Abstract
Mitochondrion is the main site of ATP production in animal cells and also orchestrates signaling pathways associated with cell survival and death. Mitochondrial dysfunction has been linked to bioenergetics and redox impairment in human diseases, such as neurodegeneration and cardiovascular disease. Protective agents able to attenuate mitochondrial impairment are of pharmacological interest. Gastrodin (GAS; 4-hydroxybenzyl alcohol 4-O-beta-d-glucoside) is a phenolic glucoside obtained from the Chinese herbal medicine Gastrodia elata Blume and exhibits antioxidant, anti-inflammatory, and antiapoptotic effects in several cell types. GAS is able to cross the blood-brain barrier, reducing the impact of different stressors on the cognition of experimental animals. In the present work, we investigated whether GAS would protect mitochondria of human SH-SY5Y neuroblastoma cells against an exposure to a pro-oxidant agent. The cells were treated with GAS at 25 μM for 30 min before the administration of hydrogen peroxide (H2O2) at 300 μM for an additional 3 or 24 h, depending on the assay. We evaluated both mitochondrial redox state and function parameters and analyzed the mechanism by which GAS protected mitochondria in this experimental model. Silencing of the nuclear factor erythroid 2-related factor 2 (Nrf2) transcription factor suppressed the GAS-induced mitochondrial protection seen here. Moreover, Nrf2 knockdown abrogated the effects of GAS on cell viability, indicating a potential role for Nrf2 in both mitochondrial and cellular protection promoted by GAS. Further research would be necessary to investigate whether GAS would be able to induce similar effects in in vivo experimental models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mitochondria are double-membrane organelles in which the oxidative phosphorylation (OXPHOS) system is the major source of ATP in mammalian cells (Arnold 2012; Jonckheere et al. 2012). The tricarboxylic acid (TCA) cycle occurs in the mitochondrial matrix and generates reducing power (mainly in the form of NADH and FADH2) that is utilized by the respiratory chain, which is located in the inner mitochondrial membrane (Robinson and Srere 1985). The flux of electrons between the respiratory chain complexes I–IV generates an electrochemical gradient across the inner mitochondrial membrane that is utilized by complex V (ATP synthase/ATPase) to produce ATP from ADP and Pi (Flippo and Strack 2017; Letts and Sazanov 2017). Oxygen (O2) is the final acceptor of electrons in the respiratory chain, and inhibition of O2 utilization by mitochondria leads to general cellular impairment causing, for example, cell death (Enríquez 2016). The integrity of mitochondrial membranes is crucial for the maintenance of the OXPHOS function (Letts and Sazanov 2017). Redox impairment in the mitochondria, for example, causes loss of membrane integrity in the organelles and affects OXPHOS directly (de Oliveira et al. 2012; de Oliveira 2015). In this context, mitochondria are a major source of reactive species in virtually any cell type in mammals (Chong et al. 2014; Sies et al. 2017). Mitochondria-related redox disruption and OXPHOS dysfunction have been seen during the intrinsic apoptotic pathway, which is dependent on the release of the mitochondria-located electron transfer cytochrome c (Green et al. 2014). Oxidation of cardiolipin, which is a lipid responsible for the association of cytochrome c in the inner mitochondrial membrane, leads to cytochrome c release from the organelle and consequent activation of the apoptosome complex in the cytosol (Ott et al. 2007). The exposure of mammalian cells to certain toxicants leads to mitochondrial dysfunction, redox impairment, and cell death, as previously reported (Oliveira 2015; de Oliveira 2016a; de Oliveira and Jardim 2016). Moreover, mitochondrial dysfunction has been observed during neurodegeneration and cardiovascular disease (Witte et al. 2010; Peixoto et al. 2012; Tocchi et al. 2015; Anandhan et al. 2017; Erpapazoglou et al. 2017; Kanaan and Harper 2017).
There is evidence showing that natural and synthetic compounds can protect mitochondria against a myriad of toxicants and also during the progression of some types of diseases (de Oliveira et al. 2015a, 2016a, b, c; de Oliveira 2016b; Picard et al. 2016; Jardim et al. 2017). Gastrodin (GAS; 4-hydroxybenzyl alcohol 4-O-bata-d-glucoside; C13H18O7) is a phenolic glucoside found in the Chinese herbal medicine Gastrodia elata Blume (Yang et al. 2007). GAS exerts antioxidant, antiapoptotic, and anti-inflammatory actions in mammalian cells (Peng et al. 2013; Xiao et al. 2016; Chen et al. 2017a). Recently, Jiang et al. (2014) demonstrated that GAS suppressed the mitochondria-related triggering of cell death in the human SH-SY5Y neuroblastoma cells by activating the p38/nuclear factor erythroid 2-related factor 2 (Nrf2) axis. Similar antioxidative and antiapoptotic effects were seen in in vivo experimental models, as shown by Peng et al. (2015). Moreover, Chen et al. (2017b) published that GAS alleviated seizures induced by pentylenetetrazole in C57BL/6 mice. GAS also exhibited antidepressant effects in experimental animals, as demonstrated by Lee et al. (2016) and Chen et al. (2016). As previously reported by Wang et al. (2008), GAS crosses the blood-brain barrier (BBB) and was found in several rat brain regions. Thus, GAS may serve as a potential neuroprotective agent.
Nrf2 is a major modulator of the redox environment in mammals (Lu 2013; Costa et al. 2016; Kim and Keum 2016). Nrf2, after being released from the Nrf2-Kelch-like ECH-associated protein 1 (Keap1) complex in the cytosol, migrates to the cell nucleus and mediates the transcription of genes involved in both antioxidant defense and metabolism of xenobiotics and takes a role in controlling the expression of genes related to mitochondria-associated bioenergetics, by binding to the antioxidant responsive element (ARE) found in these genes (Nguyen et al. 2009). Disruption in the Nrf2-mediated signaling is associated with generalized cell dysfunction, as observed experimentally and in human diseases (Jin et al. 2013; Sachdeva et al. 2014).
Even though efforts have been made aiming to elucidate how GAS would cause cytoprotective effects in several experimental models, it remains to be demonstrated exactly how this phenolic glucoside promotes mitochondrial protection in mammalian cells. Therefore, we investigated here whether and how GAS would prevent mitochondria-related bioenergetics and redox impairment in SH-SY5Y cells exposed to the pro-oxidant agent hydrogen peroxide (H2O2). This reactive species is produced by mitochondria and by other reactions occurring in several cell types and also plays a role during the progression of neurodegeneration, as previously reported (Coombes et al. 2011; Koppenhöfer et al. 2015). Furthermore, we analyzed whether Nrf2 would take a role in the protection elicited by GAS in this experimental model.
Materials and Methods
Materials
The cell culture-related plastic materials were obtained from Corning, Inc. (NY, USA) and Beckton Dickson (NJ, USA). Culture analytical grade chemicals were purchased from Sigma (MO, USA). The other chemicals and assay kits used in the present work were acquired as described.
Cell Culture and Chemical Treatment
The human neuroblastoma SH-SY5Y cell line was acquired from the American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F-12 HAM nutrient medium (1:1 mixture; supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 1000 units/mL penicillin, 1000 μg/mL streptomycin, and 2.5 μg/mL amphotericin B) in a 5% CO2-humidified incubator at 37 °C, as previously described (de Oliveira et al. 2015b, 2016d).
Cytotoxicity and mitochondrial dysfunction were induced by exposing the SH-SY5Y cells to H2O2 at 300 μM for 24 h, as previously described by our research group (de Oliveira et al. 2017a). The cells were treated with gastrodin at 5–25 μM for 30 min before exposure to H2O2 for an additional 3 or 24 h, as described in details according to each experiment.
Cell Viability and Cytotoxicity Analyses
Cell viability was analyzed through the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Mosmann 1983). The release of the lactate dehydrogenase (LDH) enzyme was utilized as an index of cytotoxicity, and we performed this assay based on the protocol of the manufacturer of the kit (CytoTox 96-Non-Radioactive Cytotoxicity Assay, Promega).
Malondialdehyde, Protein Carbonyl, and 8-Oxo-dG Level Measurement
The levels of MDA and protein carbonyl, as well as the amounts of nuclear 8-oxo-dG, were measured by using commercial kits following the instructions of the manufacturer (Abcam, MA, USA), as previously published by us (de Oliveira et al. 2017b).
3-Nitrotyrosine Level Quantification
We evaluated the levels of 3-nitrotyrosine by utilizing a polyclonal antibody to 3-nitrotyrosine (Calbiochem, Germany) in an indirect ELISA assay, as previously described (de Oliveira et al. 2015b, 2016d).
Mitochondrial Isolation
We isolated mitochondria from the human SH-SY5Y neuroblastoma cells by using a previously published protocol (Wang et al. 2014). Briefly, the cells were washed and re-suspended in a buffer containing 250 mM sucrose, 10 mM KCl, 1 mM EGTA, 1 mM EDTA, 1 mM MgCl2, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 1 mM pepstatin A, 10 mg/mL leupeptin, 2 mg/mL aprotinin, and 20 mM HEPES (pH 7.4). After several differential centrifugations, samples containing purified mitochondria were obtained and used in posterior assays.
Submitochondrial Particle Isolation
In order to isolate SMP from SH-SY5Y cells, the mitochondria obtained by using the abovementioned protocol were frozen and thawed (three times), causing rupture of mitochondrial membranes and leakage of mitochondrial matrix-located enzymes, such as Mn-superoxide dismutase. The samples containing SMP were washed (twice) with a buffer containing 140 mM KCl, 20 mM Tris-HCl (pH 7.4), leading to the complete leakage of Mn-superoxide dismutase leakage from the organelles. This enzyme would interfere in the quantification of the radical anion superoxide (O2−•) produced by the organelle. Therefore, we used this protocol to verify the O2−• production by mitochondria and to study the redox-related effects of H2O2 and/or gastrodin in mitochondrial membranes (Poderoso et al. 1996).
Intracellular Reactive Oxygen Species Production Measurement
We investigated the production of intracellular ROS by using the nonpolar compound 2′-7′-dichlorodihydrofluorescein diacetate (DCFH-DA) assay, as reported (LeBel et al. 1992).
O2 −• Production Measurement
The production of O2−• by mitochondria was studied by measuring the auto-oxidation of adrenaline to adrenochrome in a plate reader (Molecular Devices, CA, USA) at 480 nm at 32 °C, as described by others (Poderoso et al. 1996).
Nitric Oxide Production Evaluation
We measured the production of NO• by utilizing an assay kit based on the instructions of the manufacturer (Abcam, MA, USA).
Enzyme Activity Analyses
The activity of the mitochondria-located enzymes aconitase, α-ketoglutarate dehydrogenase (α-KGDH), succinate dehydrogenase (SDH), complex I, and complex V were quantified through the utilization of commercial kits according to the instructions of the manufacturer (Abcam, MA, USA).
ATP Level Measurement
The levels of ATP were measured by utilizing a commercial kit based on the instructions of the manufacturer (Abcam, MA, USA).
MMP Determination
MMP was investigated by using a commercial kit applying the tetraethylbenzimidazolylcarbocyanide iodine (JC-1) according to the instructions of the manufacturer (Abcam, MA, USA).
Nrf2 Silencing
The silencing of the transcription factor Nrf2 was obtained by performing transient transfection of SH-SY5Y cells with siRNA targeting Nrf2 based on the recommendations of the manufacturer (Santa Cruz, CA, USA) and as previously described (Quesada et al. 2011; Jin et al. 2015).
Statistical Analyses
We utilized the GraphPad 5.0 software in order to perform statistical analyses in the herein presented work. Data are demonstrated as the mean ± standard error of the mean (S.E.M.) of three or five independent experiments each done in triplicate; p values were considered significant when p < 0.05. The differences between the experimental groups were checked by one-way ANOVA followed by the post hoc Tukey’s test.
Results
GAS Attenuated Loss of Cell Viability and Cytotoxicity in SH-SY5Y Cells Treated with H2O2
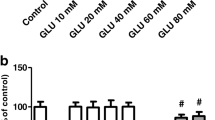
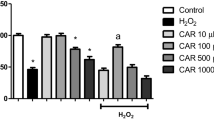
As depicted in Fig. 1, GAS pretreatment for 30 min at 5 or 25 μM reduced the effect of H2O2 on the viability of SH-SY5Y cells (p < 0.05). Thus, we next investigated whether GAS would prevent H2O2-induced cytotoxicity in SH-SY5Y cells. We found that GAS pretreatment at 25 μM abrogated cytotoxicity elicited by H2O2 in this experimental model (Fig. 2).
The effect of GAS on the viability of SH-SY5Y cells challenged with H2O2. The cells were treated with GAS at 1–25 μM during 30 min before exposure to H2O2 at 300 μM for additional 24 h. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from H2O2-treated group
The effect of GAS on the H2O2-induced cytotoxicity in SH-SY5Y cells. The cells were treated with GAS at 25 μM during 30 min before exposure to H2O2 at 300 μM for an additional 24 h. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from the H2O2-treated group
GAS Exerted an Antioxidant Effect in SH-SY5Y Cells Exposed to H2O2
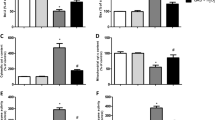
Based on the data demonstrating the cytoprotective effect induced by GAS in SH-SY5Y cells, we examined whether gastrodin would affect redox-related parameters in cells treated with the pro-oxidant agent H2O2. As may be viewed in Fig. 3, GAS pretreatment for 30 min at 25 μM decreased lipid peroxidation (Fig. 3a; p < 0.05), protein carbonylation (Fig. 3b; p < 0.05), protein nitration (Fig. 3c; p < 0.05), and DNA oxidation (Fig. 3d; p < 0.05) in H2O2-treated SH-SY5Y cells.
The effects of GAS on the total levels of lipid peroxidation (a), protein carbonylation (b), 3-nitrotyrosine (c), and 8-oxo-dG (d) in SH-SY5Y cells exposed to H2O2. The cells were treated with GAS at 25 μM during 30 min before exposure to H2O2 at 300 μM for additional 24 h. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from the H2O2-treated group
GAS Promoted Mitochondria-Related Antioxidant Effects in H2O2-Treated SH-SY5Y Cells
We next evaluated whether GAS would be able to protect mitochondria of SH-SY5Y cells exposed to H2O2. GAS pretreatment for 30 min at 25 μM significantly reduced the levels of lipid peroxidation (Fig. 4a; p < 0.05), protein carbonylation (Fig. 4b; p < 0.05), and protein nitration (Fig. 4c; p < 0.05) in the membranes of mitochondria obtained from SH-SY5Y cells.
The effects of GAS on levels of lipid peroxidation (a), protein carbonylation (b), and 3-nitrotyrosine (c) in mitochondrial membranes obtained from cultured SH-SY5Y cells exposed to H2O2. The cells were treated with GAS at 25 μM during 30 min before exposure to H2O2 at 300 μM for an additional 24 h. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from the H2O2-treated group
GAS Alleviated the Production of Reactive Species in SH-SY5Y Cells Treated with H2O2
As demonstrated in Fig. 5a, GAS pretreatment for 30 min at 25 μM alleviated the production of reactive species in SH-SY5Y cells treated with H2O2. Based on this finding, we investigated whether GAS would exert an effect on the production of specific reactive species. GAS reduced the production of O2−• (Fig. 5a; p < 0.05) and NO• (Fig. 5c; p < 0.05) in H2O2-treated SH-SY5Y cells.
The effects of GAS on the production of reactive species (a), O2−• (b), and NO• in cultured SH-SY5Y cells challenged with H2O2. The cells were treated with GAS at 25 μM during 30 min before exposure to H2O2 at 300 μM for an additional 3 h. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from the H2O2-treated group
GAS Prevented H2O2-Induced Mitochondria-Related Bioenergetics Effects in SH-SY5Y Cells
Since GAS induced an antioxidant effect in the mitochondria obtained from SH-SY5Y cells exposed to a pro-oxidant agent, we evaluated whether GAS would promote a benefit regarding the function of mitochondria. As may be observed in Fig. 6, GAS pretreatment for 30 min at 25 μM reduced the H2O2-induced effects on the activity of aconitase (Fig. 6a; p < 0.05), α-KGDH (Fig. 6b; p < 0.05), and SDH (Fig. 6c; p < 0.05).
The effects of GAS on the activity of enzymes of the tricarboxylic acid cycle aconitase (a), α-ketoglutarate dehydrogenase (b), and succinate dehydrogenase (c) in H2O2-treated SH-SY5Y cells. The cells were treated with GAS at 25 μM during 30 min before exposure to H2O2 at 300 μM for additional 24 h. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from the H2O2-treated group
Furthermore, GAS prevented the H2O2-elicited inhibition in the activities of the OXPHOS complexes I (Fig. 7a; p < 0.05) and V (Fig. 7b; p < 0.05) and prevented the reduction in the levels of ATP in SH-SY5Y cells (Fig. 7c; p < 0.05). GAS also blocked the H2O2-induced loss of MMP in this experimental model, as depicted in Fig. 8 (p < 0.05).
The effects of GAS on the activity of the oxidative phosphorylation system complex I (a) and complex V (b) and on the levels of ATP (c). The cells were treated with GAS at 25 μM during 30 min before exposure to H2O2 at 300 μM for an additional 24 h. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from the H2O2-treated group
The effect of GAS on the mitochondrial membrane potential (MMP) in SH-SY5Y cells exposed to H2O2. The cells were treated with GAS at 25 μM during 30 min before exposure to H2O2 at 300 μM for additional 24 h. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the control group; # different from the H2O2-treated group
GAS Exerted Mitochondrial Protection by an Nrf2-Related Mechanism
In order to analyze the mechanism underlying the mitochondria-related benefits elicited by GAS in SH-SY5Y cells, we silenced Nrf2 in these cells by using siRNA targeting Nrf2. We observed that knockdown of Nrf2 attenuated the effects of GAS pretreatment on the activity of the mitochondrial complexes I (Fig. 9a; p < 0.05) and V (Fig. 9; p < 0.05). Moreover, the protective effect elicited by GAS regarding MMP was alleviated by Nrf2 silencing in the cells exposed to H2O2 (Fig. 10; p < 0.05).
The effects of Nrf2 knockdown (for 48 h) on the activity of complex I (a) and complex V (b) in SH-SY5Y cells treated with GAS and/or H2O2. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the cells transfected with scrambled control (NC) siRNA and treated with GAS and H2O2
The effects of Nrf2 knockdown (for 48 h) on the mitochondrial membrane potential (MMP) in SH-SY5Y cells treated with GAS and/or H2O2. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the cells transfected with scrambled control (NC) siRNA and treated with GAS and H2O2
GAS Promoted Cytoprotection by a Mechanism Involving Nrf2
Finally, we evaluated the role of Nrf2 in GAS-treated cells exposed to H2O2. We found that Nrf2 silencing abrogated the cytoprotection induced by a pretreatment (for 30 min) with GAS (at 25 μM) in H2O2-treated SH-SY5Y cells (Fig. 11; p < 0.05).
The effects of Nrf2 knockdown (for 48 h) on the viability of SH-SY5Y cells treated with GAS and/or H2O2. Data are shown as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 different from the cells transfected with scrambled control (NC) siRNA and treated with GAS and H2O2
Discussion
In the presented work, we demonstrate that a pretreatment with GAS attenuated the H2O2-induced mitochondrial dysfunction in SH-SY5Y cells. GAS exerted cytoprotective and antioxidative effects by reducing the levels of markers of lipid peroxidation and protein carbonylation and nitration in the mitochondrial membranes obtained from cultured SH-SY5Y cells. GAS also attenuated the production of reactive species in H2O2-treated cells. Moreover, GAS suppressed the effects of H2O2 on the activity of mitochondria-located enzymes involved in the maintenance of TCA and OXPHOS system. GAS caused mitochondrial protection, at least in part, by a mechanism associated with Nrf2, since the silencing of this transcription factor abrogated the GAS-elicited benefits regarding mitochondrial function. This is the first work demonstrating a direct role between Nrf2 and mitochondrial protection (involving redox state and function of the organelle) in GAS-treated cells.
Nrf2, in addition to the modulation of the redox environment, takes a role in the bioenergetics homeostasis in mammalian cells, as indicated by an increasing body of evidence (Kim et al. 2011; Ludtmann et al. 2014). Nrf2 modulates the expression of genes whose products are involved in the consumption of reactive species, such as Mn-SOD and glutathione peroxidase (GPx), and coordinates the expression of enzymes that participate in the metabolism of xenobiotics, such as glutathione-S-transferase (GST) (Ma 2013). Additionally, Nrf2 activation has been linked to anti-inflammatory effects induced by natural compounds, as evidenced in several experimental models (Ahmed et al. 2017). Nrf2 also demonstrated the ability to modulate cell fate by preventing death of cells exposed to some toxicants (de Oliveira et al. 2016e, 2017c). Therefore, there is increasing interest in investigating natural and synthetic agents able to promote Nrf2 activation in mammalian cells.
The maintenance of mitochondrial function is necessary physiologically and specially during exposure of cells to chemical or physical stressors (Broadley and Hartl 2008). During a mitochondria-dependent cell death event, for example, it is important to maintain some mitochondria-producing ATP in order to preserve the function of the apoptosome and, consequently, the activation of the caspases, which execute reactions that lead to the formation of apoptotic bodies (Green et al. 2014). Otherwise, absence of ATP at sufficient levels may block the activation of caspases and cell death would occur by necrosis, causing inflammation (Elmore 2007). Thus, mitochondria play a pivotal role in both triggering and sustaining the intrinsic apoptotic pathway in mammals. Mitochondria exposed to some types of toxicants may present increased levels of protein carbonylation and nitration and augmented lipid peroxidation, in their membranes (de Oliveira and Moreira 2007; de Oliveira et al. 2009, 2011). Redox impairment in the organelles may be associated with morphological alterations and impaired mitochondrial dynamics (Klamt et al. 2005; Ito and Di Polo 2017).
In this context, GAS efficiently prevented H2O2-induced mitochondrial dysfunction regarding redox state and bioenergetics by a mechanism associated with the transcription factor Nrf2. Further research would be necessary in order to evaluate whether GAS would act in a similar way in in vivo experimental models.
References
Ahmed SM, Luo L, Namani A, Wang XJ, Tang X (2017) Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta 1863(2):585–597. https://doi.org/10.1016/j.bbadis.2016.11.005
Anandhan A, Jacome MS, Lei S, Hernandez-Franco P, Pappa A, Panayiotidis MI, Powers R, Franco R (2017) Metabolic dysfunction in Parkinson’s disease: bioenergetics, redox homeostasis and central carbon metabolism. Brain Res Bull 133:12–30. https://doi.org/10.1016/j.brainresbull.2017.03.009
Arnold S (2012) The power of life—cytochrome c oxidase takes center stage in metabolic control, cell signalling and survival. Mitochondrion 12(1):46–56. https://doi.org/10.1016/j.mito.2011.05.003
Broadley SA, Hartl FU (2008) Mitochondrial stress signaling: a pathway unfolds. Trends Cell Biol 18(1):1–4. https://doi.org/10.1016/j.tcb.2007.11.003
Chen WC, Lai YS, Lin SH, Lu KH, Lin YE, Panyod S, Ho CT, Sheen LY (2016) Anti-depressant effects of Gastrodia elata Blume and its compounds gastrodin and 4-hydroxybenzyl alcohol, via the monoaminergic system and neuronal cytoskeletal remodeling. J Ethnopharmacol 182:190–199. https://doi.org/10.1016/j.jep.2016.02.001
Chen J, Gu YT, Xie JJ, Wu CC, Xuan J, Guo WJ, Yan YZ, Chen L, Wu YS, Zhang XL, Xiao J, Wang XY (2017a) Gastrodin reduces IL-1β-induced apoptosis, inflammation, and matrix catabolism in osteoarthritis chondrocytes and attenuates rat cartilage degeneration in vivo. Biomed Pharmacother 97:642–651. https://doi.org/10.1016/j.biopha.2017.10.067
Chen L, Liu X, Wang H, Qu M (2017b) Gastrodin attenuates pentylenetetrazole-induced seizures by modulating the mitogen-activated protein kinase-associated inflammatory responses in mice. Neurosci Bull 33(3):264–272. https://doi.org/10.1007/s12264-016-0084-z
Chong SJ, Low IC, Pervaiz S (2014) Mitochondrial ROS and involvement of Bcl-2 as a mitochondrial ROS regulator. Mitochondrion 19 Pt A:39–48. https://doi.org/10.1016/j.mito.2014.06.002
Coombes E, Jiang J, Chu XP, Inoue K, Seeds J, Branigan D, Simon RP, Xiong ZG (2011) Pathophysiologically relevant levels of hydrogen peroxide induce glutamate-independent neurodegeneration that involves activation of transient receptor potential melastatin 7 channels. Antioxid Redox Signal 14(10):1815–1827. https://doi.org/10.1089/ars.2010.3549
Costa SL, Silva VD, Dos Santos Souza C, Santos CC, Paris I, Muñoz P, Segura-Aguilar J (2016) Impact of plant-derived flavonoids on neurodegenerative diseases. Neurotox Res 30(1):41–52. https://doi.org/10.1007/s12640-016-9600-1
de Oliveira MR (2015) Vitamin A and retinoids as mitochondrial toxicants. Oxidative Med Cell Longev 2015:140267–140213. https://doi.org/10.1155/2015/140267
de Oliveira MR (2016a) Fluoxetine and the mitochondria: a review of the toxicological aspects. Toxicol Lett 258:185–191. https://doi.org/10.1016/j.toxlet.2016.07.001
de Oliveira MR (2016b) Evidence for genistein as a mitochondriotropic molecule. Mitochondrion 29:35–44. https://doi.org/10.1016/j.mito.2016.05.005
de Oliveira MR, Jardim FR (2016) Cocaine and mitochondria-related signaling in the brain: a mechanistic view and future directions. Neurochem Int 92:58–66. https://doi.org/10.1016/j.neuint.2015.12.006
de Oliveira MR, Moreira JC (2007) Acute and chronic vitamin A supplementation at therapeutic doses induces oxidative stress in submitochondrial particles isolated from cerebral cortex and cerebellum of adult rats. Toxicol Lett 173(3):145–150. https://doi.org/10.1016/j.toxlet.2007.07.002
de Oliveira MR, Soares Oliveira MW, Müller Hoff ML, Behr GA, da Rocha RF, Fonseca Moreira JC (2009) Evaluation of redox and bioenergetics states in the liver of vitamin A-treated rats. Eur J Pharmacol 610(1-3):99–105. https://doi.org/10.1016/j.ejphar.2009.03.046
de Oliveira MR, da Rocha RF, Stertz L, Fries GR, de Oliveira DL, Kapczinski F, Moreira JC (2011) Total and mitochondrial nitrosative stress, decreased brain-derived neurotrophic factor (BDNF) levels and glutamate uptake, and evidence of endoplasmic reticulum stress in the hippocampus of vitamin A-treated rats. Neurochem Res 36(3):506–517. https://doi.org/10.1007/s11064-010-0372-3
de Oliveira MR, da Rocha RF, Pasquali MA, Moreira JC (2012) The effects of vitamin A supplementation for 3 months on adult rat nigrostriatal axis: increased monoamine oxidase enzyme activity, mitochondrial redox dysfunction, increased β-amyloid(1-40) peptide and TNF-α contents, and susceptibility of mitochondria to an in vitro H2O2 challenge. Brain Res Bull 87(4-5):432–444. https://doi.org/10.1016/j.brainresbull.2012.01.005
de Oliveira MR, Nabavi SF, Habtemariam S, Erdogan Orhan I, Daglia M, Nabavi SM (2015a) The effects of baicalein and baicalin on mitochondrial function and dynamics: a review. Pharmacol Res 100:296–308. https://doi.org/10.1016/j.phrs.2015.08.021
de Oliveira MR, Ferreira GC, Schuck PF, Dal Bosco SM (2015b) Role for the PI3K/Akt/Nrf2 signaling pathway in the protective effects of carnosic acid against methylglyoxal-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Chem Biol Interact 242:396–406. https://doi.org/10.1016/j.cbi.2015.11.003
de Oliveira MR, Nabavi SF, Manayi A, Daglia M, Hajheydari Z, Nabavi SM (2016a) Resveratrol and the mitochondria: from triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim Biophys Acta 1860(4):727–745. https://doi.org/10.1016/j.bbagen.2016.01.017
de Oliveira MR, Jardim FR, Setzer WN, Nabavi SM, Nabavi SF (2016b) Curcumin, mitochondrial biogenesis, and mitophagy: exploring recent data and indicating future needs. Biotechnol Adv 34(5):813–826. https://doi.org/10.1016/j.biotechadv.2016.04.004
de Oliveira MR, Nabavi SM, Braidy N, Setzer WN, Ahmed T, Nabavi SF (2016c) Quercetin and the mitochondria: a mechanistic view. Biotechnol Adv 34(5):532–549. https://doi.org/10.1016/j.biotechadv.2015.12.014
de Oliveira MR, Ferreira GC, Schuck PF (2016d) Protective effect of carnosic acid against paraquat-induced redox impairment and mitochondrial dysfunction in SH-SY5Y cells: role for PI3K/Akt/Nrf2 pathway. Toxicol In Vitro 32:41–54. https://doi.org/10.1016/j.tiv.2015.12.005
de Oliveira MR, Peres A, Ferreira GC, Schuck PF, Bosco SM (2016e) Carnosic acid affords mitochondrial protection in chlorpyrifos-treated Sh-Sy5y cells. Neurotox Res 30(3):367–379. https://doi.org/10.1007/s12640-016-9620-x
de Oliveira MR, da Costa Ferreira G, Peres A, Bosco SM (2017a) Carnosic acid suppresses the H2O2-induced mitochondria-related bioenergetics disturbances and redox impairment in SH-SY5Y cells: role for Nrf2. Mol Neurobiol. https://doi.org/10.1007/s12035-016-0372-7
de Oliveira MR, Schuck PF, Bosco SMD (2017b) Tanshinone I induces mitochondrial protection through an Nrf2-dependent mechanism in paraquat-treated human neuroblastoma SH-SY5Y cells. Mol Neurobiol 54(6):4597–4608. https://doi.org/10.1007/s12035-016-0009-x
de Oliveira MR, Brasil FB, Andrade CMB (2017c) Naringenin attenuates H2O2-induced mitochondrial dysfunction by an Nrf2-dependent mechanism in SH-SY5Y cells. Neurochem Res 42(11):3341–3350. https://doi.org/10.1007/s11064-017-2376-8
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516. https://doi.org/10.1080/01926230701320337
Enríquez JA (2016) Supramolecular organization of respiratory complexes. Annu Rev Physiol 78(1):533–561. https://doi.org/10.1146/annurev-physiol-021115-105031
Erpapazoglou Z, Mouton-Liger F, Corti O (2017) From dysfunctional endoplasmic reticulum-mitochondria coupling to neurodegeneration. Neurochem Int 109:171–183. https://doi.org/10.1016/j.neuint.2017.03.021
Flippo KH, Strack S (2017) Mitochondrial dynamics in neuronal injury, development and plasticity. J Cell Sci 130(4):671–681. https://doi.org/10.1242/jcs.171017
Green DR, Galluzzi L, Kroemer G (2014) Metabolic control of cell death. Science 345(6203):1250256. https://doi.org/10.1126/science.1250256
Ito YA, Di Polo A (2017) Mitochondrial dynamics, transport, and quality control: a bottleneck for retinal ganglion cell viability in optic neuropathies. Mitochondrion 36:186–192. https://doi.org/10.1016/j.mito.2017.08.014
Jardim FR, de Rossi FT, Nascimento MX, da Silva Barros RG, Borges PA, Prescilio IC, de Oliveira MR (2017) Resveratrol and brain mitochondria: a review. Mol Neurobiol. https://doi.org/10.1007/s12035-017-0448-z
Jiang G, Hu Y, Liu L, Cai J, Peng C, Li Q (2014) Gastrodin protects against MPP(+)-induced oxidative stress by up regulates heme oxygenase-1 expression through p38 MAPK/Nrf2 pathway in human dopaminergic cells. Neurochem Int 75:79–88. https://doi.org/10.1016/j.neuint.2014.06.003
Jin YN, Yu YV, Gundemir S, Jo C, Cui M, Tieu K, Johnson GV (2013) Impaired mitochondrial dynamics and Nrf2 signaling contribute to compromised responses to oxidative stress in striatal cells expressing full-length mutant huntingtin. PLoS One 8(3):e57932. https://doi.org/10.1371/journal.pone.0057932
Jin X, Liu Q, Jia L, Li M, Wang X (2015) Pinocembrin attenuates 6-OHDA-induced neuronal cell death through Nrf2/ARE pathway in SH-SY5Y cells. Cell Mol Neurobiol 35(3):323–333. https://doi.org/10.1007/s10571-014-0128-8
Jonckheere AI, Smeitink JA, Rodenburg RJ (2012) Mitochondrial ATP synthase: architecture, function and pathology. J Inherit Metab Dis 35(2):211–225. https://doi.org/10.1007/s10545-011-9382-9
Kanaan GN, Harper ME (2017) Cellular redox dysfunction in the development of cardiovascular diseases. Biochim Biophys Acta 1861(11):2822–2829. https://doi.org/10.1016/j.bbagen.2017.07.027
Kim J, Keum YS (2016) NRF2, a key regulator of antioxidants with two faces towards cancer. Oxidative Med Cell Longev 2016:2746457. https://doi.org/10.1155/2016/2746457
Kim TH, Hur EG, Kang SJ, Kim JA, Thapa D, Lee YM, Ku SK, Jung Y, Kwak MK (2011) NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1α. Cancer Res 71:2260–2275. https://doi.org/10.1158/0008-5472.CAN-10-3007
Klamt F, Roberto de Oliveira M, Moreira JC (2005) Retinol induces permeability transition and cytochrome c release from rat liver mitochondria. Biochim Biophys Acta 1726(1):14–20. https://doi.org/10.1016/j.bbagen.2005.07.016
Koppenhöfer D, Kettenbaum F, Susloparova A, Law JK, Vu XT, Schwab T, Schäfer KH, Ingebrandt S (2015) Neurodegeneration through oxidative stress: monitoring hydrogen peroxide induced apoptosis in primary cells from the subventricular zone of BALB/c mice using field-effect transistors. Biosens Bioelectron 67:490–496. https://doi.org/10.1016/j.bios.2014.09.012
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5(2):227–231. https://doi.org/10.1021/tx00026a012
Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm DH (2016) Gastrodin reversed the traumatic stress-induced depressed-like symptoms in rats. J Nat Med 70(4):749–759. https://doi.org/10.1007/s11418-016-1010-4
Letts JA, Sazanov LA (2017) Clarifying the supercomplex: the higher-order organization of the mitochondrial electron transport chain. Nat Struct Mol Biol 24(10):800–808. https://doi.org/10.1038/nsmb.3460
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta 1830(5):3143–3153. https://doi.org/10.1016/j.bbagen.2012.09.008
Ludtmann MH, Angelova PR, Zhang Y, Abramov AY, Dinkova-Kostova AT (2014) Nrf2 affects the efficiency of mitochondrial fatty acid oxidation. Biochem J 457(3):415–424. https://doi.org/10.1042/BJ20130863
Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53(1):401–426. https://doi.org/10.1146/annurev-pharmtox-011112-140320
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1-2):55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Nguyen T, Nioi P, Pickett CB (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284(20):13291–13295. https://doi.org/10.1074/jbc.R900010200
Oliveira MR (2015) The neurotoxic effects of vitamin A and retinoids. An Acad Bras Cienc 87(2 suppl):1361–1373. https://doi.org/10.1590/0001-3765201520140677
Ott M, Zhivotovsky B, Orrenius S (2007) Role of cardiolipin in cytochrome c release from mitochondria. Cell Death Differ 14(7):1243–1247. https://doi.org/10.1038/sj.cdd.4402135
Peixoto PM, Dejean LM, Kinnally KW (2012) The therapeutic potential of mitochondrial channels in cancer, ischemia-reperfusion injury, and neurodegeneration. Mitochondrion 12(1):14–23. https://doi.org/10.1016/j.mito.2011.03.003
Peng Z, Wang H, Zhang R, Chen Y, Xue F, Nie H, Chen Y, Wu D, Wang Y, Wang H, Tan Q (2013) Gastrodin ameliorates anxiety-like behaviors and inhibits IL-1beta level and p38 MAPK phosphorylation of hippocampus in the rat model of posttraumatic stress disorder. Physiol Res 62(5):537–545
Peng Z, Wang S, Chen G, Cai M, Liu R, Deng J, Liu J, Zhang T, Tan Q, Hai C (2015) Gastrodin alleviates cerebral ischemic damage in mice by improving anti-oxidant and anti-inflammation activities and inhibiting apoptosis pathway. Neurochem Res 40(4):661–673. https://doi.org/10.1007/s11064-015-1513-5
Picard M, Wallace DC, Burelle Y (2016) The rise of mitochondria in medicine. Mitochondrion 30:105–116. https://doi.org/10.1016/j.mito.2016.07.003
Poderoso JJ, Carreras MC, Lisdero C, Riobó N, Schöpfer F, Boveris A (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys 328(1):85–92. https://doi.org/10.1006/abbi.1996.0146
Quesada A, Ogi J, Schultz J, Handforth A (2011) C-terminal mechano-growth factor induces heme oxygenase-1-mediated neuroprotection of SH-SY5Y cells via the protein kinase Cϵ/Nrf2 pathway. J Neurosci Res 89(3):394–405. https://doi.org/10.1002/jnr.22543
Robinson JB Jr, Srere PA (1985) Organization of Krebs tricarboxylic acid cycle enzymes in mitochondria. J Biol Chem 260(19):10800–10805
Sachdeva MM, Cano M, Handa JT (2014) Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp Eye Res 119:111–114. https://doi.org/10.1016/j.exer.2013.10.024
Sies H, Berndt C, Jones DP (2017) Oxidative stress. Annu Rev Biochem 86(1):715–748. https://doi.org/10.1146/annurev-biochem-061516-045037
Tocchi A, Quarles EK, Basisty N, Gitari L, Rabinovitch PS (2015) Mitochondrial dysfunction in cardiac aging. Biochim Biophys Acta 1847(11):1424–1433. https://doi.org/10.1016/j.bbabio.2015.07.009
Wang Q, Chen G, Zeng S (2008) Distribution and metabolism of gastrodin in rat brain. J Pharm Biomed Anal 46(2):399–404. https://doi.org/10.1016/j.jpba.2007.10.017
Wang K, Zhu L, Zhu X, Zhang K, Huang B, Zhang J, Zhang Y, Zhu L, Zhou B, Zhou F (2014) Protective effect of paeoniflorin on Aβ25-35-induced SH-SY5Y cell injury by preventing mitochondrial dysfunction. Cell Mol Neurobiol 34(2):227–234. https://doi.org/10.1007/s10571-013-0006-9
Witte ME, Geurts JJ, de Vries HE, van der Valk P, van Horssen J (2010) Mitochondrial dysfunction: a potential link between neuroinflammation and neurodegeneration? Mitochondrion 10(5):411–418. https://doi.org/10.1016/j.mito.2010.05.014
Xiao MM, Zhang YQ, Wang WT, Han WJ, Lin Z, Xie RG, Cao Z, Lu N, Hu SJ, Wu SX, Dong H, Luo C (2016) Gastrodin protects against chronic inflammatory pain by inhibiting spinal synaptic potentiation. Sci Rep 6(1):37251. https://doi.org/10.1038/srep37251
Yang XD, Zhu J, Yang R, Liu JP, Li L, Zhang HB (2007) Phenolic constituents from the rhizomes of Gastrodia elata. Nat Prod Res 21(2):180–186. https://doi.org/10.1080/14786410601081997
Acknowledgments
This work was supported by CNPq. FBB receives financial support from the FOPESQ/UFF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
de Oliveira, M.R., Brasil, F.B. & Fürstenau, C.R. Evaluation of the Mitochondria-Related Redox and Bioenergetics Effects of Gastrodin in SH-SY5Y Cells Exposed to Hydrogen Peroxide. J Mol Neurosci 64, 242–251 (2018). https://doi.org/10.1007/s12031-018-1027-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-018-1027-0