissolved organic compounds present in natural waters. The structure of humic substances is reported to be a crosslinked network of organic chains containing functional groups such as carboxylic, aliphatic, aromatic, hydroxyl, and amino, with empty spaces that can trap and bind other organic and inorganic compounds present in aquatic system (Avena et al. 1999). The normal content of these compounds could vary from 1 to 20 mg L−1 in natural waters (de Wuilloud et al. 2003; Li and Lee 2001). In the experiments, humic acid was spiked into water samples with the concentration of 0, 5, 10, 15, and 20 mg L−1. From Fig. 6d, the existence of humic acid has no significant effect on the recoveries of Sudan dyes. This proved that the adsorbent synthesized in this study had strong anti-interference ability. Therefore, this method can be used for the extraction of Sudan dyes from the complex natural water samples containing humic acid.
Desorption solvents
The desorption of Sudan dyes from hemimicelles-based Fe3O4@TiO2 NPs was studied with different organic solvents, including acetonitrile, methanol, and ethanol. The best recoveries were obtained when acetonitrile was used. Desorption solution containing a small amount of acetic acid was also reported (Chen et al. 2010). Thus, acetonitrile-acetic acid (99.5:0.5, v/v) and acetonitrile-acetic acid (99:1, v/v) also served as desorption solution. Figure 6e shows that acetonitrile has much stronger desorption power compared with other elution solvents. Quantitative recoveries of Sudan dyes ranging from 87.4 to 96.4 % were obtained with 3 × 1 mL acetonitrile assisted with sonication.
Maximal extraction volume
In order to obtain high enrichment factor of this method, the effect of the volume of water samples (300–700 mL) was also investigated. As can be seen in Fig. 6f, quantitative recoveries of Sudan dyes (87.4–96.4 %) were obtained up to 500 mL. When the aqueous solution volume was 600 and 700 mL, the adsorption rate decreased to 81.5–90.0 and 74.5–82.3 %, respectively. Thus, 500 mL was considered to be the maximal enrichment volume for water samples. By enriching 500 mL of aqueous solutions, drying 3 mL of desorption solution and redissolving the analytes in 0.5 mL of chromatographic mobile phase, concentration factor of 1,000 was achieved.
Adsorption kinetics
Kinetics studies which provide valuable insights about the adsorption rates and mechanisms (Salgueiro et al. 2013) were conducted according to experiment results in “Optimization of extraction conditions” section (Fig. 6b). The adsorption kinetics was investigated and the experimental data were fitted with two well-known kinetic models, the pseudo-first order and pseudo-second order kinetic equations, given by Eqs. (1) and (2), respectively (Shen et al. 2009; Chen et al. 2009; Chowdhury et al. 2011; Fasfous et al. 2010),
where Qt (μg g−1) represents the amount adsorbed of Sudan dyes at time t, Q eq (μg g−1) at equilibrium, Q 1cal (μg g−1), and Q 2cal (μg g−1) are the theoretic absorption capacity of the first-order and second-order kinetic model, respectively, k 1 (min−1) and k 2 (g μg−1 min−1) are the rate constant of pseudo-first order and pseudo-second order which can be calculated from the plot of ln (Q eq − Q t) versus t and t/Q t versus t, respectively. The analysis results including the regression equation, correlation coefficients (R 2), and the calculated value of Q 1cal and Q 2cal fitted by pseudo-first order and pseudo-second order kinetic equations were shown in Table 1. Through the calculation, R 2 obtained in pseudo-second order kinetic model was ranging from 0.9993–0.9996, and Q 2cal was more close to Q e. Therefore, the adsorption data are well represented by pseudo-second order kinetic model.
Analytical performance
Quantitative parameters such as linear range, correlation coefficient, limit of detection (LOD), and limit of quantification (LOQ) were evaluated. LOD and LOQ of Sudan dyes were estimated as the minimum concentration producing a signal-to-noise ratio of 3 and 10, respectively. The linearity was in a range of 25–5,000 ng L−1 with a good linearity (R 2 > 0.999). The LODs obtained were 7.3, 3.1, 2.9, and 6.8 ng L−1 for Sudan I, II, III, and IV, respectively. The LOQs obtained were 24.7, 10.4, 9.8, and 22.8 ng L−1 for Sudan I, II, III, and IV, respectively.
In order to evaluate precision, relative standard deviation (RSD) of intra- and inter-day tests were measured by analyzing six replicates of quality control samples at three different spiked concentrations of 25, 250, and 2,500 ng L−1. The intra-day precision was evaluated by assaying one real water sample six times in 1 day. The inter-day precision was evaluated by assaying this sample once a day on six consecutive days. RSDs of intra- and inter-day ranging from 2.5 to 6.9 % and from 3.9 to 9.3 % were obtained, respectively. In all three spiked levels, recoveries of Sudan dyes were in the range of 86.9–93.6 % (Table 2).
Environmental water sample analysis
In this work, the method was applied to three environmental water samples obtained at random from different rivers in Harbin, China. No Sudan dyes at detectable levels were found in these water samples. The recovery study was carried out by spiking environmental water samples with Sudan dyes at the level of 25 ng L−1. In all three fortified levels, recoveries of Sudan dyes are in the range of 80.9–85.6 %.
The analytical results obtained by this method were compared with those of previous literatures for analyzing Sudan dyes in environmental water samples (Jiang et al. 2012; Zhao et al. 2010; Wang et al. 2013). As can be seen from Table 3, the extraction time, concentration factor, LOD, recovery, and precision obtained in this work were comparable with other methods. Moreover, the synthetic method of Fe3O4@TiO2 in this work was simple and time-saving; in addition, the dosage used (20 mg per 100-mL sample) was lower than that of other studies. It indicates that Fe3O4@TiO2 modified by SDS hemimicelles system used in our work has high adsorption ability for Sudan dyes.
Conclusion
In this study, Fe3O4@TiO2 nanoparticles were modified with SDS and then were applied for convenient, fast, and efficient enrichment of Sudan dyes from environmental water samples. The new adsorbents were prepared easily with inexpensive materials and utilized conveniently. The magnetic materials can be reused for five times under the premise of satisfactory recoveries of Sudan dyes. Thus, the analysis cost was decreased. A high enrichment factor of 1,000 can be obtained in this method to extract 500 mL of water samples. The analytes were then determined by HPLC. The low LOD and satisfactory recoveries of Sudan dyes were obtained. Moreover, this method avoided the time-consuming elution from a column or filtration potential in the separation, purification, and concentration of analytes in large volume of environmental water samples. It can be considered that this method is promising and may be a good alternative to be applied in the extraction of other trace organic pollutants in environmental water.
References
Afkhami A, Moosavi R, Madrakian T (2010) Preconcentration and spectrophotometric determination of low concentrations of malachite green and leuco-malachite green in water samples by high performance solid phase extraction using maghemite nanoparticles. Talanta 82:785–789
Avena MJ, Vermeer AWP, Koopal LK (1999) Volume and structure of humic acids studied by viscometry pH and electrolyte concentration effects. Colloids Surf A 151:213–224
Chen GC, Shan XQ, Zhou YQ, Shen XE, Huang HL, Khan SU (2009) Adsorption kinetics, isotherms and thermodynamics of atrazine on surface oxidized multiwalled carbon nanotubes. J Hazard Mater 169:912–918
Chen L, Zhang X, Xu Y, Du X, Sun X, Sun L, Wang H, Zhao Q, Yu A, Zhang H, Ding L (2010) Determination of fluoroquinolone antibiotics in environmental water samples based on magnetic molecularly imprinted polymer extraction followed by liquid chromatography–tandem mass spectrometry. Anal Chim Acta 662:31–38
Chowdhury S, Mishra R, Saha P (2011) Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 265:159–168
de Wuilloud JCA, Wuilloud RG, Sadi BBM, Caruso JA (2003) Trace humic and fulvic acid determination in natural water by cloud point extraction/preconcentration using non-ionic and cationic surfactants with FI-UV detection. Analyst 128:453–458
Fan Y, Ma C, Li W, Yin Y (2012) Synthesis and properties of Fe3O4/SiO2/TiO2 nanocomposites by hydrothermal synthetic method. Mat Sci Semicon Proc 15:582–585
Fasfous II, Radwan ES, Dawoud JN (2010) Kinetics, equilibrium and thermodynamics of the sorption of tetrabromobisphenol A on multiwalled carbon nanotubes. Appl Surf Sci 256:7246–7252
Ghaedi M, Tavallali H, Shokrollahi A, Zahedi M, Montazerozohori M, Soylak M (2009) Flame atomic absorption spectrometric determination of zinc, nickel, iron and lead in different matrixes after solid phase extraction on sodium dodecyl sulfate (SDS)-coated alumina as their bis (2-hydroxyacetophenone)-1,3-propanediimine chelates. J Hazard Mater 166:1441–1448
Hu X, Cai Q, Fan Y, Ye T, Cao Y, Guo C (2012) Molecularly imprinted polymer coated solid-phase microextraction fibers for determination of Sudan I–IV dyes in hot chili powder and poultry feed samples. J Chromatogr A 1219:39–46
Ji Y, Liu X, Guan M, Zhao C, Huang H, Zhang H, Wang C (2009) Preparation of functionalized magnetic nanoparticulate sorbents for rapid extraction of biphenolic pollutants from environmental samples. J Sep Sci 32:2139–2145
Jiang C, Sun Y, Yu X, Zhang L, Sun X, Gao Y, Zhang H, Song D (2012) Removal of sudan dyes from water with C18-functional ultrafine magnetic silica nanoparticles. Talanta 89:38–46
Kim B, Lee J, Um W, Kim J, Joo J, Lee J, Kwak J, Kim J, Lee C, Lee H, Addleman RS, Hyeon T, Gu M, Kim J (2011) Magnetic mesoporous materials for removal of environmental wastes. J Hazard Mater 192:1140–1147
Kosmulski M (2002) The significance of the difference in the point of zero charge between rutile and anatase. Adv Colloid Interface 99:255–264
Li N, Lee HK (2001) Solid-phase extraction of polycyclic aromatic hydrocarbons in surface water Negative effect of humic acid. J Chromatogr A 921:255–263
López-Jiménez F, Rubio S, Pérez-Bendito D (2005) Determination of phthalate esters in sewage by hemimicelles-based solid-phase extraction and liquid chromatography–mass spectrometry. Anal Chim Acta 551:142–149
Noguerol-Cal R, López-Vilariño JM, Fernández-Martínez G, Barral-Losada L, González-Rodríguez MV (2008) High-performance liquid chromatography analysis of ten dyes for control of safety of commercial articles. J Chromatogr A 1179:152–160
Ojamäe L, Aulin C, Pedersen H, Käll P (2006) IR and quantum-chemical studies of carboxylic acid and glycine adsorption on rutile TiO2 nanoparticles. J Colloids Interface 296:71–78
Parida SK, Dash S, Patel S, Mishra BK (2006) Adsorption of organic molecules on silica surface. Adv Colloids Interface 121:77–110
Puoci F, Garreffa C, Iemma F, Muzzalupo R, Spizzirri UG, Picci N (2005) Molecularly imprinted solid phase extraction for detection of sudan I in food matrices. Food Chem 93:349–353
Qi P, Zeng T, Wen Z, Liang X, Zhang X (2011) Interference-free simultaneous determination of Sudan dyes in chili foods using solid phase extraction coupled with HPLC–DAD. Food Chem 125:1462–1467
Qiao F, Geng Y, He C, Wu Y, Pan P (2011) Molecularly imprinted microspheres as SPE sorbent for selective extraction of four Sudan dyes in catsup products. J Chromatogr B 879:2891–2896
Ravikumar K, Krishnan S, Ramalingam S, Balu K (2007) Optimization of process variables by the application of response surface methodology for dye removal using a novel adsorbent. Dyes Pigments 72:66–74
Salgueiro AM, Daniel-da-Silva AL, Girão AV, Pinheiro PC, Trindade T (2013) Unusual dye adsorption behavior of κ-carrageenan coated superparamagnetic nanoparticles. Chem Eng J 229:276–284
Shen XE, Shan XQ, Dong DM, Hua XY, Owens G (2009) Kinetics and thermodynamics of sorption of nitroaromatic compounds to as-grown and oxidized multiwalled carbon nanotubes. J Colloids Interf Sci 330:1–8
Sun L, Zhang C, Chen L, Liu J, Jin H, Xu H, Ding L (2009) Preparation of alumina-coated magnetite nanoparticle for extraction of trimethoprim from environmental water samples based on mixed hemimicelles solid-phase extraction. Anal Chim Acta 638:162–168
Wang Y, Sun Y, Wang Y, Jiang C, Yu X, Gao Y, Zhang H, Song D (2013) Determination of Sudan dyes in environmental water by magnetic mesoporous microsphere-based solid phase extraction ultra fast liquid chromatography. Anal Methods 5:1399–1406
Yan H, Qiao J, Pei Y, Long T, Ding W, Xie K (2012) Molecularly imprinted solid-phase extraction coupled to liquid chromatography for determination of Sudan dyes in preserved beancurds. Food Chem 132:649–654
Yu X, Sun Y, Jiang CZ, Gao Y, Wang YP, Zhang HQ, Song DQ (2012) Magnetic solid-phase extraction and ultrafast liquid chromatographic detection of Sudan dyes in red wines, juices, and mature vinegars. J Sep Sci 35:3403–3411
Zargar B, Parham H, Hatamie A (2009) Fast removal and recovery of amaranth by modified iron oxide magnetic nanoparticles. Chemosphere 76:554–557
Zhang L, Zhai Y, Chang X, He Q, Huang X, Hu Z (2009) Determination of trace metals in natural samples by ICP-OES after preconcentration on modified silica gel and on modified silica nanoparticles. Microchim Acta 165:319–327
Zhao C, Zhao T, Liu X, Zhang H (2010) A novel molecularly imprinted polymer for simultaneous extraction and determination of sudan dyes by on-line solid phase extraction and high performance liquid chromatography. J Chromatogr A 1217:6995–7002
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (No. 2572014EB06) and National Natural Science Foundation of China (No. 21205010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Li, C., Chen, L. & You, X. Extraction of Sudan dyes from environmental water by hemimicelles-based magnetic titanium dioxide nanoparticles. Environ Sci Pollut Res 21, 12382–12389 (2014). https://doi.org/10.1007/s11356-014-3153-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3153-8