Abstract
Data on the dural invasiveness of pituitary adenomas have been correlated to the expression of matrix metalloproteinases (e.g. MMP-9). Serine proteases have not yet been investigated in human pituitary adenomas. In this study, paraffin-embedded material from 84 human pituitary adenomas (acromegaly n=18, Cushing's disease n=21, prolactinoma n=18, thyroid-stimulating hormone-secreting adenoma n=1, nonsecreting adenoma n=26) and 9 nontumourous anterior pituitary lobes (obtained from patients with prostate cancer) was immunohistochemically analysed for expression of MMP-2, MMP-9, tissue inhibitor of metalloproteinases-2 (TIMP-2), urokinase-type plasminogen activator (uPA), uPA receptor (uPAR), tissue-type plasminogen activator (tPA), plasminogen activator inhibitor-1 (PAI-1), and interleukin-6 (IL-6). Cavernous sinus invasion was determined by assessment of preoperative magnetic resonance imaging and intraoperative inspection (invasive n=50, noninvasive n=34). In pituitary adenomas, reactions were positive (diffuse expression) to MMP-2 (74% of cases), MMP-9 (49%), TIMP-2 (88%), uPA (89%), uPAR (90%), tPA (69%), and PAI-1 (87%). A weak expression of IL-6 was found in 12% of the adenomas. All reactions were positive (focal expression) in every sample of anterior lobe tissue, except for uPA (negative in 3 out of 9 cases), and IL-6 (faintly positive in 5 out of 8 cases). Adenomas showed remarkably greater expression of uPA than anterior lobe tissue (Chi-square P<0.05). Nonsecreting adenomas exhibited a stronger tendency towards overexpression of uPA in invasive tumours when compared to noninvasive adenomas (Chi-square P=0.053). We found no correlation of MMP-9 expression and tumour invasion. TIMP-2 was overexpressed in noninvasive as compared to invasive adenomas (Chi-square P<0.05). The interrelationship between MMPs and serine proteinases in pituitary adenomas remains to be elucidated. From our data, a correlation between IL-6 and an activation of MMP-9 cannot be proven. The uPA-system may, however, play a role in dural invasion of pituitary adenomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pituitary adenomas are regarded as benign tumours but may invade the adjacent dura and bone. Up to now, it is not clear which factors are responsible for the invasiveness of pituitary adenomas. Several biological markers for dural invasiveness have been investigated and recently it was found, that expression of matrix metalloproteinase-9 (MMP-9) may play a role in the invasion of neighbouring structures [13, 14]. Other groups, however, could not confirm these results [3, 28]. Serine proteases, e.g. urokinase-type plasminogen activator (uPA), uPA receptor (uPAR) or its modulator plasminogen activator inhibitor-1 (PAI-1) have not been investigated in regard to their expression in invasive pituitary adenomas.
The uPA system is considered to be a marker for malignancy in several types of cancer [11]. Furthermore, it plays a role in progression of malignant melanoma [25]. Its importance for invasion in human gliomas is controversial [5, 8].
Another factor that might be involved in tumour invasion is interleukin-6 (IL-6). This is released by human pituitary adenomas in vitro [12] and may play a role in the their development [17]. IL-6 stimulates both MMP-2 and MMP-9 in different pituitary tumours and cell lines (M. Pàez Pereda, personal communication).
In this study, we examined the expression of uPA, uPAR, tissue-type plasminogen activator (tPA), PAI-1, MMP-2, MMP-9, tissue inhibitor of metalloproteinases-2 (TIMP-2), and IL-6 in human pituitary adenomas and in human anterior lobe tissue (obtained during transsphenoidal surgery) with reference to the invasiveness of the tumours.
Materials and methods
Tissues
Pituitary adenomas were obtained by transsphenoidal surgery from 84 patients (male:female ratio 1:1.5; mean age 47 years, range 17–79 years): acromegaly, n= 18; Cushing's disease, n= 21; prolactinoma, n= 18; thyroid-stimulating hormone (TSH)-secreting adenoma, n= 1; nonsecreting adenoma, n= 26. In 6 cases primary and recurrent tumours were investigated, in 2 of these adenoma tissue was obtained at three time points.
Tumour size was measured from preoperative magnetic resonance imaging (MRI). Parasellar invasiveness of pituitary adenomas was evaluated by preoperative MRI [1] and verified by intraoperative inspection of the medial wall of the cavernous sinus using the previously described mirror technique [16]: 50 cases were found to be invasive, and 34 tumours were noninvasive.
Anterior pituitary lobe tissue was obtained by transsphenoidal surgery for pain relief in 9 male patients with osseus metastasis originating from prostate cancer (mean age 63 years, range 46–81 years).
All surgical specimen were fixed in formalin (4%), embedded in paraffin, and stained with hematoxylin-eosin (HE), reticulin stain, and periodic acid-Schiff stain (PAS). Routine immunohistochemistry was performed with antibodies for FSH, LH, ACTH, PRL, STH, TSH, alpha-subunit, pan-cytokeratin, Ki67-antigen (MIB-1 antibody, n= 50), S-100, and GFAP using the streptavidin-biotin peroxidase method. All pituitary adenomas were evaluated histologically according to the WHO-classification [19, 21].
In 12 cases additional fresh tumour tissue was snap frozen in liquid nitrogen and stored at –70°C for quantitative detection of uPA and PAI-1.
Antibodies and dilutions
Murine monoclonal antibodies to human uPA (B-chain, dilution 1:100), to human uPAR (CD 87, 1:20), to human PAI-1 (1:20), to tPA (1:20; all from American Diagnostica Inc./LOXO, Dossenheim, Germany), to MMP-2 (1:25), to human MMP-9 (1:30), to TIMP-2 (1:30; Novocastra, Newcastle, UK) and polyclonal antibodies to human IL-6 (1:700; Rockland Inc,/DPC Biermann, Bad Nauheim, Germany) were used.
Immunohistochemical staining procedure
Paraffin sections (5 µm thick) were dewaxed in xylene, rehydrated by passing through a series of graded alcohols and rinsed in TRIS-buffered-saline/Triton (0.05 M, pH 7.6, 0.1% Triton X-100).
Rehydrated sections were pretreated for uPA using microwave heating (750 W) for 12 min in 0.01 M citrate buffer (pH 6.0). Pretreatment for MMP-2, MMP-9, and TIMP-2 with microwave heating (360 W) for 2×10 min in EDTA solution (2.92 g EDTA; E5134, Sigma, Germany) in 100 ml distilled water was followed by titration with NaOH (Merck, Germany) to pH 8.0, (1:100). For demonstration of IL-6, slides were preincubated with 1% protease (Sigma, P5147) in TRIS buffer for 20 min at 37°C followed by blocking unspecific binding sites with goat serum for 30 min (Dako, Germany, 1:10 in 50 mM TRIS). Primary antibodies were used according to the dilutions mentioned above. Secondary anti-mouse antibody (Sigma, B7264) 1:60 in TBS/Triton was applied for 1 h at room temperature. Streptavidin-biotin complex (Dako, K377) was employed for 30 min, and sections were stained with DAB and counterstained with hemalum.
In three cases of anterior pituitary lobe tissue, double staining was performed. We used the labelled streptavidin-biotin method (Dako LSAB TM kit) to demonstrate hormones with monoclonal antibodies for uPA, uPAR, and PAI-1 as well as for beta-FSH (murine monoclonal antibody) 1:100 (0373, Immunotec, France) and beta-LH (murine monoclonal antibody) 1:200 (Immunotec, 0374). The two-layer indirect technique stained with 4-chloro-naphthol was used as reported previously [4].
Analysis of immunohistochemical results
The slides were analysed independently by four observers (W.W., W.S., U.J.K., and C.H.) blinded for tumour invasiveness. There was general agreement between the observers in most cases. For the discrepancies a second evaluation course was run to reach agreement. Unblinded results were only revealed after definitive histochemical classification.
For uPA, uPAR, and PAI-1 the expression was scored as: distinct (+++) if more than 50% of cells were positive or a strong diffuse reaction was seen; moderate (++) if less than 50% of cells were positive, or a moderate diffuse reaction was observed; slightly positive (+) if immunoreactions were found in small clusters of tumour cells or the diffuse reaction was weak; and negative (−).
For MMP-2, MMP-9, TIMP-2, tPA, and IL-6 it was only practicable to differentiate between positive and negative. A positive reaction was assumed if more than 5% of tumour cells were labelled intensely or the diffuse reaction was at least moderate.
Statistical analysis of the sample groups was accomplished using the Chi-square test, Fisher's exact test or the Spearman correlation test.
Positive controls
A malignant melanoma and glioblastoma multiforme served as positive controls for uPA, uPAR, and PAI-1; a colon carcinoma for MMP-2, MMP-9, TIMP-2, and tPA; and a breast carcinoma for IL-6.
Evaluation of uPA and PAI-1 cytosol levels
In 12 cases additional fresh tumour material was snap-frozen and stored in liquid nitrogen immediately after excision. In a standardized fashion approximately 300 mg frozen specimen were pulverized using the 'Micro-Dismembrator' (Braun-Melsungen, Germany). The tissue powder was suspended in TRIS-buffered saline containing 0.1% Triton X-100 and centrifuged at 100,000 g for 1 h at 4°C. The supernatant was stored in liquid nitrogen until use. Determination of uPA and PAI-1 was done by enzyme-linked immunosorbent assay (American Diagnostica, Greenwich, CT; uPA, Imubind 894; PAI-1, Imubind 821) as described in detail by Jänicke et al. [11]. The protein content was determined by a BCA protein assay kit (Pierce, Rockford, IL). The uPA and PAI-1 concentrations were expressed as ng/mg protein.
In three of the pituitary adenomas less than 50 mg tissue material was available. Therefore, dilutions had to be prepared.
Results
Expression of proteases, inhibitors, uPAR, and IL-6
In pituitary adenomas a diffuse labelling of proteases was found in a variable number of cases (Fig. 1). MMP-9 was expressed in 49% of the investigated cases, MMP-2 in 74%, TIMP-2 in 88%, uPA in 89%, uPAR in 90%, tPA in 69%, and PAI-1 in 87%. A weak expression of IL-6 was found in 12% of the adenomas. The data are summarized in Table 1.
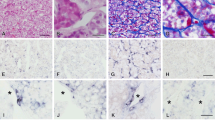
Corresponding slices of a nonsecreting, invasive pituitary adenoma. Border of adenoma tissue to fibrous capsule. A Diffuse expression of uPA is classified as strong (+++, see text). B uPAR is weakly expressed (+). C PAI-1 is expressed strongly (+++). D MMP-9, and E TIMP-2 are positive. No detection of specific antigens in fibrous tissue. Vessel walls are positive only for uPA and slightly positive for MMP-9 (A, D). In this case expression of MMP-2 is weak, but specific for tumour cells. IL-6 is expressed only by some cells (not shown). F Immunohistochemistry for uPA in human non-tumourous anterior pituitary tissue obtained during hypophysectomy in a case of metastasising prostate cancer. Expression of uPA is focal and restricted to pituicytes. No expression of uPA in fibrous stroma (uPA urokinase-type plasminogen activator, uPAR uPA receptor, PAI plasminogen activator inhibitor, MMP matrix metalloproteinase, TIMP tissue inhibitor of metalloproteinase, IL interleukin). A–E ×250, F ×120
In non-tumourous anterior pituitary lobe tissue, expression of uPA was negative in three cases and slightly positive (+) in six cases. In all specimen of anterior pituitary lobe tissue (n=9) all other proteases, uPAR, and inhibitors showed rather focal expression (Fig. 1). In five out of eight cases (63%) only weak expression of IL-6 could be detected. False-positive staining for uPA, uPAR, and PAI-1 in cells activated by castration was excluded by double staining for gonadotropins.
Expression of markers versus demographic data and tumour size
We found no correlation between expression of any investigated protease, uPAR, inhibitor, or IL-6 and age or sex of the patients, or the size of the tumour.
Expression of markers versus tumour type
We found no correlation between expression of the respective antigen of uPA, tPA, PAI-1 and any tumour type (data not shown). When compared to all other adenomas ACTH-secreting adenomas exhibited an overexpression of uPAR (Chi-square P< 0.01, Table 2), MMP-2 (P <0.05) and MMP-9 (P <0.05), as well as their inhibitor TIMP-2 (P<0.05) and IL-6 (P< 0.05). Moreover, when compared to nonsecreting adenomas alone, significant overexpression was found in ACTH-secreting adenomas for MMP-2 (P<0.01), MMP-9 (P<0.01), and IL-6 (P<0.05). When compared to GH-secreting tumours, IL-6 was also overexpressed in ACTH-secreting adenomas (P<0.05). In nonsecreting adenomas there was significantly less expression of MMP-2 (P<0.05) and MMP-9 (P<0.05) when compared to all other groups together. For this evaluation the one case with a TSH-secreting adenoma has been excluded. The statistical analysis was done with Fisher's test.
Expression of markers versus invasiveness of the tumours
Table 1 shows the data concerning the expression of the different markers in relationship to the invasiveness of all adenomas. Here, for uPA, uPAR, and PAI-1, the distinguishing criteria slightly/moderately/distinctly positive were pooled as "positive". An evaluation of all tumours showed no correlation between invasiveness of the adenomas and the expression of uPAR, PAI-1, tPA, MMP-2, MMP-9, and IL-6. TIMP-2 was overexpressed in noninvasive (n=31) as compared to invasive adenomas (n=44, Chi-square P=0.049).
In ACTH-secreting adenomas tPA was overexpressed in noninvasive adenomas (n=8) as compared to invasive tumours (n=13, Fisher's test P= 0.029, data not shown).
Nonsecreting adenomas exhibited a stronger tendency towards overexpression of uPA in invasive tumours (n=19) when compared to noninvasive adenomas (n=7, Chi-square P=0.053) (Table 3).
Expression of markers in primary and recurrent adenomas
Table 4 summarizes the data retrieved from six cases with persistent or recurrent tumours, which had been operated on and evaluated histologically at different time points: four ACTH-secreting tumours, one prolactinoma, and one nonsecreting adenoma. None of the tumours showed progression of expression of any investigated protease. Only case 5 exhibited a loss of MMP-2 and MMP-9 within 1 year. This patient died 4 years later. At autopsy a pancreatic metastasis was discovered, leading to the diagnosis of pituitary carcinoma. This specimen was negative for the investigated antigens (uPA, uPAR, and PAI-1).
Correlation of marker expression in pituitary adenomas
The PAI-1 expression correlated to expression of uPA and uPAR (Spearman correlation, 2-sided, P< 0.01), and MMP-9 (P<0.05). Expression of uPAR also correlated to expression of MMP-9 (P<0.01), MMP-2 (P<0.05), and IL-6 (P<0.05). MMP-9 expression also correlated to expression of MMP-2 and TIMP-2 (P<0.01). For this analysis, the immunohistochemical classification −/+/++/+++ was used for uPA, uPAR, and PAI-1, and −/+ for the remaining antigens.
Expression of markers in adenomas versus anterior pituitary lobe tissue
Overexpression of uPA was found in all adenomas (n=84), invasive adenomas (n=50), and noninvasive adenomas (n=34) when compared to anterior pituitary lobe tissue (Chi-square P<0.05). Expression of MMP-9 in pituitary adenomas was less frequent than in anterior pituitary lobe tissue, irrespective of tumour invasiveness (Chi-square P< 0.05). No difference was detected for uPAR, PAI-1, tPA, MMP-2, and TIMP-2. In pituitary adenomas a loss of expression of IL-6 could be observed (12% versus 63%, Fisher's test P< 0.01) when compared to anterior pituitary lobe tissue.
Quantitative evaluation of uPA and PAI-1
The mean level of uPA in eight invasive tumours was 0.26 ng/mg protein (SD 0.21) and 0.3 ng/mg protein in four noninvasive adenomas (SD 0.08). In the eight invasive adenomas, the mean value of PAI-1 in the cytosol was 4.1 ng/mg protein (SD 1.9) and 3.2 ng/mg protein (SD 2.4) in the four noninvasive tumours. None of the markers correlated with tumour invasiveness (Student's t-test) or to the immunohistological profile of the respective antigen expression (Fig. 2). In pituitary adenomas both parameters are clearly under the threshold value of breast cancer tissue.
Graph demonstrating quantitative analysis of uPA (stars) and PAI-1 (circles) (ng/mg protein) in tissue of 12 human pituitary adenomas related to the classification of immunohistochemical expression of the respective antigens. There was no correlation of immunohistochemical classification (0, negative; 1+, slightly positive; 2+, moderately positive; 3+, distinctly positive) and quantitative detection of uPA and PAI-1 in the cytosol. In pituitary adenomas all values for uPA and PAI-1 are clearly below the cut off levels of breast cancer tissue (3.0 ng/mg protein for uPA, 14 ng/mg protein for PAI-1)
Expression of Ki-67 nuclear antigen with MIB-1 antibody
Specimen of 50 pituitary adenomas (invasive n=28, noninvasive n=22) were investigated with MIB-1. Only in 5 cases more than 0.5% of the nuclei were labelled. In 2 noninvasive adenomas the labelling index was 1% and 2%, respectively. In 3 invasive tumours, it was 3% in 1 case and 7% in 2 cases.
Discussion
For the first time, we have demonstrated the presence of urokinase (uPA), its receptor (uPAR), and one of its inhibitors (PAI-1), as well as tPA, in human pituitary adenomas and anterior pituitary lobe tissue. By cleaving plasminogen into its active form, uPA activates a cascade of proteolytic activity including several MMPs [2]. Its activity is mediated by a cellular surface receptor (uPAR). This is mostly co-expressed with uPA in different malignant tumours [26, 27, 29]. In late stages of progression in malignant melanomas both uPA and uPAR have been identified in invasive parts of the tumours [25]. Both uPA and tPA are inactivated by PAI-1 and PAI-2 [18].
Jänicke et al. [11] demonstrated a strong relationship between the concentration of both uPA and PAI-1 in tumours and an unfavourable prognosis in primary breast cancer patients: in patients with node-negative breast cancer, those with uPA levels above 3.0 ng/mg protein and PAI-1 levels above 14.0 ng/mg protein had a significantly higher risk of tumour progression and dissemination. These findings have been confirmed by independent studies [6, 7]. In the 12 pituitary adenomas investigated here by the same technique, we only found very low levels of uPA and PAI-1 compared to breast cancer tissue, and no correlation of either marker with invasiveness of adenomas could be demonstrated. This is in accordance with our immunohistochemical results, which also showed no correlation of uPA and PAI-1 with invasiveness of adenomas.
Sampling of both tumour tissue and pituitary capsule is done close to the opening in the intrasellar parts of the tumour during transsphenoidal surgery [20] and the areas of lateral invasion are cleaned by suction rather than excision [16]. Therefore, we could not investigate differences of expression of proteases in distinct areas of the tumours, e.g. the relevant lateral/parasellar areas of possible dural invasiveness, nor compare them with central intrasellar areas of the tumours.
In anterior pituitary lobe tissue, we were able to exclude selective expression in cells that had been stimulated by castration of the patients with metastasising prostate cancer, by double staining for the uPA system and gonadotropins. We could thus show that uPA, uPAR, tPA, and PAI-1 are expressed physiologically in human anterior pituitary lobe tissue.
Irrespective of invasiveness of the adenomas, uPA was overexpressed in adenomas compared to anterior pituitary lobe tissue. From these data we conclude that uPA may play a role in formation of human pituitary adenomas.
MMPs are Zn-containing proteases, which are divided into several subgroups according to their substrate specificity. TIMPS are specific inhibitors of MMPs and, like PAIs, have a permissive role in angiogenesis by preventing inappropriate matrix degradation [18].In 22 adenomas investigated by immunoblot analysis, MMP-9 levels were found to be significantly higher in invasive adenomas than in noninvasive ones [14]. In this study, TIMP-1 secretion was not detected in any adenoma [14]. Turner et al. [24] reported that expression of MMP-9 did not differ between noninvasive tumours and normal pituitary glands, but was related to aggressive tumour behaviour. It was higher in invasive when compared to noninvasive macroprolactinomas. Samples of recurrent nonsecreting adenomas were more likely to express MMP-9. In contrast, Yokoyama et al. [28] could not find any difference in immunoexpression of MMP-9 in a relatively small number of nonsecreting adenomas with or without encasement of the carotid artery within the cavernous sinus. Beaulieu et al. [3] found no correlation between the expression of MMP-1, MMP-2, and MMP-3 in 28 human pituitary adenomas analysed by Western blot analysis and the invasive potential of the tumours. However, they stated an inverse correlation between invasiveness and expression of TIMP-2 and TIMP-3. Our data confirm the overexpression of TIMP-2 in noninvasive tumours as compared to invasive ones. In a large number of samples, we found no correlation between immunohistochemical expression of MMP-2 and MMP-9 on one hand, and invasiveness of the adenomas on the other, and in addition, no difference between the expression of MMP-2 in adenomas as compared to anterior pituitary lobe tissue was found. Expression of MMP-9 was less frequent in adenomas compared to anterior pituitary lobe tissue. These data suggest that the loss of modulation of protease activity by TIMP-2 may play a role in development of invasiveness of pituitary adenomas. However, our data concerning expression of MMP-9 are contradictory to the findings of Kawamoto et al. [13, 14] and Turner et al. [24]. Moreover, we found no differences in expression of MMP-9 in recurrent tumours.
We found expression of MMP-2, MMP-9, and TIMP-2 in anterior pituitary lobe tissue. It has been previously described that normal pituitary cells contain MMPs and lesser amounts of TIMPs, whereas far fewer MMPs and TIMPs were identified in pituitary adenomas [22]. In the same study, no correlation was observed between localization of pituitary hormones, MMPs, and TIMPs in anterior pituitary lobe tissue obtained at autopsy [22]. This was confirmed by our data of nontumourous anterior pituitary tissue. Our findings in pituitary adenomas, however, suggest differences in expression of proteases between tumour types.
In ACTH-secreting adenomas we detected an overexpression of uPAR, MMP-2, MMP-9, and TIMP-2 compared to the other tumour types. This may contribute to the relatively high portions of ACTH-secreting adenomas that are invasive, although small in growth [15]. In nonsecreting adenomas, expression of MMP-2 and MMP-9 was lower compared to all other tumours. These data suggest that different types of pituitary adenomas may encompass different mechanisms of tumour development and invasive growth. As anticipated, expression of uPA and uPAR correlated positively to PAI-1, as did MMP-9 to TIMP-2. Moreover, expression of MMP-2, uPAR, and PAI-1 was correlated to presence of MMP-9, and uPAR to MMP-2. These findings substantiate possible interactions between plasminogen activators and MMPs [2, 10].
In 14 out of 15 human pituitary adenomas, immunostaining for IL-6 demonstrated clusters of IL-6-positive cells [23] and different human pituitary adenomas released IL-6 in vitro [12]. IL-6 stimulates both MMP-2 and MMP-9 in different pituitary tumours and cell lines (M. Pàez Pereda, personal communication). Therefore, we analysed our tissue samples in terms of immunohistochemical expression of IL-6. We could not find any significant correlation between the expression of MMP-2, MMP-9, and IL-6 in pituitary adenomas. Only the expression of uPAR weakly correlated to the expression of IL-6. ACTH-secreting adenomas, which overexpress MMP-2 and MMP-9, showed a significantly higher expression of IL-6 when compared to all other tumours. The expression of IL-6 in our material was significantly lower in pituitary adenomas when compared to normal anterior pituitary lobe tissue. The interactions between interleukins and proteases in human pituitary adenomas remain to be identified.
Proliferative activity, determined by labelling of the Ki-67 antigen, did not correlate with tumour invasiveness.
In conclusion our data suggest that:
-
1.
proteases may play a role in tumour invasiveness of pituitary adenomas. The proteases differ for distinct types of pituitary tumours
-
2.
uPA may be related to invasiveness of nonsecreting adenomas
-
3.
Presence of TIMP-2 in tumour tissue protects the integrity of the pituitary capsule in all groups of pituitary adenomas
-
4.
In non-tumorous anterior pituitary lobe tissue serine proteases, MMPs, and the balance with their specific inhibitors seem to play a role in tissue remodelling.
References
Abe T, Lüdecke DK (2001) Effects of preoperative octreotide treatment on different subtypes of 90 GH-secreting pituitary adenomas and outcome in one surgical centre. Eur J Endocrinol 145:137–145
Andreasen PA, Kjoller L, Christensen L, Duffy MJ (1997) The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 72:1–22
Beaulieu E, Kachra Z, Mousseau N, Delbecchi L, Hardy J, Béliveau R (1999) Matrix metalloproteinases and their inhibitors in human pituitary tumors. Neurosurgery 45:1432–1441
Behncken A, Saeger W (1991) Lectin bindings in pituitary adenomas and normal pituitaries. Pathol Res Pract 187:629–631
Bindal AK, Hammoud M, Shi WM, Wu SZ, Sawaya R, Rao JS (1994) Prognostic significance of proteolytic enzymes in human brain tumors. J Neurooncol 22:101–110
Duffy MJ (1996) Proteases as prognostic markers in cancer. Clin Cancer Res 2:613–618
Foekens JA, Peters HA, Look MP, Portengen H, Schmitt M, Kramer MD, Brunner N, Jänicke F, Meijer-van Gelder ME, Henzen-Logmans SC, Putten WL van, Klijn JG (2000) The urokinase system of plasminogen activation and prognosis in 2,780 breast cancer patients. Cancer Res 60:636–643
Giese A, Westphal M (1996) Glioma invasion in the central nervous system. Neurosurgery 39:235–253
Gladson CL, Pijuan-Thompson V, Olman MA, Gillespie GY, Yacoub IZ (1995) Up-regulation of urokinase receptor genes in malignant astrocytoma. Am J Pathol 146:1150–1160
Inuzuka K, Ogata Y, Nagase H, Shirouzu K (2000) Significance of coexpression of urokinase-type plasminogen activator and matrix metalloproteinase 3 (stromelysin) and 9 (gelatinase B) in colorectal carcinoma. J Surg Res 93:211–218
Jänicke F, Schmitt M, Pache L, Ulm K, Harbeck N, Hofler H (1993) Urokinase (uPA) and its inhibitor PAI-1 are strong and independent prognostic factors in node-negative breast cancer. Breast Cancer Res Treat 24:195–208
Jones TH, Daniels M, James RA, Justice SK, McCorkle R, Price A, Kendall-Taylor P, Weetman AP (1994) Production of bioactive and immunoreactive interleukin-6 (IL-6) and expression of IL-6 messenger ribonucleic acid by human pituitary adenomas. J Clin Endocrinol Metab 78:180–187
Kawamoto H, Uozumi T, Kawamoto K, Arita K, Yano Y, Hirohata T (1996) Type IV collagenase activity and cavernous sinus invasion in human pituitary adenomas. Acta Neurochir (Wien) 138:390–395
Kawamoto H, Kawamoto K, MizoueT, Uozumi T, Arita K, Kurisu K (1996) Matrix metalloproteinase-9 secretion by human pituitary adenomas detected by cell immunoblot analysis. Acta Neurochir (Wien) 138:1442–1448
Knappe UJ, Lüdecke DK (1996) Persistent and recurrent hypercortisolism after transsphenoidal surgery for Cushing's disease. Acta Neurochir (Wien) 65 (Suppl):31–34
Lüdecke DK, Flitsch J, Knappe UJ, Saeger W (2001) Cushing's disease: a surgical view. J Neurooncol 54:151–166
Pàez-Pereda M, Goldberg V, Chervin A, Carrizo G, Molina A, Andrada J, Sauer J, Renner U, Stalla GK, Arzt E (1996) Interleukin-2 (IL-2) and IL-6 regulate c-fos protooncogene expression in human pituitary adenoma explants. Mol Cell Endocrinol 124:33–42
Pepper MS, Montesano R (1990) Proteolytic balance and capillary morphogenesis. Cell Differ Dev 32:319–328
Saeger W, Schröder S, Klöppel G (2001) Pathologie wichtiger Erkrankungen endokriner Organe (Schilddrüse ausgenommen). Pathologe 22:296–309
Selman WR, Laws ER Jr, Scheithauer BW, Carpenter SM (1986) The occurrence of dural invasion in pituitary adenomas. J Neurosurg 64:402–407
Solcia E, Klöppel G, Sobin LH, Capella C, Lells RA de, Heitz PU, Horvath E, Kovacs K, Lack EE, Lloyd RJ, Rosai J, Scheithauer BW (2000) Histological typing of endocrine tumors, 2nd edn. Springer, Berlin Heidelberg New York, pp 1–149
Tomita T (1997) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in pituitary adenomas: possible markers of neuroendocrine cells. Endocr Pathol 8:305–313
Tsagarakis S, Kontogeorgos G, Giannou P, Thalassinos N, Woolley J, Besser GM, Grossman A (1992) Interleukin-6, a growth promoting cytokine, is present in human pituitary adenomas: an immunocytochemical study. Clin Endocrinol (Oxf) 37:163–167
Turner HE, Nagy Z, Esiri MM, Harris AL, Wass JAH (2000) Role of matrix metalloproteinase 9 in pituitary tumor behaviour. J Clin Endocrinol Metab 85:2931–2935
Weidle UH, Wöllisch E, Ronne E, Ploug M, Behrendt N, Vries TJ de, Quax PH, Verheijen JH, Muijen GNP van, Ruiter DJ, Hoyer-Hansen G, Dano K (1994) Studies on functional and structural role of urokinase receptor and other components of the plasminogen activation system in malignancy. Ann Biol Clin 52:775–782
Yamamoto M, Sawaya R, Mohanam S, Rao VH, Bruner JM, Nicolson GL, Ohshima K, Rao JS (1994) Activities, localizations, and roles of serine proteases and their inhibitors in human brain tumor progression. J Neurooncol 22:139–151
Yamamoto M, Sawaya R, Mohanam S, Bindal AK, Bruner JM, Oka K, Rao VH, Tomonaga M, Nicolson GL, Rao JS (1994) Expression and localization of urokinase-type plasminogen activator in human astrocytomas in vivo. Cancer Res 54:3656–3661
Yokoyama S, Hirano H, Moroki K, Goto M, Imamura S, Kuratsu J-I (2001) Are nonfunctioning adenomas extending into the cavernous sinus aggressive and/or invasive? Neurosurgery 49:857–863
Xu YC, Hagede J, Doublet JD, Callard P, Sraer JD, Ronne E, Rondeau E (1997) Endothelial and macrophage upregulation of urokinase receptor in human renal cell carcinoma. Hum Pathol 2:206–213
Acknowledgements
The authors acknowledge the help of Mrs.Ulrike Rumpf and Mrs. Petra Wolf for preparation of the immunohistological slides, Mrs. Antje Andreas and Mrs. Barbara Baack for the quantitative analysis by Triton extraction, and Dr. Birgit Jödicke for kindly revising the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Knappe, U.J., Hagel, C., Lisboa, B.W. et al. Expression of serine proteases and metalloproteinases in human pituitary adenomas and anterior pituitary lobe tissue. Acta Neuropathol 106, 471–478 (2003). https://doi.org/10.1007/s00401-003-0747-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-003-0747-5