Abstract
Purpose of Review
Cardiac arrests constitute a leading cause of mortality in the adult population and cardiologists are often tasked with the management of patients following cardiac arrest either as a consultant or primary provider in the cardiac intensive care unit. Familiarity with evidence-based practice for post-cardiac arrest care is a requisite for optimizing outcomes in this highly morbid group. This review will highlight important concepts necessary to managing these patients.
Recent Findings
Emerging evidence has further elucidated optimal care of post-arrest patients including timing for routine coronary angiography, utility of therapeutic hypothermia, permissive hypercapnia, and empiric aspiration pneumonia treatment.
Summary
The complicated state of multi-organ failure following cardiac arrest needs to be carefully optimized by the clinician to prevent further neurologic injury and promote systemic recovery. Future studies should be aimed at understanding if these findings extend to specific patient populations, especially those at the highest risk for poor outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
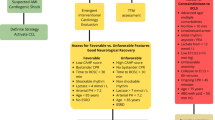
The rising incidence of cardiac arrest (CA) contributes substantially to cardiovascular mortality and reduced quality of life [1, 2]. The American Heart Association (AHA) 2022 Annual Update and Cardiac Arrest Registry to Enhance Survival (CARES) data report a surge in the annual incidence of CA with reduced survival for out-of-hospital cardiac arrests (OHCA) compared to pre-pandemic statistics [1, 3]. Understanding the pathophysiologic, metabolic, and reperfusion-injury cascades post-arrest is imperative to provide optimal care and improve survival. Cardiologists with or without critical care training are often engaged in the care of these complex patients as consultants or the primary care team. Early in-hospital care requires a knowledge of when and in whom revascularization and circulatory support strategies should be used, ventilation techniques, and therapies for multisystem organ dysfunction. Additionally, the post-resuscitation care continuum requires close neurological monitoring, targeted temperature management, supportive care, and neuroprognostication (Fig. 1). In this review, we explore evidence-based practices and multimodal perspectives in post-arrest cares in the intensive care unit (ICU).
Post Cardiac Arrest Care in the Cardiac ICU. Created with BioRender.com
Cardiovascular Specific Interventions
Timing of Coronary Angiography
Acute coronary syndrome (ACS) is a common etiology of CA, accounting for 59–71% of OHCA cases presenting with a shockable rhythm [3,4,5,6,7], and 30–35% of in-hospital cardiac arrest (IHCA) cases [8, 9]. The current guidelines recommend emergent coronary angiography (CAG) for OHCA with suspected cardiac etiology and ST-elevation myocardial infarction (STEMI), cardiogenic shock (CS), or recurrent arrhythmias. There is no delineation on timing of CAG among patients with non-ST segment elevation myocardial infarction (NSTEMI) [10••, 11••, 12]. The likelihood of coronary artery disease (CAD) increases up to 73% in patients with shockable refractory CA [13] potentially increasing the benefit of CAG regardless of EKG findings in this subgroup. Because serologic and echocardiographic markers of coronary ischemia may not be reliable immediately post-arrest [14, 15] and outcomes are heavily reliant on neurologic recovery [16•], predicting who will benefit from early CAG can be difficult. Several trials have compared a delayed (24–96 h or following neurologic recovery) versus an immediate (< 2 h) strategy for timing of CAG in patients without STEMI after OHCA with shockable [17,18,19,20] and non-shockable [18,19,20] rhythms. Neither these trials nor meta-analyses have shown a difference in outcomes with either strategy [17,18,19,20,21,22]. These findings can be reconciled with a non-emergent CAG strategy among OHCA survivors without STEMI, evidence of post-arrest CS, or refractory/recurrent arrhythmias following resuscitation. A more nuanced assessment of ACS risk in conjunction with the use of early neuroprognostication scores may better identify those patients most likely to have neurologic recovery that would allow them to benefit from an early intervention approach (Table 1) [23].
Hemodynamic Management
Post-arrest hypotension and shock occurs in 50–70% of patients [24,25,26]. Hypotension and malperfusion are multifactorial, owing to a combination of acute myocardial injury, myocardial stunning, vascular dysfunction due to systemic inflammatory response, reperfusion injury, metabolic perturbations related to poor perfusion peri-arrest, trauma from cardiopulmonary resuscitation (CPR), and relative adrenal insufficiency. Laurent et al. described the hemodynamics associated with this combination of early myocardial stunning paired with vasoplegia in great detail [27].
Post-arrest myocardial dysfunction or myocardial stunning can occur in addition to or in the absence of myocardial ischemia and may be considered a form of stress-induced cardiomyopathy occurring in up to 69% of survivors of CA [28, 29]. Risk factors include the history of hypertension, prior myocardial infarction (MI), cardiac etiology of arrest, duration of resuscitation, repeated defibrillations, and higher cumulative epinephrine dose [30]. Post-arrest myocardial stunning and associated reduction in cardiac output (CO) may be completely reversible within 48–72 h following ROSC [27, 30]. Vasoplegia following global ischemia–reperfusion injury can compound myocardial stunning. Elevation in cytokines including TNF-α and IL-6 [31], neutrophil activation, coagulation cascade activation, and translocation of endotoxins [32] leads to endothelial cell dysfunction, activation of inducible nitric oxide-synthase, and resultant vascular smooth muscle relaxation [33]. Lactic acidosis from poor end-organ perfusion and respiratory acidosis from hypoventilation during CA may result in reduced responsiveness to endogenous and exogenous catecholamines [34]. This sepsis-like state results in micro-circulatory failure and contributes to post-arrest hypotension [31]. In addition to cardiogenic and vasodilatory shock, Hékimian et al. reported that 42% of post-arrest patients have relative adrenal insufficiency [35] due to direct ischemic adrenal injury and/or inhibitory effects of circulating cytokines [36]. Despite relative adrenal insufficiency among these patients, there is conflicting data regarding the benefit of exogenous steroids [37,38,39,40] and current guidelines do not recommend their routine use [10••].
Initial management of post-arrest shock begins with identifying the presence of cardiogenic and/or vasoplegic components. As with traditional septic shock, post-arrest vasoplegia is treated with fluid resuscitation and vasopressors. There is little evidence comparing vasopressor agents for post-arrest patients; however, guidelines recommend norepinephrine as a first-line agent [10••]. A recent observational comparing epinephrine and norepinephrine in patients with post-arrest shock found epinephrine administration was associated with higher all-cause mortality [41•]. When CS contributes to hemodynamic compromise, either in isolation or with vasoplegia, the addition of inotropes and temporary mechanical circulatory support (tMCS) may be warranted. Dobutamine’s inotropic properties mitigate left ventricular systolic and diastolic dysfunction that occurs as part of post-arrest myocardial stunning [42] while milrinone has not been well studied in this population. Dobutamine having a shorter half-life is better suited for post-arrest management of CS where rapid uptitration is necessary, and as discussed later, renal failure is highly prevalent in this population making milrinone a less ideal agent as its excretion is highly dependent on renal function [43, 44]. As a more established treatment, dobutamine is the inotrope of choice per the European Resuscitation Council guidelines [10••].
Invasive Hemodynamic Monitoring Devices
The approach to hemodynamic monitoring varies based on institutional expertise. Given fluctuations in hemodynamics during the post-resuscitation period, patients often necessitate invasive hemodynamic monitoring including peripheral arterial catheterization, central venous catheterization, arterial pulse waveform analysis, and pulmonary artery catheterization (PAC).
While PAC use has not specifically been studied following CA, its use in the management of undifferentiated shock in ICUs and decompensated heart failure has not shown significant differences in outcomes [45,46,47]. However, more recent data offers a nuanced view of the utility of the PAC in a modern cardiac ICU (CICU) [48, 49]. The use of PAC in patients following CA may provide nuanced hemodynamic profiling in mixed shock states as vasoplegia and myocardial dysfunction are often concomitant [29]; however, more data in this population is necessary.
Temporary Mechanical Circulatory Support
In patients where fluid resuscitation, vasopressor agents, and inotropes are not sufficient to maintain adequate perfusion, and treatable syndromes such as cardiac tamponade and pulmonary embolus have been addressed, the addition of tMCS, including intra-aortic balloon pump (IABP), percutaneous ventricular assist device (VAD) (Impella©), or veno-arterial extracorporeal membranous oxygenation (VA-ECMO), may effectively augment CO to reach hemodynamic goals [50••]. Device use and selection are based largely on the amount of cardiovascular support needed as well as provider and institutional access and expertise. In addition, device selection is patient specific, and patient characteristics may present contraindications to certain devices. For example, a prior mechanical aortic valve replacement or LV thrombus would preclude Impella© or significant aortic insufficiency will preclude the use of IABP and VA ECMO. More general recommendations regarding the decision to initiate tMCS, device selection, and how to escalate tMCS are available [50••]; however, few studies have evaluated the superiority of one circulatory support device over another in the post-arrest setting. A retrospective study of patients from the PROCAT (Parisian Region Out of Hospital Cardiac Arrest) registry with post-arrest shock compared the use of IABP with Impella© and showed no difference in survival; however, patients supported with the Impella© trended towards higher rates of serious bleeding (26% versus 9%), albeit in the setting of therapeutic hypothermia (TH) [49].
VA-ECMO provides the highest level of cardiovascular and pulmonary support [51, 52]. There is conflicting data regarding the efficacy of VA-ECMO as a salvage therapy in the setting of ongoing refractory CA. With the promise of saving 40–45% of patients with refractory shockable OHCA [53], there has been an increased use of VA-ECMO for extracorporeal cardiopulmonary resuscitation (ECPR) [54]. The successful implementation of ECPR depends on appropriate patient selection and dedicated centers with high levels of expertise [13, 55, 56]. Generally, patients who are placed on ECMO during CA remain supported through the aforementioned cardiac stunning period until cardiac function improves.
Implantable Cardioverter Defibrillator
ICD implantation is recommended in patients following successful resuscitation from VF or hemodynamically unstable VT without any completely reversible causes [57, 58]. Approximately, 55% of OHCA patients will have a reversible cause; most commonly MI (55–58%) or electrolyte abnormalities (10–20%) [59, 60], and do not necessarily require an ICD. Despite addressing reversible causes, some patients continue to have ventricular arrhythmias. If these tachyarrhythmias continue > 48 h following an MI in the absence of ongoing ischemia [61] patients are at an increased risk of death compared to those with ventricular tachyarrhythmia < 48 h following MI (HR 20.7 versus 7.45) [62] requiring consideration of ICDs. Current guidelines do not address ICD implantation in post-arrest survivors with reversible causes but persistent arrhythmia after > 48 h [10••, 11••].
Ventilatory Management
Pulmonary complications are common after CA and have a variety of etiologies including cardiogenic pulmonary edema, aspiration pneumonitis, ischemia–reperfusion injury, acute respiratory distress syndrome (ARDS), atelectasis, pneumonia, pulmonary contusion from cardiac compressions, and ventilator-associated lung injuries [63]. In this setting, lung protective mechanical ventilation is necessary for the optimization of lung mechanics, while simultaneously avoiding hyperoxemic free radical production and ameliorating acid–base disturbances to improve the homeostatic milieu for neurologically favorable survival [64].
Tidal Volume
Mechanical ventilation strategies are particularly important post-arrest due to the complex interplay between positive pressure ventilation and intra-thoracic hemodynamics as well as right and left ventricular preload and afterload [65]. The majority of CA patients are supported with lung protective ventilation settings, including low tidal volumes (VT), and minimizing driving and plateau pressures (PPLAT) [66]. Current evidence in post-arrest patients without ARDS is limited with variable findings. The Protective Ventilation in Patients without ARDS (PReVENT) trial randomized patients who received invasive ventilation for indications other than ARDS and comprised nearly 25% of CA patients (N = 230). Low VT strategy, 4–6 mL/kg predicted body weight (PBW), did not result in a difference in number of ventilator-free days or mortality at 28 days compared to an intermediate VT strategy, 10 mL/kg PBW, decreasing by 1 mL/kg PBW per hour if PPLAT > 25 cmH2O [67]. However, observational studies and retrospective reviews have shown that lower VT (≤ 8 mL/kg PBW) is associated with favorable neurological outcomes and more ventilator-free days among OHCA patients [68, 69]. Current expert consensus for post-arrest patients suggests VT between 6 and 8 mL/kg PBW is reasonable [10••] with a primary CO2 goal of normocarbia (PaCO2 35–45 mmHg) and avoidance of hypo or hypercarbia [64].
Positive End-expiratory Pressure (PEEP)
PEEP is the positive pressure that remains in the airway at the end of the respiratory cycle and has important therapeutic implications following CA. Inadequate PEEP increases the risk of atelectasis [70] while excessive PEEP increases intrathoracic pressure causing an unwanted decrease in right ventricular venous return. In addition, PEEP has a complicated relationship with RV afterload and LV hemodynamics that can result in a dramatic reduction in CO and hemodynamic collapse. The net effect of PEEP on ventricular function and CO is unique to each patient and their pathology. Alviar et al. illustrate the principles governing these interactions well in their article published in JACC in 2018 [65]. In a recent sub-analysis of the TTM2 trial comprising 1848 post-arrest patients, PEEP alone was not an independent predictor of mortality in OHCA [71].
Plateau and Driving Pressure
PPLAT and driving pressure (ΔP) reflect end-inspiratory small airway pressure when there is no air movement and distending pressure of the lungs as measured as the difference between PPLAT or and PEEP. Excessive PPLAT or ΔP has been correlated with barotrauma and ventilator-associated lung injuries [72•, 73]. Changes in VT directly affect PPLAT and ΔP, where direction and magnitude are dependent on underlying static compliance of the lung [74]. High ΔP has been independently associated with 6-month mortality among patients after CA [71]. Mechanical power (MP) is a measure of energy transferred from the ventilator to the respiratory system per unit time, computed by minute ventilation, inspiratory flow, peak airway pressure, and PEEP [75, 76]. High MP has independently been associated with higher ICU and hospital mortality, fewer ventilator-free days, and longer ICU and hospital length of stay [77]. Additionally, high MP has shown to be an independent predictor of 6-month mortality in patients following CA [71].
Neurologic Support: Preventing Secondary Brain Injury
Neurologic injury is the largest contributor to mortality and poor neurologic outcomes in patients after CA [25]. As care for patients who have suffered CA has improved, our understanding of primary and secondary brain injury has increased.
Primary brain injury occurs following cessation of cerebral blood flow (CBF) due to depletion of neuronal glucose and oxygen delivery. Anaerobic respiration causes mitochondrial dysfunction, reactive oxygen species (ROS) formation, ATP depletion, and intracellular Ca2+ accumulation. These processes lead to widespread cellular damage, loss of cell polarity integrity, cytotoxic edema, and programmed cell death. Ca2+ release causes the release of the glutamate resulting in neuronal excitotoxicity and further injury [78,79,80] Secondary brain injury occurs after restoration of CBF with return of spontaneous circulation (ROSC). Immediately after ROSC, CBF is characterized by early relative hyperemia followed by hypoperfusion resulting in endothelial dysfunction and microcirculatory abnormalities [78, 80, 81]. Additionally, cerebral autoregulation becomes significantly impaired post-arrest, leading to instability in CBF susceptible to fluctuations in MAP and CO2 [82, 83]. Compounding the ischemic injury, endothelial dysfunction caused by cytotoxic damage leads to microthombi and increased cellular permeability worsening cerebral edema [78]. The goal of post-arrest neurologic care is to minimize secondary brain injury.
Mean Arterial Pressure Goals
Optimal blood pressure targets in this population are unknown; however, they may be of particular importance to ensure adequate CBF in the setting of abnormal cerebral autoregulation and cerebral edema that occurs after anoxic brain injury [84••]. Guidelines recommend avoiding hypotension, SBP < 90 mmHg, or mean arterial pressures (MAP) < 65 mmHg, but lack specific MAP targets. Some have hypothesized MAP targets > 80 mmHg may improve cerebral perfusion [85]; however, the Neuroprotect trial randomized 112 OHCA patients to “early goal-directed hemodynamic optimization” (EGDO) defined as MAP 85–100 mmHg and SvO2 65–75% versus MAP of 65 mmHg. The EGDO group showed improved estimates of cerebral perfusion and oxygenation but failed to reduce anoxic brain injury on MRI or improve neurologic outcomes when compared to the standard-of-care group [86]. Similarly, the BOX trial evaluated MAP targets of 63 mmHg versus 77 mmHg in comatose adults after OHCA and noted no difference between the groups for all-cause death, severe neurological disability, or coma [84••]. This suggests that at a population level, elevated MAP goals do not improve outcomes; however, there are ongoing studies to identify patients who may benefit from higher MAP goals [87]. Generally, hemodynamics should be maintained to optimize end-organ perfusion as demonstrated by urine output > 0.5 mL/kg/h and normal or decreasing lactate [10••, 11••].
Therapeutic Hypothermia and Targeted Temperature Management
TH has been theorized to mitigate secondary brain injury with several experimental models and early human studies suggesting improved neurologic outcomes [88,89,90,91,92]. However, these results have not translated to large randomized human studies. The Targeted Temperature Management (TTM) and TTM2 trials showed no difference in all-cause mortality when comparing TH (32-34°C) versus normothermia (36–37.5°C) for 28 h following ROSC [93, 94••] (Table 2). Hyperthermia is associated with worse neurologic injury following CA [95, 96]. As such, avoidance of hyperthermia has been considered a standard of practice; however, the optimal duration of strict fever avoidance is unknown [97,98,99,100]. Hassanger et al. investigated the role of device-based hyperthermia prevention for 24 h versus 72 h. Their randomized control trial (RCT) of 393 patients showed no difference in mortality or severe disability/coma between the groups (33.6% versus 32.3%) [101]. The guidelines synthesize this data by advocating for TTM with a target temperature of 32 to 36 °C for at least 24 h followed by fever prevention for at least 72 h though adjudication with TTM trial data is expected in future iterations [10••, 11••].
Oxygenation and Ventilation Targets
Post-arrest oxygen targets balance the deleterious effects of tissue hypoxia and reperfusion-related oxygen-derived free radicals. Observational evidence demonstrates higher in-hospital mortality among OHCA patients with hypoxemia (partial pressure of arterial oxygen; PaO2 < 60 mmHg) related to organ tissue hypoxia and hyperoxemia (PaO2 > 300 mmHg) within 24 h following ROSC [102, 103]. In the BOX trial, Schmidt et al. observed similar composite outcomes of death and severe disability/coma, among patients who were treated with restrictive oxygen target (PaO2 68–75 mmHg) after ROSC versus liberal oxygen targets (PaO2 98–105 mmHg) [104••]. Current guidelines recommend a 100% fraction of inspired oxygen (FiO2) initially followed by titration to SpO2 94–98% or a PaO2 75–100 mmHg after reliable pulse oximetry or blood gas values are available [10••, 11••].
Partial pressure of arterial carbon dioxide (PaCO2) is a key determinant of cerebral hemodynamics [105]. Acute hypocapnia induces cerebral vasoconstriction, a rise in cerebral vascular resistance, and a fall in cerebral perfusion [105]. In contrast, an acute rise in PaCO2 induces the opposite effect with increased blood flow to the brain increasing the total intracranial volume potentially exacerbating cerebral edema’s compressive effects [106, 107]. The TAME trial, a large RCT, showed that targeted mild hypercapnia (PaCO2 50–55 mmHg) did not lead to better neurologic outcomes compared to targeted normocapnia in OHCA patients [108••]. A meta-analysis by McKenzie et al. suggested both hyper and hypocarbia were associated with an increased mortality compared to normocarbia in post-arrest patients [109].
Sedation
Sedation selection post-arrest has not been well studied. Guidelines recommend short-acting sedatives and analgesics to not interfere with neuroprognostication [10••, 64]. Ketamine has been described as neuroprotective through its action as an NMDA receptor antagonist [110,111,112,113]. It may be useful in preventing secondary brain injury driven by the upregulation of NMDA receptors, increased intracellular Ca2+, ROS production, and activation of programmed cell death. Several small preclinical studies have shown promise [110, 114]; however, no human trials have investigated this potential mechanism.
Neuroprognostication
Neuroprognostication is a challenge for clinicians and a dynamic process requiring frequent evaluation and multiple testing modalities. Prognostication should be delayed at least 72 h post ROSC and rewarming if TH is used and residual sedation and metabolic abnormalities should have resolved [115••]. Persistent coma 72 h post-arrest does not necessarily equate with poor neurologic prognosis. About 10–22% of patients will awaken after 72 h post-arrest [116,117,118,119,120,121]; with case reports of awakenings after 2 and even 4 weeks [121,122,123,124,125,126,127]. While survival with neurologically favorable outcomes remains modest in the post-arrest population [1, 3], a meticulous understanding of neuroprognostication may help prevent premature withdrawal of life-sustaining treatment in patients who may go on to have a favorable recovery.
Many factors determine the extent of brain injury including time without CPR, time to first responder, length and quality of CPR, comorbid conditions, baseline neurologic function, and post-arrest care during the vulnerable period for secondary brain injury. Many scores have been developed to quantify initial risk which include the CAHP, OHCA, MIRACLE2, and PCAC scores (Table 1) [23, 128,129,130]. Patients with catastrophic brain injury or signs of neurologic recovery may declare themselves early with catastrophic findings on head imaging such as herniation and/or wakefulness respectively. Thus, the goal of neuroprognostic tools focuses on those indeterminate comatose patients without a clear neurologic trajectory.
Neurologic Examination
The neurologic exam remains one of the most useful tools for patients following resuscitation and a daily exam is recommended [10••]. The level of consciousness, pupillary and ocular findings, best motor response, and myoclonus are clinical exam features used in prognostication post-arrest.
Bilaterally absent pupillary light response at least 72 h following ROSC and rewarming, if applicable, portends poor neurologic outcomes. Bilateral absence of the corneal reflex at 72 h is less specific and should not be considered a reliable predictor of outcome due to the high false positive rate [115••]. Similarly, 72 h after ROSC, an absence of the ability to follow commands or extension response should not be considered a reliable predictor of poor outcome [115••].
Myoclonus is the sudden and involuntary contraction of muscle frequently seen in ICU patients [131]. In post-arrest patients, it was once thought to be a sign of extremely poor neurologic outcomes [132, 133]. However, it is now clear this is not the case [134], and myoclonus is not a reliable predictor of poor outcomes [115••]. Status myoclonus, a similar but sustained entity, is defined as spontaneous repetitive generalized multifocal myoclonus in comatose patients lasting ≥ 30 min within 72 h of CA involving the face, limbs, and axial musculature and has traditionally been associated with a poor prognosis [133]. Electroencephalography (EEG) may be able to help identify salvageable subgroups with early or status myoclonus [135].
Neuroimaging
Shortly (< 2 h) after ROSC, a computer tomography (CT) may be obtained to assess for a neurologic etiology of the arrest and signs of catastrophic neurologic injury [11••]; however, early scans are often too soon to see ischemic changes from anoxic injury. Guidelines recommend imaging within 72 h to assess for brain edema which can be quantified with the ratio of the density of the grey matter and the white matter at pre-specified locations [136, 137]. A diffuse pattern of loss of grey-white differentiation with sulcal effacement at least 48 h from ROSC is a moderately reliable predictor of poor outcome [115••].
Similarly, MRI studies can detect neuronal cytotoxic edema. Hyperintensities on diffusion-weighted images (DWI) days 2–7 after ROSC are a moderately reliable predictor of poor outcomes [115••]; however, this modality is limited by conditions such as hyperammonemia, seizures, and status epilepticus as these can also cause cytotoxic edema and DWI hyperintensity [115••].
Electroencephalography
EEG is recommended in all post-arrest patients [10••, 11••, 138]. EEG suppression (background voltage < 10 µV [139]) and burst suppression pattern have been classified as “highly malignant” at 72 h following CA with high specificity (100%) for poor neurologic outcomes but lacked sensitivity (50%) [115••, 140]. The presence of status epilepticus and status myoclonus are no longer invariably associated with poor outcomes [115••]. EEG tracings must be interpreted in the absence of confounders such as hypothermia, ongoing sedation, and metabolic derangements.
Seizures are common in survivors of CA occurring in 10–35% of this population [64, 78, 141]. Aggressive treatment of seizures based on current practice guidelines [142] is recommended [10••]. The TELSTAR trial showed that suppressing rhythmic and periodic EEG activity (non-seizure activity) with the use of anti-seizure medication in survivors of CA showed no benefit [143].
Somatosensory Evoked Potential (SSEP)
SSEP testing is a recommended neurophysiologic study used to neuroprognosticate following CA [10••, 11••, 113]. SSEPs assess the afferent functionality of thalamocortical connections in comatose patients. At least 48 h after ROSC bilateral absence of the cortical response with preservation of the cervical spine response is associated with poor outcome with high specificity and variable sensitivities [115••].
Serum Biomarkers of Neurologic Injury
Several biomarkers have been shown to be markers for severe neuronal injury and poor neurologic outcomes [144]. Neuron-specific enolase (NSE), from neurons and glial cells, and s-100B, from astrocytes, are the only serum biomarkers given a recommendation in published guidelines [10••]. Both are structural proteins released in the setting of hypoxic brain injury. NSE has delayed release following injury thus presenting levels can be normal despite severe injury. Levels at 24 h have the highest specificity for poor neurologic outcomes [145]. Several studies have shown that serial measurements at 48 h and 72 h can be even better predictors and an increase of NSE between any two time points being associated with poor outcomes. On the contrary, patients with decreasing levels of NSE at 48 h are more likely to have a good neurologic recovery [144,145,146]. The ERC/ESICM 2021 guidelines state an NSE > 60 µg/L at 48 h may be associated with poor neurologic outcomes [10••]; while the other guidelines caution against the use of NSE alone, describing it as an unreliable predictor of functional outcome owing to the inconsistency of its predictive value related to various and unclear thresholds [11••, 113].
Multimodal Approach
Neuroprognostication is challenging for clinicians and requires a pragmatic approach. Guidelines strongly recommend a multimodal approach as no single test has sufficient positive predictive value [10••]. This approach includes a combination of clinical exams, neuroimaging, neurophysiological (EEG and SSEP), and biomarker data. This data should be interpreted with assistance from experienced providers familiar with recommended testing modalities.
Other Supportive Measures
Empiric Antibiotic Use
While bacteremia may be common (13–38%) [147, 148], empiric antibiotic use in this setting is not well understood. Pneumonia is even more common occurring in up to 61% of patients [148, 149]. Several RCTs and meta-analyses indicate that the use of prophylactic antibiotics in post-arrest patients does not significantly reduce the length of ICU stay or overall mortality rate [150,151,152]. However, an empiric 2-day course of amoxicillin-clavulanate decreases the incidence of early-onset pneumonia in OHCA patients treated with TH, presumably related to aspiration events during the CA [150, 151]. While the 2015 AHA guidelines do not comment on prophylactic antibiotic use, the ERC/ESICM 2021 guidelines advise against them [10••].
Nutrition
There is scarce data regarding the optimal timing of nutrition initiation in post-arrest patients. Post-resuscitation care including the use of vasopressors and TH if applicable may lead to hypo-perfusion and ischemia of the gastrointestinal system [153, 154]. Furthermore, TH can result in decreased absorption and peristalsis leading to increased gastric residuals and aspiration [155]. On the other hand, early initiation of feeding (within 24 h to 72 h) in general critically ill patients is associated with decreased mortality, decreased infection, and favorable outcomes [156,157,158,159]. In the post-arrest population specifically, studies have shown conflicting results regarding the superiority of early (< 48 h after admission to the ICU) versus delayed (> 48 h after admission to the ICU) feeding in patients treated with TH [160, 161]. The guidelines recommend starting enteral trophic feeding during TH and increasing the rate after rewarming if TH is implemented [10••].
Renal Replacement Therapy (RRT)
Acute kidney injury (AKI) occurs in more than 45% of patients after CA and at least a third of these patients require RRT [162, 163]. AKI has been associated with an increased risk of mortality; however, the relationship between RRT and mortality post-arrest is not as clear [164,165,166,167,168]. Risk factors for AKI post-arrest include increased age, poor baseline renal function, increased resuscitation time, OHCA outside the public setting, initial non-shockable rhythm, and post-resuscitation shock. Currently, there are no specific guidelines regarding the timing of RRT post-arrest and general indications apply. Notably, renal recovery occurs in most survivors [166].
Usual ICU Care
Best practices in general intensive care management should be used, including deep venous thrombosis and stress ulcer prophylaxis [10••, 169, 170]. Optimum blood glucose concentration is unknown in the post-arrest period, but strict glucose control (72–108 mg/dL) has no survival benefit and may be harmful secondary to hypoglycemia [171]. Guidelines have recommended a target glucose level of 140–180 mg/dL [10••, 11••, 172].
Conclusion
Research in post-arrest care and our understanding of ideal management to promote neurologic recovery continues to grow yet much work remains. As our understanding of these complex patients continues to deepen, it is imperative that cardiologists engage in multidisciplinary team approaches to patient care and remain vigilant to their commitment to evidence-based practices and contributing to research endeavors that advance the field.
References
Paudel R, Trinkle CA, Waters CM, Robinson LE, Cassity E, Sturgill JL, et al. Mechanical power: a new concept in mechanical ventilation. Am J Med Sci. 2021;362:537–45.
Giosa L, Busana M, Pasticci I, et al. Mechanical power at a glance: a simple surrogate for volume-controlled ventilation. Intensive Care Med Exp. 2019;7:61.
Tonna JE, Peltan I, Brown SM, Herrick JS, Keenan HT. Mechanical power and driving pressure as predictors of mortality among patients with ARDS. Intensive Care Med. 2020;46:1941–3.
Sandroni C, Cronberg T, Sekhon M. Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis. Intensive Care Med. 2021;47:1393–414.
Bano D, Nicotera P. Ca2+ Signals and neuronal death in brain ischemia. Stroke. 2007;38:674–6.
Sekhon MS, Ainslie PN, Griesdale DE. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a “two-hit” model. Crit Care. 2017;21:90.
van den Brule JMD, van der Hoeven JG, Hoedemaekers CWE. Cerebral perfusion and cerebral autoregulation after cardiac arrest. BioMed Res Int. 2018;2018:4143636.
Sundgreen C, Larsen FS, Herzog TM, Knudsen GM, Boesgaard S, Aldershvile J. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke. 2001;32:128–32.
Battisti-Charbonney A, Fisher J, Duffin J. The cerebrovascular response to carbon dioxide in humans. J Physiol. 2011;589:3039–48.
•• Kjaergaard J, Møller JE, Schmidt H, et al. Blood-pressure targets in comatose survivors of cardiac arrest N Engl J Med. 2022;387:1456–1466. The BOX trial explores liberal vs restrictive mean arterial blood pressure (MAP) targets in patients resuscitated from cardiac arrest, and their association with death, severe disability, or coma.
Niemelä V, Siddiqui F, Ameloot K, et al. Higher versus lower blood pressure targets after cardiac arrest: systematic review with individual patient data meta-analysis. Resuscitation. 2023. https://doi.org/10.1016/j.resuscitation.2023.109862.
Ameloot K, De Deyne C, Eertmans W, et al. Early goal-directed haemodynamic optimization of cerebral oxygenation in comatose survivors after cardiac arrest: the Neuroprotect post-cardiac arrest trial. Eur Heart J. 2019;40:1804–14.
Griesdale DEG, Sekhon MS, Wood MD, et al. Near-infrared spectroscopy to assess cerebral autoregulation and optimal mean arterial pressure in patients with hypoxic-ischemic brain injury: a prospective multicenter feasibility study. Crit Care Explor. 2020;2: e0217.
Leonov Y, Sterz F, Safar P, Radovsky A. Moderate hypothermia after cardiac arrest of 17 minutes in dogs. Effect on cerebral and cardiac outcome. Stroke. 1990;21:1600–6.
Leonov Y, Sterz F, Safar P, Radovsky A, Oku K-I, Tisherman S, et al. Mild cerebral hypothermia during and after cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow Metab. 1990;10:57–70.
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63.
Lascarrou J-B, Merdji H, Le Gouge A, et al. Targeted temperature management for cardiac arrest with nonshockable rhythm. N Engl J Med. 2019;381:2327–37.
Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556.
Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 °C versus 36 °C after cardiac arrest. N Engl J Med. 2013;369:2197–206.
•• Dankiewicz J, Cronberg T, Lilja G, et al. Hypothermia versus normothermia after out-of-hospital cardiac arrest. N Engl J Med. 2021;384:2283–94. The TTM2 trial evaluates the association of targeted hypothermia versus normothermia with death and functional outcomes among patients following cardiac arrest.
Zeiner A, Holzer M, Sterz F, Schörkhuber W, Eisenburger P, Havel C, et al. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch Intern Med. 2001;161:2007–12.
Busto R, Dietrich WD, Globus MY-T, Valdés I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987;7:729–38.
Gebhardt K, Guyette FX, Doshi AA, Callaway CW, Rittenberger JC, Post Cardiac Arrest Service. Prevalence and effect of fever on outcome following resuscitation from cardiac arrest. Resuscitation. 2013;84:1062–7.
Leary M, Grossestreuer AV, Iannacone S, Gonzalez M, Shofer FS, Povey C, et al. Pyrexia and neurologic outcomes after therapeutic hypothermia for cardiac arrest. Resuscitation. 2013;84:1056–61.
Winters SA, Wolf KH, Kettinger SA, Seif EK, Jones JS, Bacon-Baguley T. Assessment of risk factors for post-rewarming “rebound hyperthermia” in cardiac arrest patients undergoing therapeutic hypothermia. Resuscitation. 2013;84:1245–9.
Bro-Jeppesen J, Hassager C, Wanscher M, Søholm H, Thomsen JH, Lippert FK, et al. Post-hypothermia fever is associated with increased mortality after out-of-hospital cardiac arrest. Resuscitation. 2013;84:1734–40.
Hassager C, Schmidt H, Møller JE, et al. Duration of device-based fever prevention after cardiac arrest. N Engl J Med. 2023;388:888–97.
Wang HE, Prince DK, Drennan IR, et al. Post-resuscitation arterial oxygen and carbon dioxide and outcomes after out-of-hospital cardiac arrest. Resuscitation. 2017;120:113–8.
Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, et al. Emergency Medicine Shock Research Network (EMShockNet) Investigators for the. association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303:2165–71.
•• Schmidt H, Kjaergaard J, Hassager C, et al. Oxygen targets in comatose survivors of cardiac arrest N Engl J Med. 2022;387:1467–1476. This paper explores restrictive vs liberal oxygenation strategies in comatose patients after resuscitation for out-of-hospital cardiac arrest.
Reivich M. Arterial Pco2 and cerebral hemodynamics. Am J Physiol-Leg Content. 1964;206:25–35.
Raichle ME, Plum F. Hyperventilation and cerebral blood flow. Stroke. 1972;3:566–75.
Yoon S, Zuccarello M, Rapoport RM. pCO2 and pH regulation of cerebral blood flow. Front Physiol. 2012;3:365.
•• Eastwood G, Nichol AD, Hodgson C, et al. Mild hypercapnia or normocapnia after out-of-hospital cardiac arrest N Engl J Med. 2023;389:45–57. The TAME trial explores ventilation targets post-arrest and association with favorable neurologic outcomes.
McKenzie N, Williams TA, Tohira H, Ho KM, Finn J. A systematic review and meta-analysis of the association between arterial carbon dioxide tension and outcomes after cardiac arrest. Resuscitation. 2017;111:116–26.
Bell JD. In Vogue: ketamine for neuroprotection in acute neurologic injury. Anesth Analg. 2017;124:1237.
Church J, Zeman S, Lodge D. The neuroprotective action of ketamine and MK-801 after transient cerebral ischemia in rats. Anesthesiology. 1988;69:702–9.
Proescholdt M, Heimann A, Kempski O. Neuroprotection of S(+) ketamine isomer in global forebrain ischemia. Brain Res. 2001;904:245–51.
Engelhard K, Werner C, Eberspächer E, Bachl M, Blobner M, Hildt E, et al. The effect of the alpha 2-agonist dexmedetomidine and the N-methyl-D-aspartate antagonist S(+)-ketamine on the expression of apoptosis-regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg. 2003;96:524–531.
Giuliano K, Etchill E, Velez AK, Wilson MA, Blue ME, Troncoso JC, Baumgartner WA, Lawton JS. Ketamine mitigates neurobehavioral deficits in a canine model of hypothermic circulatory arrest. Semin Thorac Cardiovasc Surg. 2023;35:251–8.
•• Rajajee V, Muehlschlegel S, Wartenberg KE, et al. Guidelines for neuroprognostication in comatose adult survivors of cardiac arrest Neurocrit Care. 2023;38:533–563. These recently published guidelines from the Neurocritical Care Society review best practices for neuroprognostication in post-arrest patients including a review of the literature supporting various modalities of assessment, and their reliability for predicting poor neurologic outcomes. The guideline clearly calls for a multimodal approach for assessing this patient population and avoiding early withdrawal of life-sustaining therapy in the immediate post resuscitation period.
Mulder M, Gibbs HG, Smith SW, Dhaliwal R, Scott NL, Sprenkle MD, et al. Awakening and withdrawal of life-sustaining treatment in cardiac arrest survivors treated with therapeutic hypothermia. Crit Care Med. 2014;42:2493–9.
Grossestreuer AV, Abella BS, Leary M, Perman SM, Fuchs BD, Kolansky DM, et al. Time to awakening and neurologic outcome in therapeutic hypothermia-treated cardiac arrest patients. Resuscitation. 2013;84:1741–6.
Paul M, Bougouin W, Dumas F, et al. Comparison of two sedation regimens during targeted temperature management after cardiac arrest. Resuscitation. 2018;128:204–10.
Paul M, Bougouin W, Geri G, Dumas F, Champigneulle B, Legriel S, et al. Delayed awakening after cardiac arrest: prevalence and risk factors in the Parisian registry. Intensive Care Med. 2016;42:1128–36.
Irisawa T, Vadeboncoeur TF, Karamooz M, Mullins M, Chikani V, Spaite DW, et al. Duration of coma in out-of-hospital cardiac arrest survivors treated with targeted temperature management. Ann Emerg Med. 2017;69:36–43.
Lee DH, Cho YS, Lee BK, et al. Late awakening is common in settings without withdrawal of life-sustaining therapy in out-of-hospital cardiac arrest survivors who undergo targeted temperature management*. Crit Care Med. 2022;50:235.
Estraneo A, Moretta P, Loreto V, Lanzillo B, Cozzolino A, Saltalamacchia A, et al. Predictors of recovery of responsiveness in prolonged anoxic vegetative state. Neurology. 2013;80:464–70.
Giacino JT, Katz DI, Schiff ND, et al. Comprehensive systematic review update summary: Disorders of consciousness. Neurology. 2018;91:461–70.
Lybeck A, Cronberg T, Aneman A, et al. Time to awakening after cardiac arrest and the association with target temperature management. Resuscitation. 2018;126:166–71.
Rey A, Rossetti AO, Miroz J-P, Eckert P, Oddo M. Late awakening in survivors of postanoxic coma: early neurophysiologic predictors and association with ICU and long-term neurologic recovery. Crit Care Med. 2019;47:85–92.
Ponz I, Lopez-de-Sa E, Armada E, Caro J, Blazquez Z, Rosillo S, et al. Influence of the temperature on the moment of awakening in patients treated with therapeutic hypothermia after cardiac arrest. Resuscitation. 2016;103:32–6.
Tsai M-S, Chen W-J, Chen W-T, Tien Y-T, Chang W-T, Ong H-N, et al. Should we prolong the observation period for neurological recovery after cardiac arrest? Crit Care Med. 2022;50:389–97.
Maupain C, Bougouin W, Lamhaut L, et al. The CAHP (Cardiac Arrest Hospital Prognosis) score: a tool for risk stratification after out-of-hospital cardiac arrest. Eur Heart J. 2016;37:3222–8.
Adrie C, Cariou A, Mourvillier B, Laurent I, Dabbane H, Hantala F, et al. Predicting survival with good neurological recovery at hospital admission after successful resuscitation of out-of-hospital cardiac arrest: the OHCA score. Eur Heart J. 2006;27:2840–5.
Coppler PJ, Elmer J, Calderon L, Sabedra A, Doshi AA, Callaway CW, et al. Validation of the Pittsburgh Cardiac Arrest Category illness severity score. Resuscitation. 2015;89:86–92.
Sutter R, Ristic A, Rüegg S, Fuhr P. Myoclonus in the critically ill: diagnosis, management, and clinical impact. Clin Neurophysiol. 2016;127:67–80.
Wijdicks EF, Parisi JE, Sharbrough FW. Prognostic value of myoclonus status in comatose survivors of cardiac arrest. Ann Neurol. 1994;35:239–43.
Wijdicks EFM, Hijdra A, Young GB, Bassetti CL, Wiebe S, Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–10.
Seder DB, Sunde K, Rubertsson S, et al. Neurologic outcomes and postresuscitation care of patients with myoclonus following cardiac arrest. Crit Care Med. 2015;43:965–72.
Elmer J, Rittenberger JC, Faro J, Molyneaux BJ, Popescu A, Callaway CW, et al. Clinically distinct electroencephalographic phenotypes of early myoclonus after cardiac arrest. Ann Neurol. 2016;80:175–84.
Sandroni C, D’Arrigo S, Cacciola S, et al. Prediction of poor neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med. 2020;46:1803–51.
Lee BK, Jeung KW, Lee HY, Jung YH, Lee DH. Combining brain computed tomography and serum neuron specific enolase improves the prognostic performance compared to either alone in comatose cardiac arrest survivors treated with therapeutic hypothermia. Resuscitation. 2013;84:1387–92.
Ruijter BJ, Tjepkema-Cloostermans MC, Tromp SC, et al. Early electroencephalography for outcome prediction of postanoxic coma: a prospective cohort study. Ann Neurol. 2019;86:203–14.
Hirsch LJ, Fong MWK, Leitinger M, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2021 Version. [Miscellaneous Article]. J Clin Neurophysiol. 2021;38:1–29.
Westhall E, Rossetti AO, Van Rootselaar A-F, et al. Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology. 2016;86:1482–90.
Rossetti AO, Logroscino G, Liaudet L, Ruffieux C, Ribordy V, Schaller MD, et al. Status epilepticus: an independent outcome predictor after cerebral anoxia. Neurology. 2007;69:255–60.
Glauser T, Shinnar S, Gloss D, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016;16:48–61.
Ruijter BJ, Keijzer HM, Tjepkema-Cloostermans MC, et al. Treating rhythmic and periodic EEG Patterns in comatose survivors of cardiac arrest. N Engl J Med. 2022;386:724–34.
Song H, Bang HJ, You Y, Park JS, Kang C, Kim HJ, et al. Novel serum biomarkers for predicting neurological outcomes in postcardiac arrest patients treated with targeted temperature management. Crit Care. 2023;27:113.
Stammet P, Collignon O, Hassager C, et al. Neuron-specific enolase as a predictor of death or poor neurological outcome after out-of-hospital cardiac arrest and targeted temperature management at 33 °C and 36 °C. J Am Coll Cardiol. 2015;65:2104–14.
Shinozaki K, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Abe R, et al. S-100B and neuron-specific enolase as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation: a systematic review. Crit Care. 2009;13:R121.
Coba V, Jaehne AK, Suarez A, Dagher GA, Brown SC, Yang JJ, et al. The incidence and significance of bacteremia in out of hospital cardiac arrest. Resuscitation. 2014;85:196–202.
Tsai M-S, Chiang W-C, Lee C-C, et al. Infections in the survivors of out-of-hospital cardiac arrest in the first 7 days. Intensive Care Med. 2005;31:621–6.
Perbet S, Mongardon N, Dumas F, et al. Early-onset pneumonia after cardiac arrest. Am J Respir Crit Care Med. 2011;184:1048–54.
François B, Cariou A, Clere-Jehl R, et al. Prevention of early ventilator-associated pneumonia after cardiac arrest. N Engl J Med. 2019;381:1831–42.
Couper K, Laloo R, Field R, Perkins GD, Thomas M, Yeung J. Prophylactic antibiotic use following cardiac arrest: a systematic review and meta-analysis. Resuscitation. 2019;141:166–73.
Gagnon DJ, Nielsen N, Fraser GL, et al. Prophylactic antibiotics are associated with a lower incidence of pneumonia in cardiac arrest survivors treated with targeted temperature management. Resuscitation. 2015;92:154–9.
Wood T, Thoresen M. Physiological responses to hypothermia. Semin Fetal Neonatal Med. 2015;20:87–96.
Powell RW, Dyess DL, Collins JN, Roberts WS, Tacchi EJ, Swafford AN, et al. Regional blood flow response to hypothermia in premature, newborn, and neonatal piglets. J Pediatr Surg. 1999;34:193–8.
Madden LK, Hill M, May TL, Human T, Guanci MM, Jacobi J, et al. The implementation of targeted temperature management: an evidence-based guideline from the Neurocritical Care Society. Neurocrit Care. 2017;27:468–87.
Meinert E, Bell MJ, Buttram S, Kochanek PM, Balasubramani GK, Wisniewski SR, et al. Initiating nutritional support before 72 hours is associated with favorable outcome after severe traumatic brain injury in children: a secondary analysis of a randomized, controlled trial of therapeutic hypothermia. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2018;19:345–52.
Tian F, Heighes PT, Allingstrup MJ, Doig GS. Early enteral nutrition provided within 24 hours of ICU admission: a meta-analysis of randomized controlled trials. Crit Care Med. 2018;46:1049–56.
Doig GS, Heighes PT, Simpson F, Sweetman EA. Early enteral nutrition reduces mortality in trauma patients requiring intensive care: a meta-analysis of randomised controlled trials. Injury. 2011;42:50–6.
McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40:159–211.
Gutierrez A, Carlson C, Kalra R, Elliott AM, Yannopoulos D, Bartos JA. Outcomes associated with delayed enteral feeding after cardiac arrest treated with veno-arterial extracorporeal membrane oxygenation and targeted temperature management. Resuscitation. 2021;164:20–6.
Martin M, Reignier J, Le Thuaut A, et al. Nutrition during targeted temperature management after cardiac arrest: observational study of neurological outcomes and nutrition tolerance. J Parenter Enter Nutr. 2020;44:138–45.
Tujjar O, Mineo G, Dell’Anna A, Poyatos-Robles B, Donadello K, Scolletta S, et al. Acute kidney injury after cardiac arrest. Crit Care. 2015;19:169.
Yanta J, Guyette FX, Doshi AA, Callaway CW, Rittenberger JC, Post Cardiac Arrest Service. Renal dysfunction is common following resuscitation from out-of-hospital cardiac arrest. Resuscitation. 2013;84:1371–4.
Geri G, Guillemet L, Dumas F, et al. Acute kidney injury after out-of-hospital cardiac arrest: risk factors and prognosis in a large cohort. Intensive Care Med. 2015;41:1273–80.
Beitland S, Nakstad ER, Staer-Jensen H, Draegni T, Andersen GØ, Jacobsen D, et al. Impact of acute kidney injury on patient outcome in out-of-hospital cardiac arrest: a prospective observational study. Acta Anaesthesiol Scand. 2016;60:1170–81.
Sandroni C, Dell’Anna AM, Tujjar O, Geri G, Cariou A, Taccone FS. Acute kidney injury after cardiac arrest: a systematic review and meta-analysis of clinical studies Minerva Anestesiol. 2016;82:989–999.
Winther-Jensen M, Kjaergaard J, Lassen JF, Køber L, Torp-Pedersen C, Hansen SM, et al. Use of renal replacement therapy after out-of-hospital cardiac arrest in Denmark 2005–2013. Scand Cardiovasc J SCJ. 2018;52:238–43.
Nielsen N, Sunde K, Hovdenes J, Riker RR, Rubertsson S, Stammet P, et al. Adverse events and their relation to mortality in out-of-hospital cardiac arrest patients treated with therapeutic hypothermia. Crit Care Med. 2011;39:57–64.
Krag M, Marker S, Perner A, et al. Pantoprazole in patients at risk for gastrointestinal bleeding in the ICU. N Engl J Med. 2018;379:2199–208.
Wang Y, Ge L, Ye Z, et al. Efficacy and safety of gastrointestinal bleeding prophylaxis in critically ill patients: an updated systematic review and network meta-analysis of randomized trials. Intensive Care Med. 2020;46:1987–2000.
Oksanen T, Skrifvars MB, Varpula T, Kuitunen A, Pettilä V, Nurmi J, et al. Strict versus moderate glucose control after resuscitation from ventricular fibrillation. Intensive Care Med. 2007;33:2093–100.
American Diabetes Association Professional Practice Committee. 16. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2021;45:S244–53.
Kirkegaard H, Søreide E, de Haas I, et al. Targeted temperature management for 48 vs 24 hours and neurologic outcome after out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2017;318:341–50.
Le May M, Osborne C, Russo J, et al. Effect of moderate vs mild therapeutic hypothermia on mortality and neurologic outcomes in comatose survivors of out-of-hospital cardiac arrest: The CAPITAL CHILL randomized clinical trial. JAMA. 2021;326:1494–503.
Martinell L, Nielsen N, Herlitz J, et al. Early predictors of poor outcome after out-of-hospital cardiac arrest. Crit Care. 2017;21:96.
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–639.
Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol. 2020;76:2982–3021.
Cardiac Arrest Registry to Enhance Survival. Accessed August 13, 2023. https://mycares.net.
Spaulding CM, Joly L-M, Rosenberg A, Monchi M, Weber SN, Dhainaut J-FA, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336:1629–33.
Larsen JM, Ravkilde J. Acute coronary angiography in patients resuscitated from out-of-hospital cardiac arrest—a systematic review and meta-analysis. Resuscitation. 2012;83:1427–33.
Dumas F, Cariou A, Manzo-Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv. 2010;3:200–7.
Davies MJ. Anatomic features in victims of sudden coronary death. Coronary artery pathology Circulation. 1992;85:I19-24.
Wallmuller C, Meron G, Kurkciyan I, Schober A, Stratil P, Sterz F. Causes of in-hospital cardiac arrest and influence on outcome. Resuscitation. 2012;83:1206–11.
Albert M, Herlitz J, Rawshani A, et al. Aetiology and outcome in hospitalized cardiac arrest patients. Eur Heart J Open. 2023;3:oead066.
•• Nolan JP, Sandroni C, Böttiger BW, et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post-resuscitation care. Resuscitation. 2021;161:220–69. The ERC/ESICM guidelines outline the post-resuscitation practices and standard of care post-arrest. The topics covered include etiology/diagnosis of cardiac arrest, oxygenation and ventilation targets, coronary revascularization, hemodynamic monitoring, general intensive care management, prognostication, and rehabilitation post-arrest.
•• Callaway CW, Donnino MW, Fink EL, et al. Part 8: Post–cardiac arrest care. Circulation. 2015;132:S465–82. The AHA Post cardiac guidelines outline best practices and standards of care for post-arrest patients similar to the ERC/ESICM guidelines above. The document includes a comprehensive review of the literature regarding many details of patient care up to the time of publication.
O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary. Circulation. 2013;127:529–55.
Belohlavek J, Smalcova J, Rob D, et al. Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2022;327:737–47.
Gonzalez MR, Esposito EC, Leary M, Gaieski DF, Kolansky DM, Chang G, et al. Initial clinical predictors of significant coronary lesions after resuscitation from cardiac arrest. Ther Hypothermia Temp Manag. 2012;2:73–7.
Dumas F, Manzo-Silberman S, Fichet J, et al. Can early cardiac troponin I measurement help to predict recent coronary occlusion in out-of-hospital cardiac arrest survivors? Crit Care Med. 2012;40:1777–84.
• Pareek N, Kordis P, Beckley-Hoelscher N, et al. A practical risk score for early prediction of neurological outcome after out-of-hospital cardiac arrest: MIRACLE2 Eur Heart J. 2020;41:4508–4517. This paper outlines the MIRACLE2 score, a validated severity score predictive of poor prognosis following cardiac arrest.
Lemkes JS, Janssens GN, van der Hoeven NW, et al. Coronary angiography after cardiac arrest without ST-segment elevation. N Engl J Med. 2019;380:1397–407.
Kern KB, Radsel P, Jentzer JC, Seder DB, Lee KS, Lotun K, et al. Randomized pilot clinical trial of early coronary angiography versus no early coronary angiography after cardiac arrest without ST-segment elevation: The PEARL study. Circulation. 2020;142:2002–12.
Desch S, Freund A, Akin I, et al. Angiography after out-of-hospital cardiac arrest without ST-Segment Elevation. N Engl J Med. 2021;385:2544–53.
Hauw-Berlemont C, Lamhaut L, Diehl J-L, et al. Emergency vs delayed coronary angiogram in survivors of out-of-hospital cardiac arrest: results of the randomized, multicentric EMERGE trial. JAMA Cardiol. 2022;7:700–7.
Al Lawati K, Forestell B, Binbraik Y, et al. Early versus delayed coronary angiography after out-of-hospital cardiac arrest without ST-segment elevation-a systematic review and meta-analysis of randomized controlled trials. Crit Care Explor. 2023;5: e0874.
Goel V, Bloom JE, Dawson L, et al. Early versus deferred coronary angiography following cardiac arrest. A systematic review and meta-analysis. Resusc Plus. 2023;14:100381.
Pareek N, Beckley-Hoelscher N, Kanyal R, et al. MIRACLE2 score and SCAI grade to identify patients with out-of-hospital cardiac arrest for immediate coronary angiography. JACC Cardiovasc Interv. 2022;15:1074–84.
Dumas F, Bougouin W, Geri G, et al. Emergency percutaneous coronary intervention in post-cardiac arrest patients without ST-segment elevation pattern: insights from the PROCAT II Registry. JACC Cardiovasc Interv. 2016;9:1011–8.
Lemiale V, Dumas F, Mongardon N, Giovanetti O, Charpentier J, Chiche J-D, et al. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013;39:1972–80.
Argaud L, Cour M, Dubien P-Y, et al. Effect of cyclosporine in nonshockable out-of-hospital cardiac arrest: the CYRUS randomized clinical trial. JAMA Cardiol. 2016;1:557–65.
Laurent I, Monchi M, Chiche J-D, et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–6.
Ruiz-Bailén M, Aguayo de Hoyos E, Ruiz-Navarro S, Díaz-Castellanos MA, Rucabado-Aguilar L, Gómez-Jiménez FJ, et al. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation. 2005;66:175–81.
Anderson RJ, Jinadasa SP, Hsu L, Ghafouri TB, Tyagi S, Joshua J, Mueller A, Talmor D, Sell RE, Beitler JR. Shock subtypes by left ventricular ejection fraction following out-of-hospital cardiac arrest. Crit Care. 2018;22:162.
Bougouin W, Cariou A. Management of postcardiac arrest myocardial dysfunction. Curr Opin Crit Care. 2013;19:195.
Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–8.
Adrie C, Laurent I, Monchi M, Cariou A, Dhainaou J-F, Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care. 2004;10:208.
Jozwiak M, Bougouin W, Geri G, Grimaldi D, Cariou A. Post-resuscitation shock: recent advances in pathophysiology and treatment. Ann Intensive Care. 2020;10:170.
Robba C, Siwicka-Gieroba D, Sikter A, Battaglini D, Dąbrowski W, Schultz MJ, et al. Pathophysiology and clinical consequences of arterial blood gases and pH after cardiac arrest. Intensive Care Med Exp. 2020;8:19.
Hékimian G, Baugnon T, Thuong M, et al. Cortisol levels and adrenal reserve after successful cardiac arrest resuscitation. Shock. 2004;22:116.
Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348:727–34.
Mentzelopoulos SD, Zakynthinos SG, Tzoufi M, Katsios N, Papastylianou A, Gkisioti S, et al. Vasopressin, epinephrine, and corticosteroids for in-hospital cardiac arrest. Arch Intern Med. 2009;169:15–24.
Mentzelopoulos SD, Malachias S, Chamos C, et al. Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: a randomized clinical trial. JAMA. 2013;310:270–9.
Mentzelopoulos SD, Pappa E, Malachias S, et al. Physiologic effects of stress dose corticosteroids in in-hospital cardiac arrest (CORTICA): a randomized clinical trial. Resusc Plus. 2022;10: 100252.
Donnino MW, Andersen LW, Berg KM, et al. Corticosteroid therapy in refractory shock following cardiac arrest: a randomized, double-blind, placebo-controlled, trial. Crit Care Lond Engl. 2016;20:82.
• Bougouin W, Slimani K, Renaudier M, et al. Epinephrine versus norepinephrine in cardiac arrest patients with post-resuscitation shock. Intensive Care Med. 2022;48:300–10. Bourgouin et al. explore epinephrine or norepinephrine as the continuous intravenous vasopressor of choice to treat post-resuscitation shock.
Kern KB, Hilwig RW, Berg RA, Rhee KH, Sanders AB, Otto CW, et al. Postresuscitation left ventricular systolic and diastolic dysfunction. Circulation. 1997;95:2610–3.
Stroshane RM, Koss RF, Biddlecome CE, Luczkowec C, Edelson J. Oral and intravenous pharmacokinetics of milrinone in human volunteers. J Pharm Sci. 1984;73:1438–41.
DOBUTamine Hydrochloride in 5% Dextrose Injection [Package Insert]. Deerfield, IL: Baxter Healthcare Corp.; 2012.
Richard C, Warszawski J, Anguel N, et al. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome a randomized controlled trial. JAMA. 2003;290:2713–20.
Shah MR, Hasselblad V, Stevenson LW, Binanay C, O’Connor CM, Sopko G, et al. Impact of the pulmonary artery catheter in critically ill patients meta-analysis of randomized clinical trials. JAMA. 2005;294:1664–70.
The ESCAPE Investigators and ESCAPE Study Coordinators*. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness the ESCAPE trial. JAMA. 2005;294:1625–33.
Garan AR, Kanwar M, Thayer KL, et al. Complete hemodynamic profiling with pulmonary artery catheters in cardiogenic shock is associated with lower in-hospital mortality. JACC Heart Fail. 2020;8:903–13.
Kadosh BS, Berg DD, Bohula EA, et al. Pulmonary artery catheter use and mortality in the cardiac intensive care unit. JACC Heart Fail. 2023. https://doi.org/10.1016/j.jchf.2023.04.007.
•• Geller BJ, Sinha SS, Kapur NK, et al. Escalating and de-escalating temporary mechanical circulatory support in cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2022;146:e50–68. This scientific statement from AHA provides expert opinion on the escalation and de-escalation of temporary MCS for cardiogenic shock in contemporary clinical practice.
Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol. 2014;63:2769–78.
Abrams D, MacLaren G, Lorusso R, et al. Extracorporeal cardiopulmonary resuscitation in adults: evidence and implications. Intensive Care Med. 2022;48:1–15.
Yannopoulos D, Bartos J, Raveendran G, et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. The Lancet. 2020;396:1807–16.
ELSO International Summary of Statistics | ECMO | ECLS. https://www.elso.org/registry/internationalsummaryandreports/internationalsummary.aspx. Accessed 3 Aug 2023.
Suverein MM, Delnoij TSR, Lorusso R, et al. Early extracorporeal cPR for refractory out-of-hospital cardiac arrest. N Engl J Med. 2023;388:299–309.
Ubben JFH, Heuts S, Delnoij TSR, et al. ECPR for refractory OHCA – lessons from 3 randomized controlled trials. The trialists' view. Eur Heart J Acute Cardiovasc Care. 2023 Aug;12(8):540–547.
Epstein AE, DiMarco JP, et al. ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities. Circulation. 2008;117:e350–408.
Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2022;43:3997–4126.
Ladejobi A, Pasupula DK, Adhikari S, et al. Implantable defibrillator therapy in cardiac arrest survivors with a reversible cause. Circ Arrhythm Electrophysiol. 2018;11: e005940.
Wyse DG, Friedman PL, Brodsky MA, et al. Life-threatening ventricular arrhythmias due to transient or correctable causes: high risk for death in follow-up. J Am Coll Cardiol. 2001;38:1718–24.
Kusumoto FM, Calkins H, Boehmer J, et al. HRS/ACC/AHA Expert Consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation. 2014;130:94–125.
Piccini JP, White JA, Mehta RH, et al. Sustained ventricular tachycardia and ventricular fibrillation complicating non–ST-segment–elevation acute coronary syndromes. Circulation. 2012;126:41–9.
Johnson NJ, Carlbom DJ, Gaieski DF. Ventilator management and respiratory care after cardiac arrest: oxygenation, ventilation, infection, and injury. Chest. 2018;153:1466–77.
Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: Post–cardiac arrest care. Circulation. 2010;122:S768–86.
Alviar CL, Miller PE, McAreavey D, Katz JN, Lee B, Moriyama B, Soble J, Van Diepen S, Solomon MA, Morrow DA. Positive pressure ventilation in the cardiac intensive care unit. J Am Coll Cardiol. 2018;72:1532–53.
Harmon MB, van Meenen DM, van der Veen AL, et al. Practice of mechanical ventilation in cardiac arrest patients and effects of targeted temperature management: a substudy of the targeted temperature management trial. Resuscitation. 2018;129:29–36.
Writing Group for the PReVENT Investigators. Effect of a low vs intermediate tidal volume strategy on ventilator-free days in intensive care unit patients without ARDS: a randomized clinical trial. JAMA. 2018;320:1872–80.
Sutherasan Y, Peñuelas O, Muriel A, et al. Management and outcome of mechanically ventilated patients after cardiac arrest. Crit Care. 2015;19:215.
Beitler JR, Ghafouri TB, Jinadasa SP, et al. Favorable neurocognitive outcome with low tidal volume ventilation after cardiac arrest. Am J Respir Crit Care Med. 2017;195:1198–206.
Farias LL, Faffe DS, Xisto DG, Santana MCE, Lassance R, Prota LFM, et al. Positive end-expiratory pressure prevents lung mechanical stress caused by recruitment/derecruitment. J Appl Physiol. 2005;98:53–61.
Robba C, Badenes R, Battaglini D, et al. Ventilatory settings in the initial 72 h and their association with outcome in out-of-hospital cardiac arrest patients: a preplanned secondary analysis of the targeted hypothermia versus targeted normothermia after out-of-hospital cardiac arrest (TTM2) trial. Intensive Care Med. 2022;48:1024–38.
• Anzueto A, Frutos-Vivar F, Esteban A, et al. Incidence, risk factors and outcome of barotrauma in mechanically ventilated patients. Intensive Care Med. 2004;30:612–9. This review article explores different parameters of lung-protective ventilation and strategies for optimal ventilation following cardiac arrest.
Ioannidis G, Lazaridis G, Baka S, et al. Barotrauma and pneumothorax J Thorac Dis. 2015;7:S38–43.
Battaglini D, Pelosi P, Robba C. Ten rules for optimizing ventilatory settings and targets in post-cardiac arrest patients. Crit Care. 2022;26:1–11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gonzalez, D., Dahiya, G., Mutirangura, P. et al. Post Cardiac Arrest Care in the Cardiac Intensive Care Unit. Curr Cardiol Rep 26, 35–49 (2024). https://doi.org/10.1007/s11886-023-02015-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-023-02015-0