Abstract
This chapter will review the elements of cardiac arrest resuscitation that begin after return of spontaneous circulation (ROSC). In-hospital mortality of patients who achieve ROSC long enough to be admitted to an intensive care unit (ICU) averages 60% with wide inter-institutional variability (40–80%) (Carr et al., Resuscitation 80:30–34, 2009; Straney et al., Crit Care Resusc 16:104–111, 2014; Nolan et al., Crit Care 20:219, 2016). The pathophysiology of post-cardiac arrest syndrome (PCAS) is composed of four major components: post-cardiac arrest brain injury, post-cardiac arrest myocardial dysfunction, systemic ischemia/reperfusion response, and persistent precipitating pathology (Neumar et al., Circulation 118:2452–2483, 2008). It is important to recognize that each component is potentially reversible and responsive to therapy. A comprehensive multidisciplinary management strategy that addresses all components of PCAS is critical to achieve optimal patient outcome. In addition, a reliable multimodal strategy to prognosticate neurologic outcome in comatose patients is essential to prevent premature cessation of care and enable appropriate resource utilization (Callaway et al., Circulation 132:S465–S482, 2015).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cardiac arrest
- Post-cardiac arrest syndrome
- Hypothermic targeted temperature management
- Percutaneous coronary intervention
- Cardiogenic shock
- Goal-directed resuscitation
- Neuroprognostication
Case Presentation
A 68-year-old male complained to his wife that he was having chest pain while mowing the lawn and then collapsed. She called “911”. When the first responders arrived 5 minutes later, they found the patient to be unresponsive, not breathing, and pulseless. They initiated cardiopulmonary resuscitation (CPR) and applied an automated external defibrillator (AED) on the patient. The AED performed initial rhythm analysis and delivered a shock. After two additional minutes of CPR, the paramedics arrived and found the patient with a palpable pulse and agonal respirations. His systolic blood pressure was 70 mmHg in a narrow complex sinus tachycardia at a rate of 110 beats per minute. Prior to transport, the patient was intubated with a supraglottic airway, and bag-valve ventilation was performed using 100% oxygen. In addition, one liter of normal saline was initiated intravenously. Time from 911 call to ROSC was 9 min.
On arrival to the local hospital, the patient lost his pulse again. The monitor revealed ventricular fibrillation. The patient was defibrillated with a biphasic defibrillator at 200 J. He achieved ROSC with narrow complex tachycardia at a rate of 120 bpm and a blood pressure of 75/40 mmHg. The patient was given 500 cm3 intravenous normal saline bolus, and epinephrine infusion was initiated and titrated to mean arterial pressure (MAP) > 65 mmHg. A femoral arterial line and an internal jugular central venous line were placed. Endotracheal intubation was performed, and the endotracheal tube placement was confirmed with continuous waveform capnography. The patient’s pupils were fixed and dilated, and he had no motor response to painful stimuli. Arterial blood gas demonstrated the following: pH = 7.18 PCO2 = 39 PaO2 = 340 HCO3 − = 16, SpO2 = 100%, Lactate = 8.0. FiO2 was decreased to achieve SpO2 94–96%. A temperature sensing Foley catheter was placed and read 36.0 °C. A 12-lead electrocardiogram (ECG) was obtained (Fig. 2.1).
Question
What interventions should be performed next?
Answer
Immediate coronary angiography with percutaneous coronary intervention (PCI) and hypothermic targeted temperature management (HTTM).
Twelve-lead ECG revealed an acute anteroseptal ST-segment elevation myocardial infarction (STEMI). The patient’s history of chest pain and recurrent episodes of ventricular fibrillation support acute coronary syndrome (ACS) as the cause of cardiac arrest. Interventional cardiology was consulted, and the patient was taken immediately to the coronary catheterization lab. Coronary angiography revealed left anterior descending artery occlusion that was successfully treated with balloon angioplasty and stent placement. An intravascular cooling catheter was placed in coronary angiography laboratory, and its target temperature was set at 33 °C.
Patient was admitted to the cardiac ICU. HTTM was maintained for 24 h at target temperature of 33 °C followed by rewarming to 37 °C over 16 h (0.25 °C/h). The patient was sedated with propofol and fentanyl infusions. Continuous electroencephalogram (EEG) revealed a reactive baseline with intermittent seizures after rewarming that were treated with intravenous lorazepam and valproic acid. Seventy-two hours after rewarming, patient’s neurologic exam revealed reactive pupils, positive corneal reflex, and withdrawal from painful stimuli. Patient began following commands 96 h after rewarming and was extubated on the sixth admission day. He was discharged to short-term rehabilitation on the 9th day with mild short-term memory deficits.
Overview of Post-cardiac Arrest Syndrome (PCAS)
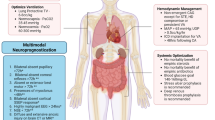
PCAS is a unique pathologic state that consists of varying degrees of post-cardiac arrest brain injury, myocardial dysfunction, systemic ischemia/reperfusion response, and persistent precipitating pathology [1]. The severity of these manifestations will vary in each patient. When ROSC is achieved rapidly, PCAS can be limited or even absent. In contrast, prolonged cardiac arrest can result in PCAS refractory to all interventions. Between these two extremes, each component of PCAS is potentially treatable and reversible. Some post-cardiac arrest interventions such as HTTM may have favorable impact on multiple components of PCAS, while more focused interventions such as early PCI specifically address the precipitating pathology. A comprehensive multidisciplinary approach that targets all PCAS components in the appropriate timeframe is the best strategy to optimize outcomes (Fig. 2.2).
Post-cardiac Arrest Brain Injury
Post-cardiac arrest brain injury is a common cause for poor outcome. One study reported neurologic injury to be the cause of death for 68% of ICU patients following out-of-hospital cardiac arrest and for 23% of patients following in-hospital cardiac arrest [2]. The unique vulnerability of the brain after cardiac arrest is attributed to its intolerance to ischemia as well as unique response to reperfusion. The brain regions most vulnerable to hypoxia and ischemia are the hippocampus, cerebellum, caudoputamen, and cortex [3,4,5,6,7]. The mechanisms of brain injury triggered by cardiac arrest and resuscitation are complex. Cellular pathways including excitotoxicity, disrupted calcium homeostasis, free radical formation, protease cascades, and cell-death signaling are activated over hours to days following ROSC [8,9,10]. The relatively protracted time course of injury cascades and histological changes suggest a broad therapeutic window for neuroprotective strategies following cardiac arrest [1]. Early clinical manifestations include coma, seizures, and myoclonus. Late manifestations range from mild short-term memory deficits to persistent vegetative state and brain death.
Post-cardiac Arrest Myocardial Dysfunction
Post-cardiac arrest myocardial dysfunction is a significant cause of morbidity and mortality after cardiac arrest [2, 11, 12]. Myocardial dysfunction manifests as tachycardia, elevated left ventricular end-diastolic pressure, decreased ejection fraction, reduced cardiac output, and hypotension. Cardiac output tends to improve within 24 h and can return to near normal by 72 h after cardiac arrest in the absence of other pathologies [12]. The responsiveness of post-cardiac arrest myocardial dysfunction to inotropic drugs is well documented in animal studies [13, 14]. However, if ACS or decompensated heart failure were the precipitating factors of the cardiac arrest, the management of post-cardiac arrest myocardial dysfunction becomes more complex.
Systemic Ischemia/Reperfusion Response
The whole body ischemia/reperfusion following cardiac arrest causes generalized activation of immunological and coagulation pathways that increase the risk of multi-organ failure and infection [15, 16]. This condition shares many common features with sepsis [17,18,19,20]. In addition, the activation of coagulation without adequate endogenous fibrinolysis may also contribute to microcirculatory reperfusion disorders after cardiac arrest [21, 22]. Finally, the stress of total body ischemia/reperfusion appears to adversely affect adrenal function [23, 24]. However, the relationship between adrenal dysfunction to cardiac arrest outcome remains controversial. Clinical manifestations of systemic ischemic/reperfusion response include intravascular volume depletion, vasoplegia, and impaired oxygen delivery and consumption.
Persistent Precipitating Pathology
PCAS is commonly associated with persistent precipitating pathology that caused or contributed to the cardiac arrest (Table 2.1). The diagnosis and treatment of precipitating pathologies such as ACS, pulmonary diseases, hemorrhage, sepsis, and drug overdose are often challenging in the setting of PCAS. However, early identification and effective therapeutic intervention are essential to improve patient outcome.
Principles of Management
Hemodynamic Optimization
Post- cardiac arrest patients typically exhibit a mixed state of cardiogenic, distributive, and hypovolemic shock. Furthermore, refractory shock can persist unless the underlying precipitating pathology has been definitively treated. Early hemodynamic optimization is essential to prevent re-arrest, secondary brain injury, and multi-organ failure. A goal-directed approach using physiologic parameters to achieve adequate oxygen delivery has been associated with improved outcomes [25, 26]. This involves optimizing preload, arterial oxygen content, afterload, ventricular contractility, and systemic oxygen utilization. Appropriate monitoring includes continuous intra-arterial pressure monitoring, arterial and central venous blood gases, urine output, lactate clearance, and bedside echocardiography. When feasible, diagnostic studies to rule out treatable persistent precipitating pathology should be performed to enable prompt treatment initiation.
The optimal MAP for post-cardiac arrest patients has not been defined by prospective clinical trials and should be individualized for each patient. Due to the disruption of cerebrovascular autoregulation in post-cardiac arrest patients, higher cerebral perfusion pressure may be needed to provide adequate cerebral blood flow [27]. In contrast, ongoing ACS and heart failure can be exacerbated by targeting a MAP higher than necessary to maintain adequate myocardial perfusion. Higher systolic and MAP during the first 24 h after ROSC are associated with better outcomes in descriptive studies [28,29,30]. In terms of goal-directed strategies, good outcomes have been achieved in published studies where the MAP target range was low as 65–75 mmHg [16] to as high as 90–100 mm [31,32,33] for patients admitted after out-of-hospital cardiac arrest. A recent prospective cohort study found that good neurologic outcome at hospital discharge was significantly higher in patients with a MAP > 90 mmHg during first 6 h after ROSC compared to MAP 70–90 mmHg, and that MAP > 90 mmHg was associated with good neurologic function [33]. Further study is necessary to determine the optimal strategy to achieve higher MAP targets personalized to each patient’s pre-cardiac arrest baseline blood pressure.
For patients with evidence of inadequate oxygen delivery or hypotension, preload optimization is the first intervention. Preload optimization is typically achieved using intravenous crystalloid boluses guided by non-invasive bedside assessment of cardiac function and volume responsiveness. Hypotonic fluids can exacerbate cerebral edema and thus should be avoided.
Vasopressor and inotrope infusions should be initiated early in severe shock states and when there is an inadequate response to preload optimization. No agent or combination of agents has been demonstrated to be superior or improve outcomes. A reasonable approach is norepinephrine as the first line vasopressor supplemented by dobutamine or epinephrine infusion for inotropic support guided by bedside echocardiography or pulmonary artery catheter.
When shock is refractory to preload optimization and vasoactive drugs, it is critical to identify and treat any acute persistent pathology that caused the cardiac arrest. If no such pathology exists or the patient is too unstable to undergo definitive intervention, then mechanical circulatory support can be considered if consistent with goals of care (see “Evidence Contour”).
Mechanical Ventilator Strategy
Optimal ventilator strategy of post-cardiac arrest patients is critical for optimal patient outcome. Abnormal arterial tensions of oxygen and CO2 have been associated with poor neurologic outcome after cardiac arrest by contributing to secondary brain injury [28, 30, 33,34,35,36,37,38]. Furthermore, cardiac arrest patients are at risk for the development of acute respiratory disease syndrome (ARDS) due to PCAS and high incidence of aspiration [39], pulmonary contusion from CPR [40], mechanical ventilation [41, 42], and infection [43, 44]. Thus, prompt optimization of mechanical ventilator strategy is paramount for the improvement of post-cardiac arrest patient outcome.
Hypoxemia can cause secondary cerebral hypoxia while hyperoxemia can increase oxidative injury in the brain. Both hypoxemia (PaO2 < 60 mmHg) and hyperoxemia (PaO2 > 300 mmHg) have been associated with worse outcomes in post-cardiac arrest patients [37]. Furthermore, there appeared to be a linear relationship between PaO2 and in-hospital mortality and functional dependence in these patients [34]. In a single-center, open-label, randomized trial conducted in Italy, ICU patients who received conservative oxygen therapy with goal PaO2 between 70 and 100 mmHg or SpO2 between 94 and 98% had lower ICU mortality than those who received conventional oxygen therapy of PaO2 up to 150 mmHg or SpO2 between 97 and 100% [45]. Given these data, it is reasonable to titrate FiO2 to maintain SpO2 ≥ 94% and PaO2 of around 100 mmHg for post-cardiac arrest patients.
The impact of PaCO2 on cerebrovascular blood flow is maintained in post-cardiac arrest patients. Therefore, hypocarbia can cause secondary brain ischemia through cerebral vasoconstriction and is associated with worse outcomes [46, 47]. In observational studies, PaCO2 in a normal range (35–45 mmHg) when measured at 37 °C is associated with better outcomes than higher or lower PaCO2 [48]. In addition, hyperventilation can also raise intrathoracic pressure by increasing intrinsic positive end-expiratory pressure (PEEP) , thereby worsen venous return, increase right ventricular afterload, and reduce cardiac output [49]. It is unknown if higher PaCO2 contributes to vasodilation, hyperemia, or cerebral edema, but one prospective observational study found both hypocarbia (PaCO2 ≤ 30 mmHg) and hypercapnia (PaCO2 ≥ 50 mmHg) during first 24 h after ROSC to be common and independently associated with poor neurologic outcome at hospital discharge [36]. Because of the temperature correction during HTTM, the actual PaCO2 may be lower than the PaCO2 measured in the laboratory at 37 °C, making 40 mmHg a safer target at all temperatures than 35 mmHg.
The epidemiology of lung injury and ARDS after cardiac arrest has not been well characterized [49]. Currently, there are no studies to specify which ventilator modes or tidal volumes are optimal for patients resuscitated from cardiac arrest. In a secondary analysis of three prospective observational studies, higher tidal volume and higher plateau pressure with lower PEEP were associated with ARDS and pneumonia during ICU stay of post-cardiac arrest patients [50].
In summary, oxygenation and ventilation goals for post-cardiac arrest patients should be normoxia and normocarbia with a low tidal volume lung protective ventilator strategy to optimize neurologic and pulmonary outcome. The detection and prompt management of pulmonary infections are also critical due to the multiple ARDS risk factors of post-cardiac arrest patients. Titration of mechanical ventilation should be aimed at achieving a PaCO2 of 40–45 mmHg, PaO2 of around 100 mmHg, and SpO2 ≥ 94%. A general approach of using assist control mode with a tidal volume goal in 6–8 cm3/kg of predicted body weight is considered standard for those without ARDS, and 4–8 cm3/kg for those with ARDS. PEEP can be uptitrated from 5 cm H2O based on patient body mass index, degree of lung injury, and hemodynamics (Table 2.2).
Management of Acute Coronary Syndrome (ACS)
ACS is a common cause of out-of-hospital cardiac arrest, but making the diagnosis in a comatose post-cardiac arrest patient presents unique challenges. A previous history of coronary artery disease, significant risk factors, and/or symptoms prior to the cardiac arrest can contribute to clinical suspicion, but the absence of these does not exclude ACS as the cause of cardiac arrest. A standard 12-lead ECG should be obtained as soon as possible after ROSC, with additional right-sided and/or posterior leads as indicated. Immediate PCI is indicated in patients meeting STEMI criteria regardless of neurologic status [51]. In addition, immediate coronary angiography should be considered in post-cardiac arrest patients whose ECG do not meet STEMI criteria but there is a high clinical suspicion for ACS (see “Evidence Contour”). Medical management of ACS is the same as for non-cardiac arrest patients.
Hypothermic Targeted Temperature Management (HTTM)
HTTM is recommended for comatose adult patients who achieve ROSC following cardiac arrest independent of presenting cardiac rhythm or location of cardiac arrest [51]. Based on current clinical evidence, providers should select a constant target temperature between 32 and 36 °C and maintain that temperature for at least 24 h [32, 52,53,54]. Rewarming should be no faster than 0.5 °C/h, and fever should be prevented for at least 72 h after ROSC [51].
When the decision is made to treat a comatose post-cardiac arrest patient with HTTM, efforts to achieve and maintain target temperature should be as soon as feasible. In the emergency department, practical methods of rapidly inducing hypothermia include cold intravenous fluid, ice packs (applied to the neck, inguinal areas, and axilla), fan cooling of dampened exposed skin, cooling blankets underneath and on top of the patient, and disabling of ventilator warming circuits [1]. Rapid intravenous infusion of limited volumes of 4 °C isotonic fluid facilitates induction of hypothermia for patients without systolic dysfunction, but additional measures are needed to maintain hypothermia. No one cooling strategy or device has been demonstrated to result in superior clinical outcomes [55]. A number of automated surface cooling devices are now available that use chest/thigh pads and continuous temperature feedback from bladder or esophageal temperature probes [56]. Although more invasive, automated endovascular cooling systems are also available that require placement of a central venous catheter and offer tighter control of temperature at target [55]. Target core body temperature is best monitored by an indwelling esophageal temperature probe, but can be monitored by a temperature-sensitive bladder catheter if adequate urine output is present.
Induction of hypothermia may lead several side effects that include shivering, bradycardia, hyperglycemia, diuresis, and electrolyte derangements such as hypokalemia and hypophosphatemia [57]. Shivering, which inhibits cooling, can be prevented with sedation and neuromuscular blockade. Due to the common occurrence of seizures in post-cardiac arrest patients, continuous EEG is strongly encouraged in comatose patient especially in those receiving sedatives and/or paralytics [51]. Although hypokalemia and hypophosphatemia are common during hypothermia, electrolyte repletion must be done with close monitoring for hyperkalemia during rewarming due to extracellular shifts.
HTTM implementation is best performed with protocol-drive therapy [25, 26]. No specific cooling technique has been demonstrated to result in superior patient outcomes. Therefore, the best strategy is the one that is most feasible to consistently implement at each institution. Although there are no absolute contraindications to HTTM after cardiac arrest, relative contraindications may include uncontrolled bleeding or refractory shock. In these cases, the target temperature can be increased to 36 °C or normothermia. Table 2.3 provides a practical checklist for HTTM implementation during each phase.
Glycemic Control
Hyperglycemia is common in post-cardiac arrest patients especially during HTTM due to insulin resistance. Both hyper- and hypoglycemia have been strongly associated with poor neurologic outcomes [58,59,60,61]. One randomized trial in post-cardiac arrest patients compared strict (72–108 mg/dL) versus moderate (108–144 mg/dL) glucose control and found no difference in 30-day mortality [62]. Based on available evidence, moderate glucose management strategies (140–180 mg/dL) in place for most critically ill patients is sufficient for post-cardiac arrest patients (Table 2.2).
Seizure Management
Seizures, nonconvulsive status epilepticus , and other epileptiform activity occur in 12–22% of comatose post-cardiac arrest patients. These neurologic complications may contribute to secondary brain injury and prevent patient awakening [51]. There is no direct evidence that post-cardiac arrest seizure prophylaxis is effective or improves outcomes for these patients. However, prolonged epileptiform discharges are associated with secondary brain injury in other conditions, making the detection and treatment of nonconvulsive status epilepticus a priority [63]. Continuous EEG, initiated as soon as possible following ROSC, should be strongly considered in all comatose survivors of cardiac arrest treated with HTTM to detect epileptiform discharges, diagnose seizures, and assist with neuroprognostication. Treatment of seizures in post-cardiac arrest patients can be challenging, as they tend to be refractory to single agent therapy due to the severity of brain injury. As there are no trials specifically comparing seizure treatments after cardiac arrest, the same antiepileptic agents and approaches for status epilepticus can be used for post-cardiac arrest seizures.
Neuroprognostication
Reliable prognostication of neurologic recovery in patients who remain comatose after cardiac arrest is a significant clinical challenge that impacts resource utilization and patient outcome. Available neuroprognostication tools include clinical examination, somatosensory evoked potential (SSEP), EEG, imaging studies, and serum biomarkers. Most experts agree that the recovery of Glasgow Motor Score (GMS) to flexion or better (i.e. >2) is a good prognostic sign. In patients who do not recover a GMS ≥ 2 by 72 h after ROSC, the most reliable predictors of poor neurologic outcome (defined by a Cerebral Performance Category score of 3–5) are listed in Table 2.4 [64].
The performance of neuroprognostic tools is highly dependent on the interval between ROSC and the measurement. For example, bilateral absence of pupillary light reflex has a false positive rate (FPR) for predicting poor neurologic outcome of 32% [95% CI 19–48%] at the time of hospital admission versus a FPR of 1% [0–3%] when measured 72 h after ROSC [64]. In addition to timing, confounders such as sedation and neuromuscular blockade, hypothermia, metabolic derangements, shock, and renal insufficiency can also cause delays in patient awakening. Recent retrospective studies have shown HTTM to be associated with variable delay in awakening beyond 3 days after ROSC or rewarming [65,66,67,68,69,70]. Taken together, decisions to limit care based on neurologic prognosis should not be made prior to 72 h after ROSC and at least 12 h after rewarming and cessation of all sedative/paralytic medications [71]. Finally, all available clinical data on the reliability of prognostication tools is limited by the potential for a self-fulfilling prophecy that occurs when these tools are used to limit care.
Neuroprognostication is best done with a multimodal approach, and the decisions to limit care should never be based on the results of a single prognostication tool [51]. Although a number of published guidelines from professional societies are available, the field is rapidly evolving. Therefore, post-cardiac arrest neuroprognostication should be guided by consultation with an experienced specialist [71, 72]. Establishing a consistent institutional approach based on available resources and multidisciplinary expertise will optimize both resource utilization and patient outcome.
Organ Donation
Patients who are initially resuscitated from cardiac arrest but subsequently die or meet brain death criteria should be evaluated as potential organ donors. Multiple studies have found no difference in immediate or long-term function of transplanted organs from donors who reach brain death after cardiac arrest compared to donors who reach brain death from other causes [73]. A meta-analysis of 23,388 patients resuscitated from cardiac arrest showed an overall 41.8% organ donation rate among the 1830 brain dead patients [74]. Patients who have withdrawal of life-sustaining therapy after initial resuscitation from cardiac arrest based on reliable neuroprognostication of futility or as part of advanced directives may also be candidates for donation after cardiac death. The list of potential organs successfully donated from the patients has expanded from primarily kidneys and liver to heart, lungs, and intestine. Finally, tissue donation (cornea, skin, and bone) is almost always possible from post-cardiac arrest patients after death.
Evidence Contour
Patient Selection for Emergent Coronary Angiography
Multiple observational studies have reported better survival in both STEMI and non-STEMI post-cardiac arrest patients who undergo immediate coronary angiography and PCI (when indicated) compared to those who did not undergo immediate coronary angiography [75,76,77]. The strength of the recommendation for post-cardiac arrest patients who meet STEMI criteria is based on extrapolation of the positive survival benefits for non-cardiac arrest patients. Conversely, the reluctance of interventional cardiologists to perform immediate coronary angiography in non-STEMI patients is similarly based on the lack of proven benefit in non-cardiac arrest patients. However, it should be recognized that the post-cardiac arrest patients are higher risk and do not always have ST-elevation with an acutely occluded culprit lesion [78]. In a large international post-cardiac arrest registry that included patients undergoing immediate coronary angiography with and without STEMI, an occluded culprit vessel was found in 74.3% of patients with STEMI and 22.9% of patients without STEMI [77]. In a single center cohort study of refractory out-of-hospital shockable cardiac arrest patients who required extracorporeal life support, 84% had significant coronary artery disease, 64% had acute thrombotic lesions, and 84% received PCI [79]. This high incidence of occluded culprit lesions in combination with post-cardiac arrest myocardial dysfunction and the need to maintain adequate cerebral perfusion pressure argues strongly for immediate coronary angiography in any post-cardiac arrest patient when there is a clinical suspicion of ACS.
The Coronary Angiography after Cardiac Arrest without ST-Segment Elevation (COACT) study found no difference in the 90-day survival of comatose out-of-hospital cardiac arrest patients with shockable rhythm and non-STEMI presenting ECG who received immediate (median average of 2.3 h from cardiac arrest) versus delayed (median average of 121.9 h from cardiac arrest) coronary angiography [80]. However, acute unstable coronary lesions were present in less than 20% of total study cohort, and PCI were performed in less than 40% of the patients. Furthermore, it was unclear how many of the study subjects truly had initial shockable rhythm because the screening data were only collected during the final phase of the study. Thus, only 10.2% patients could be confirmed as meeting inclusion criteria of initial shockable rhythm. Eight additional randomized multicenter studies are now planned or underway as of 2019 to evaluate the potential survival benefit of early coronary angiography after out-of-hospital cardiac arrest in patients without ST-segment elevation. These studies include ACCESS (NCT03119571), DISCO-2 (NCT02309151), EMERGE (NCT02876458), and others. Together, these trials will collectively enroll >5000 patients and help define clinical practice in the future [81].
Patient Selection for HTTM
Although international guidelines consistently recommend treating all comatose post-cardiac arrest patients with HTTM, this practice has not been universally implemented. The two major clinical trials demonstrating efficacy of HTTM limited enrollment to witnessed out-of-hospital cardiac arrest with shockable presenting rhythm [32, 54]. There have been no prospective randomized clinical trials comparing HTTM to normothermia (or no active temperature management) in out-of-hospital cardiac arrest patients with non-shockable presenting rhythms or in in-hospital cardiac arrest patients with any presenting rhythm. Therefore, these recommendations represent an extrapolation of the evidence in patients with witnessed out-of-hospital shockable cardiac arrest. This extrapolation is supported by clinical observational studies that suggest benefit in these patient populations and robust animal data demonstrating the neuroprotective effect of HTTM in cardiac arrest models regardless of rhythm [82, 83]. In addition, HTTM has a relatively safe side effect profile limited primarily to shivering and electrolyte shifts.
For intubated comatose cardiac arrest survivors managed in the ICU setting, the incremental intensity of care and cost is modest. One potential concern for treating all comatose post-cardiac arrest patients with HTTM is the delay in prognostication of poor outcome and decision to limit care. However, there is international consensus that neuroprognostication should not be performed before 72 h after ROSC. Therefore, awaiting completion of HTTM therapy does itself delay neuroprognostication. Moreover, HTTM could delay the metabolism of sedative and paralytic agents. Therefore, some experts have recommended delaying prognostication until 72 h after rewarming [51], which could cause significant delays in neuroprognostication. However, the initiation of HTTM should not impact decisions to limit care that are based criteria other than neurologic prognosis, such as terminal illness or previously unrecognized advanced directives. In such cases, the decision to discontinue HTTM can be considered in the same way as the decision to discontinue mechanical ventilation or intravenous vasopressor therapy.
HTTM Optimization
Most experts agree that the optimal time to the initiation, maintenance, and duration of HTTM is unknown. Animal studies have shown that earlier initiation of HTTM and time to target temperature of within 4 h after ROSC to improve survival with good neurologic function [84]. In addition, the initiation of intra-arrest hypothermia may reduce brain injury [85,86,87,88,89,90]. However, these findings have not been consistently observed in human subjects. Even in the two landmark clinical trials, the time to target temperature was discordant. The time to target temperature was reported to be ≤2 h in the study by Bernard et al., while the median time to target temperature was 8 h in the HACA Trial (IQR 4–16 h) [32, 54]. Rapid infusion of prehospital cold intravenous saline immediately after ROSC by paramedics had no impact on outcomes in two randomized clinical trials [91, 92]. Observational studies examining the time to target temperature are not informative because neurologically devastated patients are easier to cool due to their loss of temperature autoregulation. Based on available preclinical evidence [84], achieving target temperature within 4 h of ROSC is a reasonable goal.
The target temperature of 32–34 °C in the original guidelines was based on the target temperature used in the Bernard and HACA studies [32, 54]. Subsequent to that, a large multicenter studied published by Nielsen et al. showed no difference in outcome between comatose post-cardiac arrest patients who were cooled to 33 °C compared to 36 °C [53]. It is for this reason that the recommended target temperature range was expanded to 32–36 °C [51]. Subsequent retrospective studies have since reported lower compliance with target temperature, increase of average lowest temperature during first 24 h in the ICU, higher rates of fever, and associated increase of in-hospital mortality after changing target temperature from 33 to 36 °C [93, 94]. Thus, although it is theoretically easier to use a higher target temperature, doing so does not obviate the need for use of active cooling techniques guided by continuous temperature monitoring to avoid fever. For patients presenting with a nonshockable rhythm, a target temperature of 33 °C resulted in nearly twice as many individuals (10.2%) alive at 90 days with a favorable neruologic status than the group treated with targeted normothermia [102].
The recommended duration of HTTM is again based on the durations used in major clinical trials. Bernard et al. maintained their study subjects for 12 h at 33 °C, while the HACA study maintained its subjects for 24 h between 32 and 34 °C. More recently, Kirkegaard et al. compared HTTM maintenance duration of 24 h versus 48 h at 33 ± 1 °C and found no difference in the survival and neurologic outcome between the two groups [52]. The optimization of HTTM requires additional research, and it is likely that a personalized approach based on severity of neurologic injury and response to therapy will emerge as the best strategy. Upcoming trials such as TTM2 (NCT02908308), which will compare a target temperature of 33 °C to early treatment of fever (≥37.8 °C) and FROST (NCT02035839), which will compare target temperatures of 32 °C, 33 °C, and 34 °C after ROSC for out-of-hospital cardiac arrests with initial shockable rhythm will further elucidate the optimal HTTM strategy.
Mechanical Support for Refractory Cardiac Arrest or Post-cardiac Arrest Cardiogenic Shock
A number of options can be considered for mechanical circulatory support in post-cardiac arrest patients with refractory cardiogenic shock. An intra-aortic balloon pump (IABP) can enhance cardiac output by approximately 0.5 L/min in cardiogenic shock. Despite early evidence of improved outcomes when IABP was used after myocardial infarction, recent studies have not shown a 30-day or 12-month mortality benefit [95, 96]. The newer peripheral ventricular assist devices Tandem Heart™ PVAD and Impella™ Recovery are reported to augment cardiac output by 3.5 and 2.5 L/min, respectively. These devices gain access to the central circulation via large cannulas, which then use either a centrifugal pump (Tandem Heart™) or catheter motor with inlet and output (Impella™) via the aorta. In centers with specialty expertise, complete circulatory support may be performed using veno-arterial extracorporeal membrane oxygenation during cardiopulmonary resuscitation or as a bridge to transplantation or recovery [79, 97].
References
Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118(23):2452–83.
Laver S, Farrow C, Turner D, et al. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30(11):2126–8.
Pulsinelli WA. Selective neuronal vulnerability: morphological and molecular characteristics. Prog Brain Res. 1985;63:29–37.
Brierley JB, Meldrum BS, Brown AW. The threshold and neuropathology of cerebral “anoxic-ischemic” cell change. Arch Neurol. 1973;29(6):367–74.
Blomqvist P, Wieloch T. Ischemic brain damage in rats following cardiac arrest using a long-term recovery model. J Cereb Blood Flow Metab. 1985;5(3):420–31.
Taraszewska A, Zelman IB, Ogonowska W, et al. The pattern of irreversible brain changes after cardiac arrest in humans. Folia Neuropathol. 2002;40(3):133–41.
Hossmann KA, Oschlies U, Schwindt W, et al. Electron microscopic investigation of rat brain after brief cardiac arrest. Acta Neuropathol. 2001;101(2):101–13.
Neumar RW. Molecular mechanisms of ischemic neuronal injury. Ann Emerg Med. 2000;36(5):483–506.
Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79(4):1431–568.
Bano D, Nicotera P. Ca2+ signals and neuronal death in brain ischemia. Stroke. 2007;38(2):674–6.
Herlitz J, Ekstrom L, Wennerblom B, et al. Hospital mortality after out-of-hospital cardiac arrest among patients found in ventricular fibrillation. Resuscitation. 1995;29(1):11–21.
Laurent I, Monchi M, Chiche JD, et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40(12):2110–6.
Kern KB, Hilwig RW, Berg RA, et al. Postresuscitation left ventricular systolic and diastolic dysfunction. Treatment with dobutamine. Circulation. 1997;95(12):2610–3.
Huang L, Weil MH, Tang W, et al. Comparison between dobutamine and levosimendan for management of postresuscitation myocardial dysfunction. Crit Care Med. 2005;33(3):487–91.
Cerchiari EL, Safar P, Klein E, et al. Visceral, hematologic and bacteriologic changes and neurologic outcome after cardiac arrest in dogs. The visceral post-resuscitation syndrome. Resuscitation. 1993;25(2):119–36.
Adams JA. Endothelium and cardiopulmonary resuscitation. Crit Care Med. 2006;34(12):S458–65.
Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106(5):562–8.
Adrie C, Laurent I, Monchi M, et al. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care. 2004;10(3):208–12.
Gando S, Nanzaki S, Morimoto Y, et al. Out-of-hospital cardiac arrest increases soluble vascular endothelial adhesion molecules and neutrophil elastase associated with endothelial injury. Intensive Care Med. 2000;26(1):38–44.
Geppert A, Zorn G, Karth GD, et al. Soluble selectins and the systemic inflammatory response syndrome after successful cardiopulmonary resuscitation. Crit Care Med. 2000;28(7):2360–5.
Bottiger BW, Motsch J, Bohrer H, et al. Activation of blood coagulation after cardiac arrest is not balanced adequately by activation of endogenous fibrinolysis. Circulation. 1995;92(9):2572–8.
Adrie C, Monchi M, Laurent I, et al. Coagulopathy after successful cardiopulmonary resuscitation following cardiac arrest: implication of the protein C anticoagulant pathway. J Am Coll Cardiol. 2005;46(1):21–8.
Hekimian G, Baugnon T, Thuong M, et al. Cortisol levels and adrenal reserve after successful cardiac arrest resuscitation. Shock. 2004;22(2):116–9.
Schultz CH, Rivers EP, Feldkamp CS, et al. A characterization of hypothalamic-pituitary-adrenal axis function during and after human cardiac arrest. Crit Care Med. 1993;21(9):1339–47.
Gaieski DF, Band RA, Abella BS, et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80(4):418–24.
Sunde K, Pytte M, Jacobsen D, et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73(1):29–39.
Sundgreen C, Larsen FS, Herzog TM, et al. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke. 2001;32(1):128–32.
Kilgannon JH, Roberts BW, Reihl LR, et al. Early arterial hypotension is common in the post-cardiac arrest syndrome and associated with increased in-hospital mortality. Resuscitation. 2008;79(3):410–6.
Beylin ME, Perman SM, Abella BS, et al. Higher mean arterial pressure with or without vasoactive agents is associated with increased survival and better neurological outcomes in comatose survivors of cardiac arrest. Intensive Care Med. 2013;39(11):1981–8.
Kilgannon JH, Roberts BW, Jones AE, et al. Arterial blood pressure and neurologic outcome after resuscitation from cardiac arrest∗. Crit Care Med. 2014;42(9):2083–91.
Oddo M, Schaller MD, Feihl F, et al. From evidence to clinical practice: effective implementation of therapeutic hypothermia to improve patient outcome after cardiac arrest. Crit Care Med. 2006;34(7):1865–73.
Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63.
Roberts BW, Kilgannon JH, Hunter BR, et al. Association between elevated mean arterial blood pressure and neurologic outcome after resuscitation from cardiac arrest: results from a multicenter prospective cohort study. Crit Care Med. 2019;47(1):93–100.
Kilgannon JH, Jones AE, Parrillo JE, et al. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011;123(23):2717–22.
Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165–71.
Roberts BW, Kilgannon JH, Chansky ME, et al. Association between postresuscitation partial pressure of arterial carbon dioxide and neurological outcome in patients with post-cardiac arrest syndrome. Circulation. 2013;127(21):2107–13.
Roberts BW, Kilgannon JH, Hunter BR, et al. Association between early hyperoxia exposure after resuscitation from cardiac arrest and neurological disability: prospective multicenter protocol-directed cohort study. Circulation. 2018;137(20):2114–24.
Trzeciak S, Jones AE, Kilgannon JH, et al. Significance of arterial hypotension after resuscitation from cardiac arrest. Crit Care Med. 2009;37(11):2895–903. quiz 2904
Simons RW, Rea TD, Becker LJ, et al. The incidence and significance of emesis associated with out-of-hospital cardiac arrest. Resuscitation. 2007;74(3):427–31.
Cha KC, Kim YW, Kim HI, et al. Parenchymal lung injuries related to standard cardiopulmonary resuscitation. Am J Emerg Med. 2017;35(1):117–21.
Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126–36.
Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S768–86.
Perbet S, Mongardon N, Dumas F, et al. Early-onset pneumonia after cardiac arrest: characteristics, risk factors and influence on prognosis. Am J Respir Crit Care Med. 2011;184(9):1048–54.
Hellenkamp K, Onimischewski S, Kruppa J, et al. Early pneumonia and timing of antibiotic therapy in patients after nontraumatic out-of-hospital cardiac arrest. Crit Care. 2016;20:31.
Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. 2016;316(15):1583–9.
Kagstrom E, Smith ML, Siesjo BK. Cerebral circulatory responses to hypercapnia and hypoxia in the recovery period following complete and incomplete cerebral ischemia in the rat. Acta Physiol Scand. 1983;118(3):281–91.
Buunk G, van der Hoeven JG, Meinders AE. Cerebrovascular reactivity in comatose patients resuscitated from a cardiac arrest. Stroke. 1997;28(8):1569–73.
McKenzie N, Williams TA, Tohira H, et al. A systematic review and meta-analysis of the association between arterial carbon dioxide tension and outcomes after cardiac arrest. Resuscitation. 2017;111:116–26.
Johnson NJ, Carlbom DJ, Gaieski DF. Ventilator management and respiratory care after cardiac arrest: oxygenation, ventilation, infection, and injury. Chest. 2018;153(6):1466–77.
Sutherasan Y, Penuelas O, Muriel A, et al. Management and outcome of mechanically ventilated patients after cardiac arrest. Crit Care. 2015;19:215.
Callaway CW, Donnino MW, Fink EL, et al. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S465–82.
Kirkegaard H, Soreide E, de Haas I, et al. Targeted temperature management for 48 vs 24 hours and neurologic outcome after out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2017;318(4):341–50.
Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369(23):2197–206.
Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56.
Glover GW, Thomas RM, Vamvakas G, et al. Intravascular versus surface cooling for targeted temperature management after out-of-hospital cardiac arrest—an analysis of the TTM trial data. Crit Care. 2016;20(1):381.
Badjatia N. Therapeutic hypothermia protocols. Handb Clin Neurol. 2017;141:619–32.
Polderman KH. Of ions and temperature: the complicated interplay of temperature, fluids, and electrolytes on myocardial function. Crit Care. 2013;17(6):1018.
Losert H, Sterz F, Roine RO, et al. Strict normoglycaemic blood glucose levels in the therapeutic management of patients within 12h after cardiac arrest might not be necessary. Resuscitation. 2008;76(2):214–20.
Borgquist O, Wise MP, Nielsen N, et al. Dysglycemia, glycemic variability, and outcome after cardiac arrest and temperature management at 33 degrees C and 36 degrees C. Crit Care Med. 2017;45(8):1337–43.
Longstreth WT Jr, Diehr P, Cobb LA, et al. Neurologic outcome and blood glucose levels during out-of-hospital cardiopulmonary resuscitation. Neurology. 1986;36(9):1186–91.
Skrifvars MB, Pettila V, Rosenberg PH, et al. A multiple logistic regression analysis of in-hospital factors related to survival at six months in patients resuscitated from out-of-hospital ventricular fibrillation. Resuscitation. 2003;59(3):319–28.
Oksanen T, Skrifvars MB, Varpula T, et al. Strict versus moderate glucose control after resuscitation from ventricular fibrillation. Intensive Care Med. 2007;33(12):2093–100.
Friedman D, Claassen J, Hirsch LJ. Continuous electroencephalogram monitoring in the intensive care unit. Anesth Analg. 2009;109(2):506–23.
Callaway CW, Soar J, Aibiki M, et al. Part 4: advanced life support: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2015;132(16 Suppl 1):S84–145.
Grossestreuer AV, Abella BS, Leary M, et al. Time to awakening and neurologic outcome in therapeutic hypothermia-treated cardiac arrest patients. Resuscitation. 2013;84(12):1741–6.
Mulder M, Gibbs HG, Smith SW, et al. Awakening and withdrawal of life-sustaining treatment in cardiac arrest survivors treated with therapeutic hypothermia∗. Crit Care Med. 2014;42(12):2493–9.
Eid SM, Albaeni A, Vaidya D, et al. Awakening following cardiac arrest: Determined by the definitions used or the therapies delivered? Resuscitation. 2016;100:38–44.
Ponz I, Lopez-de-Sa E, Armada E, et al. Influence of the temperature on the moment of awakening in patients treated with therapeutic hypothermia after cardiac arrest. Resuscitation. 2016;103:32–6.
Paul M, Bougouin W, Geri G, et al. Delayed awakening after cardiac arrest: prevalence and risk factors in the Parisian registry. Intensive Care Med. 2016;42(7):1128–36.
Zanyk-McLean K, Sawyer KN, Paternoster R, et al. Time to awakening is often delayed in patients who receive targeted temperature management after cardiac arrest. Ther Hypothermia Temp Manag. 2017;7(2):95–100.
Sandroni C, Cariou A, Cavallaro F, et al. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Resuscitation. 2014;85(12):1779–89.
Cronberg T, Brizzi M, Liedholm LJ, et al. Neurological prognostication after cardiac arrest—recommendations from the Swedish Resuscitation Council. Resuscitation. 2013;84(7):867–72.
Orioles A, Morrison WE, Rossano JW, et al. An under-recognized benefit of cardiopulmonary resuscitation: organ transplantation. Crit Care Med. 2013;41(12):2794–9.
Sandroni C, D’Arrigo S, Callaway CW, et al. The rate of brain death and organ donation in patients resuscitated from cardiac arrest: a systematic review and meta-analysis. Intensive Care Med. 2016;42(11):1661–71.
Callaway CW, Schmicker RH, Brown SP, et al. Early coronary angiography and induced hypothermia are associated with survival and functional recovery after out-of-hospital cardiac arrest. Resuscitation. 2014;85(5):657–63.
Dumas F, Bougouin W, Geri G, et al. Emergency percutaneous coronary intervention in post-cardiac arrest patients without ST-segment elevation pattern: insights from the PROCAT II registry. JACC Cardiovasc Interv. 2016;9(10):1011–8.
Kern KB, Lotun K, Patel N, et al. Outcomes of comatose cardiac arrest survivors with and without ST-segment elevation myocardial infarction: importance of coronary angiography. JACC Cardiovasc Interv. 2015;8(8):1031–40.
Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336(23):1629–33.
Yannopoulos D, Bartos JA, Raveendran G, et al. Coronary artery disease in patients with out-of-hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol. 2017;70(9):1109–17.
Lemkes JS, Janssens GN, van der Hoeven NW, et al. Coronary angiography after cardiac arrest without ST-segment elevation. N Engl J Med. 2019;381(2):189–90.
Yannopoulos D, Bartos JA, Aufderheide TP, et al. The evolving role of the cardiac catheterization laboratory in the management of patients with out-of-hospital cardiac arrest: a scientific statement from the American Heart Association. Circulation. 2019;139(12):e530–52.
Testori C, Sterz F, Behringer W, et al. Mild therapeutic hypothermia is associated with favourable outcome in patients after cardiac arrest with non-shockable rhythms. Resuscitation. 2011;82(9):1162–7.
Perman SM, Grossestreuer AV, Wiebe DJ, et al. The utility of therapeutic hypothermia for post-cardiac arrest syndrome patients with an initial nonshockable rhythm. Circulation. 2015;132(22):2146–51.
Che D, Li L, Kopil CM, et al. Impact of therapeutic hypothermia onset and duration on survival, neurologic function, and neurodegeneration after cardiac arrest. Crit Care Med. 2011;39(6):1423–30.
Kim T, Paine MG, Meng H, et al. Combined intra- and post-cardiac arrest hypothermic-targeted temperature management in a rat model of asphyxial cardiac arrest improves survival and neurologic outcome compared to either strategy alone. Resuscitation. 2016;107:94–101.
Sterz F, Safar P, Tisherman S, et al. Mild hypothermic cardiopulmonary resuscitation improves outcome after prolonged cardiac arrest in dogs. Crit Care Med. 1991;19(3):379–89.
Kuboyama K, Safar P, Radovsky A, et al. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993;21(9):1348–58.
Abella BS, Zhao D, Alvarado J, et al. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109(22):2786–91.
Nozari A, Safar P, Stezoski SW, et al. Critical time window for intra-arrest cooling with cold saline flush in a dog model of cardiopulmonary resuscitation. Circulation. 2006;113(23):2690–6.
Zhao D, Abella BS, Beiser DG, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation. 2008;77(2):242–9.
Bernard SA, Smith K, Cameron P, et al. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation. 2010;122(7):737–42.
Kim F, Nichol G, Maynard C, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. JAMA. 2014;311(1):45–52.
Bray JE, Stub D, Bloom JE, et al. Changing target temperature from 33 degrees C to 36 °C in the ICU management of out-of-hospital cardiac arrest: a before and after study. Resuscitation. 2017;113:39–43.
Salter R, Bailey M, Bellomo R, et al. Changes in temperature management of cardiac arrest patients following publication of the target temperature management trial. Crit Care Med. 2018;46(11):1722–30.
Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–96.
Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382(9905):1638–45.
Stub D, Bernard S, Pellegrino V, et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation. 2015;86:88–94.
Carr BG, Kahn JM, Merchant RM, et al. Inter-hospital variability in post-cardiac arrest mortality. Resuscitation. 2009;80(1):30–4.
Straney LD, Bray JE, Finn J, et al. Trends in intensive care unit cardiac arrest admissions and mortality in Australia and New Zealand. Crit Care Resusc. 2014;16(2):104–11.
Nolan JP, Ferrando P, Soar J, et al. Increasing survival after admission to UK critical care units following cardiopulmonary resuscitation. Crit Care. 2016;20(1):219.
Westhall E, Rossetti A, van Rootselaar A. Standardized EEG interpretation accurately predicts progno sis after cardiac arrest. Neurology. 2016;86(16):1482–90.
Lascarrou J-B, Merdji H, Le Gouge A, et al. Targeted temperature management for cardiac arrest with nonshockable rhythm. N Engl J Med. 2019;381(24):2327–37.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hsu, C.H., Neumar, R.W. (2020). Post-cardiac Arrest Management. In: Hyzy, R.C., McSparron, J. (eds) Evidence-Based Critical Care. Springer, Cham. https://doi.org/10.1007/978-3-030-26710-0_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-26710-0_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-26709-4
Online ISBN: 978-3-030-26710-0
eBook Packages: MedicineMedicine (R0)