Abstract
Invasion of the cavernous sinus by pituitary adenomas impedes complete surgical resection, compromises biochemical remission, and increases the risk of further tumor recurrence. Accurate preoperative MRI-based diagnosis or intraoperative direct inspection of cavernous sinus invasion are essential for optimal surgical planning and for tailoring postoperative therapeutic strategies, depending on whether a total resection has been achieved, or tumoral tissue has been left in surgically inaccessible locations. The molecular mechanisms underlying the invasive behavior of pituitary adenomas remain poorly understood, hindering the development of targeted therapies. Some studies have identified genes overexpressed in pituitary adenomas invading the cavernous sinus, offering insights into the acquisition of invasive behavior. Their main limitation however lies in comparing purely intrasellar specimens obtained from invasive and non-invasive adenomas. Further, precise anatomical knowledge of the medial wall of the cavernous sinus is crucial for grasping the mechanisms of invasion. Recently, alongside the standard intrasellar surgery, extended endoscopic intracavernous surgical procedures with systematic selective resection of the medial wall of the cavernous sinus have shown promising results for invasive secreting pituitary adenomas. The first- and second-generation somatostatin agonist ligands and cabergoline are used with variable efficacy to control secretory activity and/or growth of intracavernous remnants. Tumor regrowth usually requires surgical reintervention, sometimes combined with radiotherapy or radiosurgery which is applied despite their benign nature. Unraveling the molecular pathways driving invasive behavior of pituitary adenomas and their tropism to the cavernous sinuses is the key for developing efficient innovative treatment modalities that could reduce the need for repeated surgery or radiotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cavernous sinus invasion by pituitary adenomas remains a significant predictor of incomplete surgical resection, failed clinical and biochemical remission, and tumor regrowth [1,2,3,4]. The presence of residual, inaccessible tumor within the cavernous sinus often necessitates multimodal therapeutic approaches, combining medical and radiation-based interventions [5]. Consequently, an accurate preoperative diagnosis of cavernous sinus invasion is crucial before considering any surgical strategy. Intraoperative and histopathological evaluations of cavernous sinus invasion can provide valuable insights for predicting postoperative hormonal control and tumor recurrence. Additionally, these data could potentially be further integrated into the decision-making algorithm for postoperative therapies.
Herein, we aim to summarize the current understanding of the prevalence, pathophysiology, diagnosis, clinical and therapeutic implications of cavernous sinus invasion by pituitary adenomas. Additionally, we explore the research perspectives on promising endoscopic intracavernous surgical procedures and medical therapies targeting intracavernous tumoral remnants. This comprehensive overview seeks to contribute to a deeper insight into the complexities of cavernous sinus invasion by pituitary adenomas and to shed light on evolving tools for its diagnosis and management.
Definition/diagnosis
Preoperative diagnosis
Accurate preoperative diagnosis of invasion is essential for prediction of surgical outcomes. Various radiological classifications have been proposed, with the Knosp and revised-Knosp classifications being the most widely accepted [1, 6, 7]. These classifications rely on preoperative magnetic resonance imaging (MRI) for assessment. Engelbert Knosp introduced a classification system using lines drawn thought the intracavernous portion of the internal carotid artery to estimate the likelihood of invasion preoperatively [7]. Adenomas are graded as follows: Knosp 0 if the adenoma is medial to the medial tangent of both internal carotid arteries on coronal cuts, Knosp 1 if it extends between the medial tangent and the intercarotid line, Knosp 2 if it extends between the intercarotid line and the lateral tangent, Knosp 3 if it extends lateral to the lateral tangent, and Knosp 4 if the adenoma completely encases the internal carotid artery [7]. The use of the Knosp classification has also been validated as a prognostic marker of surgical remission [8, 9]. The revised-Knosp classification further refines Knosp 3, categorizing it into Knosp 3A when the adenoma is above the intracavernous carotid artery (superior compartment) and Knosp 3B when below (inferior compartment) [1].
Occasionally, 3 Tesla MRI can directly visualize perforations of the medial wall of the cavernous sinus [7]. Seven Tesla MRI, which is currently not available for routine clinical care, has a superior spatial resolution and increased signal-to-noise and contrast-to-noise ratios, and can thus provide more accurate information on cavernous sinus invasion [10]. In clinical studies, this technique improved the agreement between intraoperative observations and preoperative radiological findings [10].
Intraoperative diagnosis
In the original Knosp classification, intraoperative assessment of cavernous sinus invasion relied on an operative microscope, which was the standard tool for transsphenoidal surgery (TSS) at that time [7]. However, the operative microscope has limitations in directly visualizing the medial wall of the cavernous sinus. With the evolution toward endoscopic endonasal approaches, significant improvement in exposure within the sellar space was achieved. This shift allowed for the direct inspection of both medial walls of the cavernous sinus, leading to the revision of their classification by Knosp et al., based on endoscopic assessment during TSS, now considered as the gold-standard [1].
More recently, thorough microanatomical studies of parasellar ligaments have enabled the safe intraoperative resection of the medial wall of the cavernous sinus when invasion is suspected [11, 12]. Meticulous histological examination has confirmed pathological invasion of the wall in 78% of the cases where invasion was intraoperatively asserted by the surgeon [13].
Epidemiology
The prevalence of cavernous invasion by pituitary adenomas remains uncertain, primarily due to inconsistencies in assessment techniques and definitions. Direct intraoperative observation reports cavernous sinus invasion in 9–30% of adenomas, while preoperative radiological assessment predicts invasion in 30–63% of cases [14,15,16,17,18]. This discrepancy suggests that the radiologically diagnosed invasion in non-operated pituitary adenomas is likely overestimated. Using histologic examination as a reference, the intraoperative endoscopic evaluation of medial wall invasions yields a positive predictive value of 78% [13]. However, histologic examination might provide false negative results due to incomplete sampling, thus underestimating the prevalence of cavernous sinus invasion. Moreover, the prevalence of cavernous sinus invasion may also vary based on the histotype of pituitary adenomas. Members of the PIT-1 family, particularly somatotrophs, have exhibited a propensity for dural invasion of the cavernous sinus, as compared to those expressing the T-box transcription factor (TPIT) and steroidogenic factor (SF-1) [13, 19].
Pathophysiology
Invasive behavior of pituitary adenomas
Pituitary adenomas are benign tumors that usually tend to displace rather than invade the surrounding anatomical structures. However, despite the benign nature of these tumors, some pituitary adenomas infiltrate beyond the physiological anatomical confines of the pituitary gland comprising the sellar diaphragm, basal dura and the medial wall of the cavernous sinuses [20]. The underpinning mechanisms of this invasive behavior remains unclear. High expressions of matrix metalloproteinase-2 (MMP-2) and -9 (MMP-9), enzymes known to degrade the extracellular matrix, have been associated with the invasiveness [21,22,23,24,25]. Beyond the MMP system, serine proteases like the urokinase-type plasminogen activator (uPA), involved in cancer cell growth, migration, epithelial-mesenchymal transition and angiogenesis, are overexpressed in pituitary tumors in comparison to normal anterior pituitary tissue. Notably, invasive non-secreting tumors exhibit an overexpression of uPA compared to their non-invasive counterparts, suggesting a potential role of the uPA in the acquisition of the invasive behaviors [26]. Microarray analysis of non-functioning pituitary adenomas has uncovered a molecular signature that grossly differentiate invasive tumors from non-invasive ones, and has highlighted MYO5A protein, a member of the myosin family implicated in actin-dependent molecular motor functions, as a potentially valuable marker of invasiveness [27]. Additionally, adenomas that invade the cavernous sinus show higher expression of vascular endothelial growth factor (VEGF) and VEGF receptor 1 (VEGFR1), both playing a role in angiogenesis, as well as of programmed death-ligand 1 (PD-L1), well-known for its roles in the maintenance of immune tolerance, along with a higher number of tumor-associated macrophages [28]. Other proteins such as bromodomain-containing protein 4 (BRD4), an epigenetic regulator that leads to the transcriptional activation of oncogenes, is overexpressed in non-secreting invasive pituitary adenomas [29]. The main limitation of these studies is the comparison of purely intrasellar specimens obtained from invasive and non-invasive adenomas as this approach does not take into consideration the intra tumoral heterogeneity of those tumor [30, 31]. Also, a deeper insight into the contribution of each component would be gained by using single cell based molecular analyses. The tumoral microenvironment of pituitary adenomas is composed of vascular structures, non-tumoral immune cells, tumor-associated fibroblast, folliculo-stellate cells and non-cellular components, namely, the extracellular matrix [25, 32]. Growing evidence suggests that the tumoral microenvironment plays significant roles in the biological behavior and therapeutic responses of invasive pituitary adenomas [24, 33, 34]. Tumor-associated macrophages, the most prevalent immune cells in pituitary adenomas, have been linked to the size, proliferation, and most importantly, their invasive characteristics [24]. Additionally, somatotroph pituitary tumors induced by mutations in the aryl hydrocarbon receptor interacting protein (AIP) exhibit a greater macrophage infiltration compared to sporadic tumors, further reinforcing the suspicion of the microenvironment’s involvement in the tumor’s invasive nature [35]. Other components, such as tumor-infiltrating lymphocytes, vessels and the extracellular matrix, have also been implicated in the invasion characteristics of pituitary adenomas, with substantial interactions between these elements [24]. Moreover, treatments targeting at various elements of the tumor microenvironment, such as immune-checkpoint inhibitors and bevacizumab, have already demonstrated effectiveness in managing invasive pituitary adenomas and carcinomas [25, 36,37,38,39,40]. Thus, a better comprehension of the underpinnings driving cavernous sinus invasion may pave the way for identifying innovative therapeutic targets.
Weakness of medial wall of the cavernous sinus
The contrast between the histologically benign nature of pituitary adenomas and their frequent extension into the adjacent cavernous sinus has prompted anatomical studies of the medial wall of the cavernous sinus. These studies have shed light on its significant mechanical weakness compared to sinus’s other walls [41, 42]. The medial wall of the cavernous sinus has two parts: the sellar and the sphenoidal. The sellar part, covering the lateral surface of the anterior lobe of the pituitary gland, is distinct from the pituitary gland’s capsule [42]. It is formed by a single thin dural layer, unlike the cavernous sinus’s other, thicker walls, which can be anatomically and histologically divided into two layers (Fig. 1) [43]. The monolayer character complicates the preoperative identification of the medial wall, as compared to anterior, inferior, and posterior surfaces [7, 44]. Additionally, the lateral aspect of the pituitary fossa lacks an osseous wall, in contrast to its anterior, inferior, and posterior surfaces. This absence of bone explains the tendency of pituitary tumors to extend laterally into the cavernous sinus [42].
Illustration of the sellar and parasellar region on a coronal section. The dura of the lateral and upper walls of the CS is shown to be divided into a meningeal layer (red) and an endosteal layer (green). The medial wall of the CS is only composed of a meningeal layer and is not adherent to the pituitary capsule (black). A Left pituitary microadenoma. B Large pituitary macroadenoma invading the left cavernous sinus and compressing the optic chiasm. C Illustration of a postoperative intracavernous tumoral remnant. (CS cavernous sinus, ICA internal carotid artery)
Further cadaveric studies have observed the disappearance of the sellar part of the medial wall at the posteroinferior face of the gland’s capsule, where a weblike network of fibrous tissue connects the capsule to the carotid artery, venous plexus, and the dura of the middle fossa [45]. Some studies have noted micro-protrusions of the pituitary gland within the cavernous sinus in healthy human cadavers, suggesting that pre-existing weaknesses in the medial wall could facilitate tumor invasion [41, 46]. While it is widely acknowledged that the medial wall of the cavernous sinus is thinner and has fewer collagen layers than the other walls, most recent studies do not identify any significant macro- or micro-scopic defects in its integrity [42, 47].
Clinical consequences of cavernous sinus invasion
The cavernous sinus houses several critical neurovascular structures, including the internal carotid artery and the oculomotor, trochlear, abducens nerves and the two first branches of the trigeminal nerve [48]. Despite the well-known vulnerability of these cranial nerves, ophthalmoplegia is only exceptionally observed in cases of cavernous sinus invasion by pituitary adenoma, except when suddenly compressed by apoplexy [49]. It has been suggested that cavernous sinus invasion may be a risk factor for pituitary apoplexy, although no convincing mechanism has yet been proposed [50, 51]. Moreover, unlike other tumors that invade the cavernous sinus, such as meningiomas, pituitary adenomas almost never cause internal carotid artery stenosis, even in cases of complete encasement [52]. Therefore, cavernous sinus invasion by pituitary adenomas is usually clinically silent.
Therapeutic approaches of cavernous sinus invasion
Standard intrasellar surgery
Historically, pituitary adenomas invading the cavernous sinus were deemed inaccessible for complete surgical resection. However, partial surgical resection of the intrasellar and suprasellar compressive tumor portions remains indicated to preserve or restore vision, regardless of the tumor’s invasive status [53]. Additionally, debulking surgery can help to reach biochemical control in secreting pituitary adenomas invading the cavernous sinus by somatostatin receptor ligands or dopaminergic agonists [54,55,56,57,58,59,60,61].
Intracavernous postoperative tumoral remnants of non-secreting adenomas show 2- and 5-years regrowth rates of 30 and 70%, respectively [62]. In case of tumor regrowth from the intracavernous remnant, novel surgical resection, medical treatments, and/or adjuvant radiotherapy may be considered [53, 63].
Intracavernous surgery
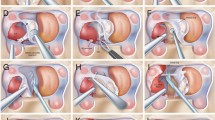
During transsphenoidal surgery of a parasellar invasive pituitary adenomas, surgeons would attempt to reduce also the volume of the intracavernous portion by tracing the tumor with great caution from the sella through the pre-existing perforations in the medial wall of the cavernous sinus [54]. Conversely, recent literature reports extended intracavernous approaches aiming at hormonal control in secreting pituitary adenomas [5]. Indeed, in these cases, some authors advocate for deliberate, systematic selective resection of the medial wall of the cavernous sinus, achieving higher resection and remission rates [12, 13]. This surgical technique can only be safely performed by experienced endonasal skull base teams. Aggressive transsphenoidal resection of intracavernous adenomas (Fig. 2) have been reported with high remissions rates and acceptable morbidity rates [8, 11,12,13, 64,65,66,67,68,69,70,71,72]. The use of high-field intraoperative MRI during transsphenoidal reoperation for non-functioning pituitary adenomas leads to higher gross total resection rates [73, 74]. Although, most studies have focused their analysis on the role of intraoperative MRI in non-functioning adenomas, some authors have also described improvement in remission rates in patients with acromegaly who underwent first-time transsphenoidal surgery using a 1.5 Tesla intraoperative MRI [75, 76]. The objective of such intracavernous surgery is not necessarily a complete cleaning of the cavernous compartment from the adenomatous tissue but this aggressive approach shows promise for more long-standing effects on improved tumor-growth control rates and remission from functioning tumors that could be valuable in some clinical situation, such as in pregnancy seeking patients with residual corticotroph adenomas, in whom steroidogenesis inhibitors are contraindicated [72, 76].
Magnetic resonance imaging in coronal cut, T1 weighted with gadolinium enhancement illustrating a patient with a pituitary macroadenoma who underwent intracavernous endonasal surgery. A Pituitary macroadenoma largely invading the left cavernous sinus. B Gross total resection of the macroadenoma after intracavernous endonasal surgery
Adjuvant therapies for intracavernous remnants
No specific drugs have been so far developed to target intracavernous tumor remnants. Two recent studies, however, suggest that dopamine agonist therapy is associated with a decreased prevalence of residual tumor enlargement in patient with non-secreting adenomas, especially when treatment is initiated before tumor remnant growth is detected [77,78,79]. The first- and second-generation somatostatin agonist ligands have a variable efficacy on growth and secretory properties of cavernous sinus remnants [80].
Finally, when tumor regrowth occurs – especially in case of aggressive tumors, external beam radiotherapy or stereotactic radiosurgical techniques may be considered as an alternative to surgical reintervention or be applied after repeated debulking surgery [32]. External beam radiotherapy, delivering a total dose of 40–45 Gy in fractions, has demonstrated long-term durability with low morbidity rates in most patients up to 30 years after treatment [5, 81, 82]. However, external beam radiotherapy cannot provide the accuracy that stereotactic radiosurgery offers [5]. For non-secreting adenomas, the antitumoral effects of conventional radiotherapy are comparable to those of stereotactic radiosurgery [83, 84]. In secreting pituitary adenomas, stereotactic radiosurgery’s efficacy appears lower than conventional radiotherapy, especially in Cushing’s disease [83, 85]. However, stereotactic radiosurgery provides a more rapid efficacy and a lower rate of hypopituitarism [83].
Conclusions and perspectives
In conclusion, cavernous sinus invasion stands as a critical prognostic indicator for both secreting and non-secreting pituitary adenomas. There is a clear need for innovative multimodal strategies to address this significant, albeit often overlooked and under-investigated clinical challenge. Recent decades have seen a rise in the use of extended endoscopic surgical procedures, rendering intracavernous aggressive resection safer in experimented hands. Nevertheless, the molecular mechanisms driving cavernous sinus invasion remain largely elusive, representing a fertile ground for the development of promising new medical therapies. A deeper understanding of these mechanisms is key to better target and manage this complex aspect of pituitary adenoma pathology.
References
A.S.G. Micko, A. Wöhrer, S. Wolfsberger, E. Knosp, Invasion of the cavernous sinus space in pituitary adenomas: endoscopic verification and its correlation with an MRI-based classification. J. Neurosurg. 122(4), 803–811 (2015). https://doi.org/10.3171/2014.12.JNS141083
K. Juraschka, O.H. Khan, B.L. Godoy et al., Endoscopic endonasal transsphenoidal approach to large and giant pituitary adenomas: institutional experience and predictors of extent of resection. J. Neurosurg. 121(1), 75–83 (2014). https://doi.org/10.3171/2014.3.JNS131679
S. Brochier, F. Galland, M. Kujas et al., Factors predicting relapse of nonfunctioning pituitary macroadenomas after neurosurgery: a study of 142 patients. Eur. J. Endocrinol. 163(2), 193–200 (2010). https://doi.org/10.1530/EJE-10-0255
G. Raverot, E. Dantony, J. Beauvy et al., Risk of recurrence in pituitary neuroendocrine tumors: a prospective study using a five-tiered classification. J. Clin. Endocrinol. Metab. 102(9), 3368–3374 (2017). https://doi.org/10.1210/jc.2017-00773
M. Rutkowski, G. Zada, Management of pituitary adenomas invading the cavernous sinus. Neurosurg. Clin. 30(4), 445–455 (2019). https://doi.org/10.1016/j.nec.2019.05.005
M. Araujo-Castro, A. Acitores Cancela, C. Vior, E. Pascual-Corrales, V. Rodríguez Berrocal, Radiological Knosp, revised-Knosp, and Hardy–Wilson classifications for the prediction of surgical outcomes in the endoscopic endonasal surgery of pituitary adenomas: study of 228 cases. Front. Oncol. 11 (2022). https://www.frontiersin.org/articles/10.3389/fonc.2021.807040
E. Knosp, E. Steiner, K. Kitz, C. Matula, Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33(4), 610–617 (1993). https://doi.org/10.1227/00006123-199310000-00008.
H. Nishioka, N. Fukuhara, K. Horiguchi, S. Yamada, Aggressive transsphenoidal resection of tumors invading the cavernous sinus in patients with acromegaly: predictive factors, strategies, and outcomes. J. Neurosurg. 121(3), 505–510 (2014). https://doi.org/10.3171/2014.3.JNS132214
A. Micko, J. Oberndorfer, W.J. Weninger et al., Challenging Knosp high-grade pituitary adenomas. J. Neurosurg. 132(6), 1739–1746 (2019). https://doi.org/10.3171/2019.3.JNS19367
F. Eisenhut, M.A. Schmidt, M. Buchfelder, A. Doerfler, S.M. Schlaffer, Improved detection of cavernous sinus invasion of pituitary macroadenomas with ultra-high-field 7 T MRI. Life13(1), 49 (2022). https://doi.org/10.3390/life13010049
H.Q. Truong, S. Lieber, E. Najera, J.T. Alves-Belo, P.A. Gardner, J.C. Fernandez-Miranda, The medial wall of the cavernous sinus. Part 1: surgical anatomy, ligaments, and surgical technique for its mobilization and/or resection. J. Neurosurg. 131(1), 122–130 (2018). https://doi.org/10.3171/2018.3.JNS18596
S. Cohen-Cohen, P.A. Gardner, J.T. Alves-Belo et al., The medial wall of the cavernous sinus. Part 2: selective medial wall resection in 50 pituitary adenoma patients. J. Neurosurg. 131(1), 131–140 (2018). https://doi.org/10.3171/2018.5.JNS18595
A. Mohyeldin, L.J. Katznelson, A.R. Hoffman et al., Prospective intraoperative and histologic evaluation of cavernous sinus medial wall invasion by pituitary adenomas and its implications for acromegaly remission outcomes. Sci. Rep. 12(1), 9919 (2022). https://doi.org/10.1038/s41598-022-12980-1
S. Dhandapani, H. Singh, H.M. Negm, S. Cohen, V.K. Anand, T.H. Schwartz, Cavernous sinus invasion in pituitary adenomas: systematic review and pooled data meta-analysis of radiologic criteria and comparison of endoscopic and microscopic surgery. World Neurosurg. 96, 36–46 (2016). https://doi.org/10.1016/j.wneu.2016.08.088
G.F. Woodworth, K.S. Patel, B. Shin et al., Surgical outcomes using a medial-to-lateral endonasal endoscopic approach to pituitary adenomas invading the cavernous sinus. J. Neurosurg. 120(5), 1086–1094 (2014). https://doi.org/10.3171/2014.1.JNS131228
A. Paluzzi, J.C. Fernandez-Miranda, S. Tonya Stefko, S. Challinor, C.H. Snyderman, P.A. Gardner, Endoscopic endonasal approach for pituitary adenomas: a series of 555 patients. Pituitary 17(4), 307–319 (2014). https://doi.org/10.1007/s11102-013-0502-4
C.P. Hofstetter, M.J. Nanaszko, L.L. Mubita, J. Tsiouris, V.K. Anand, T.H. Schwartz, Volumetric classification of pituitary macroadenomas predicts outcome and morbidity following endoscopic endonasal transsphenoidal surgery. Pituitary 15(3), 450–463 (2012). https://doi.org/10.1007/s11102-011-0350-z
M. Messerer, J.C. De Battista, G. Raverot et al., Evidence of improved surgical outcome following endoscopy for nonfunctioning pituitary adenoma removal. Neurosurg. Focus 30(4), E11 (2011). https://doi.org/10.3171/2011.1.FOCUS10308
K. Asmaro, M. Zhang, A.J. Rodrigues et al., Cytodifferentiation of pituitary tumors influences pathogenesis and cavernous sinus invasion. J. Neurosurg. 1, 1–9 (2023). https://doi.org/10.3171/2023.3.JNS221949
R. Fahlbusch, M. Buchfelder, Current management of invasive pituitary adenomas. Contemp. Neurosurg. 11(17), 1 (1989)
H.Y. Liu, W.J. Gu, C.Z. Wang, X.J. Ji, Y.M. Mu, Matrix metalloproteinase-9 and -2 and tissue inhibitor of matrix metalloproteinase-2 in invasive pituitary adenomas. Medicine 95(24), e3904 (2016). https://doi.org/10.1097/MD.0000000000003904
J. Gong, Y. Zhao, R. Abdel-Fattah et al., Matrix metalloproteinase-9, a potential biological marker in invasive pituitary adenomas. Pituitary 11(1), 37–48 (2008). https://doi.org/10.1007/s11102-007-0066-2
H. Kawamoto, T. Uozumi, K. Kawamoto, K. Arita, T. Yano, T. Hirohata, Type IV collagenase activity and cavernous sinus invasion in human pituitary adenomas. Acta Neurochir. 138(4), 390–395 (1996). https://doi.org/10.1007/BF01420300
M.D. Ilie, A. Vasiljevic, P. Bertolino, G. Raverot, Biological and therapeutic implications of the tumor microenvironment in pituitary adenomas. Endocr. Rev. 44(2), 297–311 (2023). https://doi.org/10.1210/endrev/bnac024
M.D. Ilie, A. Vasiljevic, G. Raverot, P. Bertolino, The microenvironment of pituitary tumors—biological and therapeutic implications. Cancers 11(10), 1605 (2019). https://doi.org/10.3390/cancers11101605
U.J. Knappe, C. Hagel, B.W. Lisboa, W. Wilczak, D.K. Lüdecke, W. Saeger, Expression of serine proteases and metalloproteinases in human pituitary adenomas and anterior pituitary lobe tissue. Acta Neuropathol. 106(5), 471–478 (2003). https://doi.org/10.1007/s00401-003-0747-5
F. Galland, L. Lacroix, P. Saulnier et al., Differential gene expression profiles of invasive and non-invasive non-functioning pituitary adenomas based on microarray analysis. Endocr. Relat. Cancer 17(2), 361–371 (2010). https://doi.org/10.1677/ERC-10-0018
M. Sato, R. Tamura, H. Tamura et al., Analysis of tumor angiogenesis and immune microenvironment in non-functional pituitary endocrine tumors. J. Clin. Med. 8(5), 695 (2019). https://doi.org/10.3390/jcm8050695
C. Shi, Z. Ye, J. Han et al., BRD4 as a therapeutic target for nonfunctioning and growth hormone pituitary adenoma. Neuro Oncol. 22(8), 1114–1125 (2020). https://doi.org/10.1093/neuonc/noaa084
M. Hage, S. Viengchareun, E. Brunet et al., Genomic alterations and complex subclonal architecture in sporadic GH-secreting pituitary adenomas. J. Clin. Endocrinol. Metab. 103(5), 1929–1939 (2018). https://doi.org/10.1210/jc.2017-02287
R.A. Burrell, N. McGranahan, J. Bartek, C. Swanton, The causes and consequences of genetic heterogeneity in cancer evolution. Nature 501(7467), 338–345 (2013). https://doi.org/10.1038/nature12625
G. Raverot, M.D. Ilie, H. Lasolle et al., Aggressive pituitary tumours and pituitary carcinomas. Nat. Rev. Endocrinol. 17(11), 671–684 (2021). https://doi.org/10.1038/s41574-021-00550-w
P. Marques, S. Barry, E. Carlsen et al., The role of the tumour microenvironment in the angiogenesis of pituitary tumours. Endocrine 70(3) (2020). https://doi.org/10.1007/s12020-020-02478-z
P. Marques, S. Barry, E. Carlsen et al., Chemokines modulate the tumour microenvironment in pituitary neuroendocrine tumours. Acta Neuropathol. Commun. 7(1), 172 (2019). https://doi.org/10.1186/s40478-019-0830-3
S. Barry, E. Carlsen, P. Marques et al., Tumor microenvironment defines the invasive phenotype of AIP-mutation-positive pituitary tumors. Oncogene 38(27), 5381–5395 (2019). https://doi.org/10.1038/s41388-019-0779-5
M.D. Ilie, H. Lasolle, G. Raverot, Emerging and novel treatments for pituitary tumors. J. Clin. Med. 8(8), 1107 (2019). https://doi.org/10.3390/jcm8081107
K. Osterhage, R. Rotermund, M. Droste et al., Bevacizumab in aggressive pituitary adenomas – experience with 3 patients. Exp. Clin. Endocrinol. Diabetes 129(3), 178–185 (2021). https://doi.org/10.1055/a-1260-3975
O.M. Alshaikh, S.L. Asa, O. Mete, S. Ezzat, An institutional experience of tumor progression to pituitary carcinoma in a 15-year cohort of 1055 consecutive pituitary neuroendocrine tumors. Endocr. Pathol. 30(2), 118–127 (2019). https://doi.org/10.1007/s12022-019-9568-5
M.D. Ilie, A. Vasiljevic, E. Jouanneau, G. Raverot, Immunotherapy in aggressive pituitary tumors and carcinomas: a systematic review. Endocr. Relat. Cancer 29(7), 415–426 (2022). https://doi.org/10.1530/ERC-22-0037
M.D. Ilie, C. Villa, T. Cuny et al., Real-life efficacy and predictors of response to immunotherapy in pituitary tumors: a cohort study. Eur. J. Endocrinol. 187(5), 685–696 (2022). https://doi.org/10.1530/EJE-22-0647
S. Yokoyama, H. Hirano, K. Moroki, M. Goto, S. Imamura, J.I. Kuratsu, Are nonfunctioning pituitary adenomas extending into the cavernous sinus aggressive and/or invasive? Neurosurgery 49(4), 857–862 (2001). https://doi.org/10.1097/00006123-200110000-00014.
A. Yasuda, A. Campero, C. Martins, A.L.J. Rhoton, G.C. Ribas, The medial wall of the cavernous sinus: microsurgical anatomy. Neurosurgery 55(1), 179 (2004). https://doi.org/10.1227/01.NEU.0000126953.59406.77
M.B. Gonçalves, J.G. de Oliveira, H.A. Williams, R.M.P. Alvarenga, J.A. Landeiro, Cavernous sinus medial wall: dural or fibrous layer? Systematic review of the literature. Neurosurg. Rev. 35(2), 147–154 (2012). https://doi.org/10.1007/s10143-011-0360-3
J.P. Cottier, C. Destrieux, L. Brunereau, et al., Cavernous sinus invasion by pituitary adenoma: MR imaging. Radiology (2000). https://doi.org/10.1148/radiology.215.2.r00ap18463
Y. Diao, L. Liang, C. Yu, M. Zhang, Is there an identifiable intact medial wall of the cavernous sinus? Macro- and microscopic anatomical study using sheet plastination. Neurosurgery 73, ons106–ons109 (2013). https://doi.org/10.1227/NEU.0b013e3182889f2b.
K. Shi, Z. Li, X. Wu et al., The medial wall and medial compartment of the cavernous sinus: an anatomic study using plastinated histological sections. Neurosurg. Rev. 45(5), 3381–3391 (2022). https://doi.org/10.1007/s10143-022-01846-9
S. Yilmazlar, H. Kocaeli, F. Aydiner, E. Korfali, Medial portion of the cavernous sinus: quantitative analysis of the medial wall. Clin. Anat. 18(6), 416–422 (2005). https://doi.org/10.1002/ca.20160
V.V. Dolenc (ed.), Anatomy of the cavernous sinus. in Anatomy and Surgery of the Cavernous Sinus. (Springer; 1989), pp. 3–137. https://doi.org/10.1007/978-3-7091-6942-1_2
S.H. Kim, K.C. Lee, S.H. Kim, Cranial nerve palsies accompanying pituitary tumour. J. Clin. Neurosci. 14(12), 1158–1162 (2007). https://doi.org/10.1016/j.jocn.2006.07.016
N. Cinar, Y. Tekinel, S. Dagdelen, H. Oruckaptan, F. Soylemezoglu, T. Erbas, Cavernous sinus invasion might be a risk factor for apoplexy. Pituitary 16(4), 483–489 (2013). https://doi.org/10.1007/s11102-012-0444-2
A. Hosmann, A. Micko, J.M. Frischer et al., Multiple pituitary apoplexy-cavernous sinus invasion as major risk factor for recurrent hemorrhage. World Neurosurg. 126, e723–e730 (2019). https://doi.org/10.1016/j.wneu.2019.02.138
A. Dincer, V. Sharma, N. Madan, C. Heilman, Cavernous segment internal carotid artery stenosis specific to meningiomas compared to pituitary adenomas. J. Neuroimaging 33(1), 73–78 (2023). https://doi.org/10.1111/jon.13051
S. Melmed, Pituitary-tumor endocrinopathies. N. Engl. J. Med. 382(10), 937–950 (2020). https://doi.org/10.1056/NEJMra1810772
M. Buchfelder, S.M. Schlaffer, The surgical treatment of acromegaly. Pituitary 20(1), 76–83 (2017). https://doi.org/10.1007/s11102-016-0765-7
J. Wass, Debulking of pituitary adenomas improves hormonal control of acromegaly by somatostatin analogues. Eur. J. Endocrinol. 152(5), 693–694 (2005). https://doi.org/10.1530/eje.1.01896
A. Colao, R. Attanasio, R. Pivonello et al., Partial surgical removal of growth hormone-secreting pituitary tumors enhances the response to somatostatin analogs in acromegaly. J. Clin. Endocrinol. Metab. 91(1), 85–92 (2006). https://doi.org/10.1210/jc.2005-1208
R.S. Jallad, N.R. Musolino, S. Kodaira, V.A. Cescato, M.D. Bronstein, Does partial surgical tumour removal influence the response to octreotide-LAR in acromegalic patients previously resistant to the somatostatin analogue? Clin. Endocrinol. 67(2), 310–315 (2007). https://doi.org/10.1111/j.1365-2265.2007.02885.x
N. Karavitaki, H.E. Turner, C.B.T. Adams et al., Surgical debulking of pituitary macroadenomas causing acromegaly improves control by lanreotide. Clin. Endocrinol. 68(6), 970–975 (2008). https://doi.org/10.1111/j.1365-2265.2007.03139.x
P. Petrossians, L. Borges-Martins, C. Espinoza et al., Gross total resection or debulking of pituitary adenomas improves hormonal control of acromegaly by somatostatin analogs. Eur. J. Endocrinol. 152(1), 61–66 (2005). https://doi.org/10.1530/eje.1.01824
A. Giustina, N. Biermasz, F.F. Casanueva et al., Consensus on criteria for acromegaly diagnosis and remission. Pituitary 27(1), 7–22 (2024). https://doi.org/10.1007/s11102-023-01360-1
M. Fleseriu, R. Auchus, I. Bancos et al., Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol. 9(12), 847–875 (2021). https://doi.org/10.1016/S2213-8587(21)00235-7
Y. Greenman, G. Ouaknine, I. Veshchev, I.I. Reider-Groswasser, Y. Segev, N. Stern, Postoperative surveillance of clinically nonfunctioning pituitary macroadenomas: markers of tumour quiescence and regrowth. Clin. Endocrinol. 58(6), 763–769 (2003). https://doi.org/10.1046/j.1365-2265.2003.01784.x
E.P. O’Sullivan, C. Woods, N. Glynn et al., The natural history of surgically treated but radiotherapy-naïve nonfunctioning pituitary adenomas. Clin. Endocrinol. 71(5), 709–714 (2009). https://doi.org/10.1111/j.1365-2265.2009.03583.x
C.P. Hofstetter, B.J. Shin, L. Mubita et al., Endoscopic endonasal transsphenoidal surgery for functional pituitary adenomas. Neurosurg. Focus 30(4), E10 (2011). https://doi.org/10.3171/2011.1.FOCUS10317
A. Ajlan, A.S. Achrol, A. Albakr et al., Cavernous sinus involvement by pituitary adenomas: clinical implications and outcomes of endoscopic endonasal resection. J. Neurol. Surg. B Skull Base 78(3), 273–282 (2017). https://doi.org/10.1055/s-0036-1598022
H. Borghei-Razavi, B.A. Muhsen, K. Joshi, T. Woodard, V.R. Kshettry, Endoscopic extracapsular resection of an adrenocorticotropic hormone–secreting macroadenoma with selective resection of the medial cavernous sinus wall. World Neurosurg. 144, 199 (2020). https://doi.org/10.1016/j.wneu.2020.09.087
L.J.M. de Macêdo Filho, A.V.G. Diógenes, E.G. Barreto et al., Endoscopic endonasal resection of the medial wall of the cavernous sinus and its impact on outcomes of pituitary surgery: a systematic review and meta-analysis. Brain Sci. 12(10), 1354 (2022). https://doi.org/10.3390/brainsci12101354
A. Ishida, H. Shiramizu, H. Yoshimoto et al., Resection of the cavernous sinus medial wall improves remission rate in functioning pituitary tumors: retrospective analysis of 248 consecutive cases. Neurosurgery 91(5), 775–781 (2022). https://doi.org/10.1227/neu.0000000000002109
Y. Nagata, K. Takeuchi, T. Yamamoto et al., Removal of the medial wall of the cavernous sinus for functional pituitary adenomas: a technical report and pathologic significance. World Neurosurg. 126, 53–58 (2019). https://doi.org/10.1016/j.wneu.2019.02.134
E.H. Oldfield, Cushing’s disease: lessons learned from 1500 cases. Neurosurgery 64, 27–36 (2017). https://doi.org/10.1093/neuros/nyx378
A.T. Omar, D.G. Munoz, J. Goguen et al., Resection of the medial wall of the cavernous sinus in functioning pituitary adenomas: technical note and outcomes in a matched-cohort study. Clin. Neurol. Neurosurg. 200, 106306 (2021). https://doi.org/10.1016/j.clineuro.2020.106306
H.H. Park, E.H. Kim, C.R. Ku, E.J. Lee, S.H. Kim, Outcomes of aggressive surgical resection in growth hormone–secreting pituitary adenomas with cavernous sinus invasion. World Neurosurg. 117, e280–e289 (2018). https://doi.org/10.1016/j.wneu.2018.06.012
S. Berkmann, S. Schlaffer, C. Nimsky, R. Fahlbusch, M. Buchfelder, Intraoperative high-field MRI for transsphenoidal reoperations of nonfunctioning pituitary adenoma. J. Neurosurg. 121(5), 1166–1175 (2014). https://doi.org/10.3171/2014.6.JNS131994
P.T. Sylvester, J.A. Evans, G.J. Zipfel et al., Combined high-field intraoperative magnetic resonance imaging and endoscopy increase extent of resection and progression-free survival for pituitary adenomas. Pituitary 18(1), 72–85 (2015). https://doi.org/10.1007/s11102-014-0560-2
R. Fahlbusch, B.V. Keller, O. Ganslandt, J. Kreutzer, C. Nimsky, Transsphenoidal surgery in acromegaly investigated by intraoperative high-field magnetic resonance imaging. Eur. J. Endocrinol. 153(2), 239–248 (2005). https://doi.org/10.1530/eje.1.01970
P.S. Jones, B. Swearingen, Intraoperative MRI for pituitary adenomas. Neurosurg. Clin. N. Am. 30(4), 413–420 (2019). https://doi.org/10.1016/j.nec.2019.05.003
Y. Greenman, K. Tordjman, E. Osher et al., Postoperative treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists decreases tumour remnant growth. Clin. Endocrinol. 63(1), 39–44 (2005). https://doi.org/10.1111/j.1365-2265.2005.02295.x
Y. Greenman, M.D. Bronstein, Cabergoline should be attempted in progressing non-functioning pituitary macroadenoma. Eur. J. Endocrinol. 185(4), D11–D20 (2021). https://doi.org/10.1530/EJE-21-0344
R.L. Batista, N.R.C. Musolino, V.A.S. Cescato et al., Cabergoline in the management of residual nonfunctioning pituitary adenoma: a single-center, open-label, 2-year randomized clinical trial. Am. J. Clin. Oncol. 42(2), 221–227 (2019). https://doi.org/10.1097/COC.0000000000000505
A. Colao, C. Di Somma, R. Pivonello, A. Faggiano, G. Lombardi, S. Savastano, Medical therapy for clinically non-functioning pituitary adenomas. Endocr. Relat. Cancer 15(4), 905–915 (2008). https://doi.org/10.1677/ERC-08-0181
P. Breen, J.C. Flickinger, D. Kondziolka, A.J. Martinez, Radiotherapy for nonfunctional pituitary adenoma: analysis of long-term tumor control. J. Neurosurg. 89(6), 933–938 (1998). https://doi.org/10.3171/jns.1998.89.6.0933
M.N. Hughes, K.J. Llamas, M.E. Yelland, L.B. Tripcony, Pituitary adenomas: long-term results for radiotherapy alone and post-operative radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 27(5), 1035–1043 (1993). https://doi.org/10.1016/0360-3016(93)90520-6
F. Castinetti, J. Régis, H. Dufour, T. Brue, Role of stereotactic radiosurgery in the management of pituitary adenomas. Nat. Rev. Endocrinol. 6(4), 214–223 (2010). https://doi.org/10.1038/nrendo.2010.4
P. Colin, N. Jovenin, B. Delemer et al., Treatment of pituitary adenomas by fractionated stereotactic radiotherapy: a prospective study of 110 patients. Int. J. Radiat. Oncol. Biol. Phys. 62(2), 333–341 (2005). https://doi.org/10.1016/j.ijrobp.2004.09.058
J. Estrada, M. Boronat, M. Mielgo et al., The long-term outcome of pituitary irradiation after unsuccessful transsphenoidal surgery in Cushing’s disease. N. Engl. J. Med. 336(3), 172–177 (1997). https://doi.org/10.1056/NEJM199701163360303
Acknowledgements
With financial support from the French National Research Agency (ANR) and ITMO Cancer of Aviesan within the framework of the 2021–2030 Cancer Control Strategy, on funds administred by Inserm.
Author information
Authors and Affiliations
Contributions
E.L., F.C., and P.K. wrote the main manuscript. M.B. prépare Fig. 1 and E.L. prepared Fig. 2. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lefevre, E., Chasseloup, F., Hage, M. et al. Clinical and therapeutic implications of cavernous sinus invasion in pituitary adenomas. Endocrine 85, 1058–1065 (2024). https://doi.org/10.1007/s12020-024-03877-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-024-03877-2