Abstract
Purpose
Motivated by a new randomized trial (the PEPTIC trial) that raised the issue of an increase in mortality with proton pump inhibitors (PPIs) relative to histamine-2 receptor antagonists (H2RAs), we updated our prior systematic review and network meta-analysis (NMA) addressing the impact of pharmacological gastrointestinal bleeding prophylaxis in critically ill patients.
Methods
We searched for randomized controlled trials that examined the efficacy and safety of gastrointestinal bleeding prophylaxis with PPIs, H2RAs, or sucralfate versus one another or placebo or no prophylaxis in adult critically ill patients. We performed Bayesian random-effects NMA and conducted analyses using all PEPTIC data as well as a restricted analysis using only PEPTIC data from high compliance centers. We used the GRADE approach to quantify absolute effects and assess the certainty of evidence.
Results
Seventy-four trials enrolling 39 569 patients proved eligible. Both PPIs (risk ratio (RR) 1.03, 95% credible interval 0.93 to 1.14, moderate certainty) and H2RAs (RR 0.98, 0.89 to 1.08, moderate certainty) probably have little or no impact on mortality compared with no prophylaxis. There may be no important difference between PPIs and H2RAs on mortality (RR 1.05, 0.97 to 1.14, low certainty), the 95% credible interval of the complete analysis has not excluded an important increase in mortality with PPIs. Both PPIs (RR 0.46, 0.29 to 0.66) and H2RAs (RR 0.67, 0.48 to 0.94) probably reduce clinically important gastrointestinal bleeding; the magnitude of reduction is probably greater in PPIs than H2RAs (RR 0.69, 0.45 to 0.93), and the difference may be important in higher, but not lower bleeding risk patients. PPIs (RR 1.08, 0.88 to 1.45, low certainty) and H2RAs (RR 1.07, 0.85 to 1.37, low certainty) may have no important impact on pneumonia compared with no prophylaxis.
Conclusion
This updated NMA confirmed that PPIs and H2RAs are most likely to have a similar effect on mortality compared to each other and compared to no prophylaxis; however, the possibility that PPIs may slightly increase mortality cannot be excluded (low certainty evidence). PPIs and H2RAs probably achieve important reductions in clinically important gastrointestinal bleeding; for higher bleeding risk patients, the greater benefit of PPIs over H2RAs may be important. PPIs or H2RAs may not result in important increases in pneumonia but the certainty of evidence is low.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This updated NMA confirmed that PPIs and H2RAs are most likely to have a similar effect on mortality compared to each other and compared to no prophylaxis; however, the possibility that PPIs may slightly increase mortality cannot be excluded (low certainty evidence). PPIs and H2RAs probably reduce clinically important gastrointestinal bleeding; PPIs may be more effective, and for higher bleeding risk patients the reductions are important. |
Introduction
Clinicians have long been concerned about stress ulceration as one of the reasons critically ill patients develop upper gastrointestinal bleeding [1]. To prevent stress-related gastrointestinal bleeding, clinicians often prescribe acid suppressing drugs (proton pump inhibitors (PPIs), histamine-2 receptor antagonists (H2RAs), or sucralfate) to patients at high risk during their stay in the intensive care unit (ICU) [2, 3].
The efficacy and safety of gastrointestinal bleeding prophylaxis remains a controversial topic that has motivated many randomized controlled trials (RCTs). Earlier in 2020, our team performed a systematic review and network meta-analysis (NMA) to summarize the available evidence from RCTs addressing the efficacy and safety of pharmacological interventions for gastrointestinal bleeding prophylaxis [4]. Results provided no support for an effect of any intervention (PPIs, H2RAs, sucralfate) on mortality, and provided evidence that PPIs and H2RAs likely result in important reductions in gastrointestinal bleeding for critically ill patients at higher risk of bleeding, with possibly greater reduction with PPIs. These results provided support for a weak recommendation for using gastrointestinal bleeding prophylaxis in critically ill patients at high risk (> 4%) of clinically important gastrointestinal bleeding (CIB), and for using PPIs rather than H2RAs [5].
A recently published international open-label cluster crossover randomized clinical trial (the PEPTIC trial) that compared PPIs with H2RAs for gastrointestinal bleeding prophylaxis in mechanically ventilated ICU patients reported more patients in the PPI group (18.3%) died than in the H2RA group (17.5%) [6]. Although the difference did not reach the conventional statistical significance threshold, the PEPTIC trial raised the concern that PPIs might increase mortality in critically ill patients.
To compare the potential benefits and harms of gastrointestinal bleeding prophylaxis with PPIs, H2RAs, and sucralfate in critically ill adults, we updated our systematic review and NMA including the PEPTIC trial. Recognizing the limitations of the open-label cluster crossover design of the PEPTIC trial, we regarded this updated NMA as a sensitivity analysis providing important additional information that might either change, or support, our prior conclusion.
Methods
We registered the protocol for this systematic review with PROSPERO (CRD42020169989). We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7].
Our prior published NMA provides details of our methods [4]. Here, we summarize briefly, highlighting differences in the methods between the prior and current NMA.
Data sources and searches
We conducted an electronic literature search for Medline, Embase, the Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL), International Clinical Trials Registry Platform (ICTRP), Latin American and Caribbean Health Sciences Literature (LILACS) and clinicaltrials.gov. Our prior NMA searched up to March 2019; this updated search included literature from January 2019 to February 2020. Electronic Supplementary Material (ESM) 1 details the search strategy.
Study selection
Two reviewers independently screened titles and abstracts and reviewed full articles for those deemed possibly eligible. Reviewers resolved conflicts by discussion.
We included RCTs that compared pharmacological gastrointestinal bleeding prophylaxis with PPIs, H2RAs, or sucralfate versus one another or placebo or no prophylaxis in adult critically ill patients at risk of gastrointestinal bleeding. Outcomes included mortality at the longest follow-up reported, CIB, pneumonia, Clostridioides difficile infection, overt gastrointestinal bleeding, length of ICU stay, length of hospital stay and duration of mechanical ventilation.
Data extraction
Two reviewers independently extracted data including study characteristics, population characteristics, description of interventions and comparators, outcomes and their definitions.
Risk of bias assessment
We assessed the risk of bias in duplicate with a modified Cochrane Collaboration tool [8], which include sequence generation, allocation sequence concealment, blinding, missing outcome data and other bias. We judged each criterion for each trial as definitely or probably low risk of bias, or definitely or probably high risk of bias. Reviewers resolved disagreement through discussion and consensus.
Data analysis
We calculated risk ratios (RRs) and corresponding 95% credible intervals for dichotomous outcomes, and ratio of medians with corresponding 95% credible intervals for continuous outcomes. The PEPTIC trial reported RRs or ratio of medians with 95% confidence intervals that adjusted for randomization batch, the order of administration of the treatments, and batch × order interaction [6]. We calculated RRs or ratio of medians for each study, and then pooled them with the RRs or ratio of medians in PEPTIC trial.
We performed a Bayesian random-effects pairwise meta-analysis for each direct comparison for each outcome. We performed a Bayesian random-effects NMA using Markov-chain Monte Carlo simulation, an analysis approach we also used in our prior NMA [9, 10]. We evaluated the ranking probabilities and calculated surface under the cumulative ranking curves (SUCRA). We used the node-splitting method to assess incoherence [11].
The PEPTIC trial conducted a post-hoc subgroup analysis based on ICU using adherence (low, medium, or high) [6]. High compliance ICUs were those, in periods in which the intent was the patients received H2RAs, less than 12% of patients received only PPIs [6]. Therefore, we conducted two sets of analyses—one analysis including the overall results for PEPTIC trial in the analysis (PEPTIC complete), and another analysis including only the high compliance ICU results of the PEPTIC trial (PEPTIC restricted).
We calculated absolute effects for all outcomes based on the RRs or ratio of medians and the baseline risks. We used the event rates in the placebo arm in the SUP-ICU trial [12] as the baseline risks for comparisons including placebo; for outcomes that SUP-ICU trial did not report, we used the median of the placebo group in the included studies. We used the point estimate of the PPIs group event rate in the comparison between PPIs and placebo as the baseline risk in the PPIs group for PPIs versus H2RAs and PPIs versus sucralfate, and used the point estimate of the H2RAs group event rate in the comparison between H2RAs and placebo as the baseline risk for H2RAs versus sucralfate. For CIB and overt gastrointestinal bleeding, we calculated absolute effects for four categories of risk of bleeding population – highest risk, high risk, moderate risk, and low risk according to a recently published clinical practice guideline [5] which used evidence from a systematic review of risk factors for gastrointestinal bleeding [13].
We conducted network meta-regression to explore the impact of each risk of bias criterion. If risk of bias influenced results, we included only low risk of bias studies in generating best estimates. For CIB and overt bleeding, we explored the impact of enteral nutrition, mechanical ventilation, and risk factors for bleeding by conducting network meta-regression using the proportion of patients with enteral nutrition or mechanical ventilation, or whether the studies specified inclusion of critically ill patients with risk factors as the independent variable.
With the assumption that H2RAs and placebo have the same null effect on mortality, at the suggestion of a reviewer we conducted a pairwise meta-analysis that included PPI versus placebo (or no prophylaxis) trials and PPI versus H2RA trials and combined the placebo and H2RA groups. The aim was to further explore the impact of PPIs on mortality.
The SUP-ICU trial conducted a predefined subgroup analysis for mortality based on SAPS II score (less sick subgroup: SAPS II score ≤ 53, sicker subgroup: > 53) [12]. The PEPTIC trial conducted a post-hoc subgroup analysis for mortality based on APACHE II score (0–13, 14–17, 18–23, and 24–61) [6]. We combined these two within-trial subgroups to address the possible influence of disease severity on drug impact on mortality with the assumption that H2RAs have no effect on mortality; when doing this, we categorized APACHE II scores of 0–23 as the less sick subgroup and scores of 24–61 as the sicker for the PEPTIC trial, a threshold that defines risk groups consistent with the threshold used in the SUP-ICU trial [12]. We adapted two approaches for pooling the subgroups. One was pooling the ratio of RRs (i.e., RRsicker subgroup/RRless sick subgroup) of the SUP-ICU trial and the PEPTIC trial; another was pooling the sicker subgroups as well as the less sick subgroups of these two trials separately and then addressed the subgroup difference.
All NMAs were performed using the gemtc package of R version 3.4.3 (R Core Team, Vienna, Austria), and we used networkplot command of Stata version 15.1 (StataCorp, College Station, Texas, USA) to draw the network plots [14].
Certainty of evidence
We rated the certainty of evidence for each outcome using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) approach for pairwise meta-analysis and NMA [15,16,17,18,19,20,21,22,23]. If the results differed depending on the statistical approach, or there were differences between complete PEPTIC analysis results, restricted PEPTIC results and our prior NMA results, or we were uncertain of the baseline risk or the absolute estimate, we considered rating down the certainty in the evidence.
Results
Search results and study characteristics
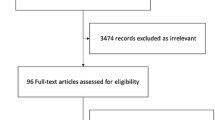
Our updated literature search identified 365 citations. After removing duplicates and screening title and abstract, 35 citations remained potentially eligible, of which 2, on full-text review, met our eligibility criteria (Fig. 1). One was the PEPTIC trial, an international open-label cluster crossover RCT that compared PPIs versus H2RAs in 26 828 patients in 50 ICUs [6]. The second RCT compared ranitidine versus sucralfate versus no prophylaxis in 81 patients [24].
Including 72 trials from our prior NMA, we ultimately included 74 trials, with sample sizes from 22 to 2 6828, enrolling a total of 39 569 patients. Only the PEPTIC trial utilized a cluster design. The most common comparisons were between H2RAs and placebo or no prophylaxis, H2RAs versus sucralfate, and PPIs versus H2RAs; the comparison that included the most patients was PPIs versus H2RAs. ESM 2 presents the characteristics and outcome definitions of each trial.
Risk of bias
ESM 3 summarizes the risk of bias assessments. The main limitations were possible lack of blinding (63.5%), lack of allocation sequence concealment (55.4%), and excessive (more than 5%) loss to follow-up (23.0%).
Outcomes
ESM 4 presents network plots for each outcome. ESM 5 presents cumulative ranking of interventions for each outcome.
Mortality
Fifty-two trials [6, 12, 25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75] enrolling 37,048 patients reported mortality. Risk ratios for all comparisons proved close to 1.0 with moderate to very low certainty. Both the complete (risk ratio 1.03, 95% credible interval 0.93 to 1.14, moderate certainty, Table 1) and restricted (1.00, 0.91 to 1.12, moderate certainty, Table 2) PEPTIC analyses demonstrated PPIs probably have little or no impact on mortality compared with no prophylaxis. Moderate certainty evidence also showed that H2RAs likely have no important effect on mortality (Tables 1 and 2). Both the complete (1.05, 0.97 to 1.14, low certainty, Table 1) and restricted (0.98, 0.91 to 1.10, low certainty, Table 2) PEPTIC analysis suggested there may be no important difference between PPIs and H2RAs on mortality, the 95% credible interval of the complete analysis (absolute difference 15, − 10 to 38 per 1000) has not excluded an important increase in mortality with PPIs. Network meta-regression failed to demonstrate an impact of risk of bias on results for mortality for both complete and restricted analyses (ESM 6).
Pairwise meta-analysis of PPIs versus placebo/H2RAs that was restricted to the high compliance ICU results of the PEPTIC trial confirmed that PPIs may not have important impact on mortality (0.98, 0.93 to 1.03, absolute difference −6 per 1000); however, the pairwise meta-analysis that used complete PEPTIC data suggested an increase in mortality with PPIs with a confidence interval bordering on no effect (1.05, 1.00 to 1.09, absolute difference 15, 0 to 27 per 1000).
A detailed credibility assessment of the hypothesis that PPIs relative to either placebo or H2RAs increase mortality in more rather than less sick patients (ESM 7) suggests the hypothesis has low credibility [76]. The statistical analysis demonstrating that any difference in effects in sicker and less sick patients is consistent with the play of chance (P = 0.16, Fig. 2) represents one important reason for this conclusion. The result is consistent with an alternative statistical approach to this analysis (P = 0.18, Fig. 1 of ESM 7).
CIB
Forty-five trials [6, 12, 24, 25, 28, 31,32,33,34, 36, 37, 40,41,42,43,44,45,46, 48, 49, 51,52,53, 55, 57, 61, 62, 64, 66,67,68,69,70,71, 73,74,75, 77,78,79,80,81,82,83,84,85] enrolling 37 005 patients reported CIB. For patients at highest or high risk of bleeding, moderate certainty evidence from the complete PEPTIC analysis (risk ratio 0.46, 95% credible interval 0.29 to 0.66, 32 fewer per 1000 for high risk patients, Table 3) and low certainty evidence from the restricted analysis (0.65, 0.38 to 1.20, 21 fewer for high risk patients, Table 4) suggested that PPIs probably reduce the risk of CIB compared with no prophylaxis. Both analyses provided moderate certainty evidence that H2RAs probably reduce CIB (complete: 0.67, 0.48 to 0.94, 20 fewer for high risk patients, Table 3). PPIs may reduce CIB relative to H2RAs (e.g. for highest risk patients: 18 fewer per 1000 patients for complete and 33 fewer for restricted, low certainty, Tables 3 and 4). PPIs, relative to sucralfate, probably reduce CIB (Tables 3 and 4). Although the point estimate of H2RAs versus sucralfate suggested a reduction in CIB with H2RAs, the magnitude of the reduction may be less than most people would think is important (Tables 3 and 4).
Network meta-regression for the restricted analysis suggested the possibility that H2RAs versus placebo had a larger relative effect for patients receiving mechanical ventilation, but the credibility of this subgroup effect is low (most important reason for low credibility: the effect was suggested by comparisons between studies) (ESM 6) [76]. Network meta-regression failed to demonstrate an impact of risk of bias or other factors on results for CIB for both complete and restricted analyses (ESM 6).
Pneumonia
Forty trials [12, 25,26,27,28, 31,32,33,34,35, 41, 42, 44, 45, 47, 49, 51,52,53,54,55, 57, 59, 61, 63, 64, 67,68,69,70,71, 73, 78, 79, 81,82,83,84, 86,87,88] enrolling 9 288 patients reported on pneumonia (PEPTIC did not). Network meta-regression suggested risk of bias on blinding significantly influences the results (ESM 6) so, in generating best estimates for pneumonia, we included only 15 blinded trials [12, 25, 31,32,33, 41, 44, 45, 55, 57, 68,69,70, 79, 81, 87] with 6 572 patients. PPIs (risk ratio 1.08, 95% credible interval 0.88 to 1.45, low certainty, Table 1 of ESM 8), H2RAs (1.07, 0.85 to 1.37, low certainty, Table 1 of ESM 8) and sucralfate (0.93, 0.65 to 1.38, low certainty, Table 1 of ESM 8) may have no important impact on pneumonia compared with no prophylaxis; however, the results differ depending on the statistical approach used (for pneumonia, although no new trials were included in this updated NMA, the network meta-regression suggested blinding influenced the results, so we used only the blinded studies in generating best estimates for pneumonia in the updated NMA; our prior NMA did not find risk of bias influences results and suggested that PPIs and H2RAs may increase pneumonia compared with no prophylaxis). There may be no important difference in pneumonia incidence between PPIs and H2RAs (1.02, 0.80 to 1.33, low certainty, Table 1 of ESM 8).
C. difficile infection
Six trials [6, 12, 25, 36, 68, 85] that enrolled 30 677 patients reported C. difficile infection. For H2RAs versus placebo and PPIs versus H2RAs, although the risk ratio of the complete analysis (e.g. for PPIs versus H2RAs, risk ratio 0.76, 95% credible interval 0.28 to 2.16, low certainty, Table 2 of ESM 8) and the risk ratio of restricted analysis (e.g. for PPIs versus H2RAs, 1.05, 0.30 to 3.65, low certainty, Table 3 of ESM 8) differ, the confidence intervals were widely overlapping due to the low risk of C. difficile infection (1.5%). Network meta-regression failed to demonstrate an impact of risk of bias on results for C. difficile infection (ESM 6).
Overt gastrointestinal bleeding
Sixty-seven trials [6, 12, 24,25,26, 28,29,30,31,32,33,34,35,36,37,38, 40,41,42,43,44,45,46, 48,49,50,51,52,53,54,55,56,57, 59,60,61,62,63,64,65,66,67,68,69,70,71, 73,74,75, 77,78,79,80,81,82,83,84,85, 87,88,89,90,91,92,93,94,95,96] including 38,571 patients reported overt bleeding. Network meta-regression suggested risk of bias on allocation sequence concealment significantly influences the results in both complete and restricted analyses (ESM 6), so we included only allocation concealed studies in generating best estimates for overt bleeding, which included 31 trials [12, 25, 28, 29, 31,32,33,34, 36, 38, 40, 41, 43,44,45, 52, 55, 57, 60, 64, 68,69,70,71, 79, 81, 87, 91,92,93,94, 96] with 8258 patients. Both PPIs (risk ratio 0.50, 95% credible interval 0.31 to 0.72, moderate certainty, Table 4 of ESM 8) and H2RAs (0.66, 0.48 to 0.89, moderate certainty, Table 4 of ESM 8) probably reduce overt bleeding. Sucralfate possibly has no impact on overt bleeding (1.00, 0.61 to 1.68, low certainty, Table 4 of ESM 8). PPIs probably reduce overt bleeding more than H2RAs (0.76, 0.49 to 1.09, moderate certainty, Table 4 of ESM 8).
Network meta-regression suggested the possibility that H2RAs versus placebo had a larger relative effect when patients received enteral nutrition, but the credibility of this subgroup effect is low (most important reason for low credibility: the effect was suggested by comparisons between studies) (ESM 6) [76].
Length of ICU stay, length of hospital stay and duration of mechanical ventilation
Eighteen trials [6, 25, 33, 34, 36, 39, 44, 48, 51, 52, 59, 61, 65, 68,69,70, 75, 78, 85] including 30 342 patients reported length of ICU stay. Eight trials [6, 25, 27, 36, 47, 61, 68, 84] including 27 619 patients reported length of hospital stay. Twenty-four trials [6, 25,26,27,28, 30, 33, 36, 41, 42, 44, 47, 49, 52, 53, 59, 61, 66, 67, 69, 70, 73, 79, 84, 85] including 15 110 patients reported duration of mechanical ventilation. Results demonstrated no important difference between any of interventions (Tables 5–9 of ESM 8). Network meta-regression failed to demonstrate an impact of risk of bias on results (ESM 6).
Discussion
Our analysis using complete PEPTIC data provides important new evidence that it is unlikely that either PPIs (risk ratio 1.03, 95% credible interval 0.93 to 1.14, moderate certainty, Table 1) or H2RAs (RR 0.98, 0.89 to 1.08, moderate certainty, Table 1) compared with no prophylaxis influence mortality in critically ill patients, a result consistent with an analysis restricted to PEPTIC centers with higher compliance (Table 2). Both the complete (1.05, 0.97 to 1.14, low certainty, Table 1) and the restricted (0.98, 0.91 to 1.10, low certainty, Table 2) PEPTIC analysis suggested there may be no important difference between PPIs and H2RAs on mortality, while the 95% credible interval of the complete analysis (absolute difference 15, -10 to 38 per 1000) has not excluded an important increase in mortality with PPIs.
Both PPIs and H2RAs probably reduce CIB; the magnitude of reduction is probably greater in PPIs than H2RAs, and the difference may be important in higher, but not lower bleeding risk patients (Tables 3 and 4).
Low certainty evidence suggested that neither PPIs nor H2RAs have an important impact on pneumonia relative to no prophylaxis (Table 1 of ESM 8) and there may be no important difference between PPIs and H2RAs on pneumonia (Table 1 of ESM 8). An absolute effect on C. difficile infection for any interventions was small, because the baseline risk of C. difficile infection was very low (Tables 2 and 3 of ESM 8). Results demonstrated that there may be no important difference between any of interventions on length of ICU stay, length of hospital stay or duration of mechanical ventilation (Tables 5–9 of ESM 8).
Overall, our interpretation of current available evidence is that results support using gastrointestinal bleeding prophylaxis in critically ill patients at higher risk (> 4%) of CIB and using PPIs rather than H2RAs. At the same time, clinicians should be aware that we have only low confidence in some of the key evidence (mortality and CIB of PPIs versus H2RAs).
Strength and limitations
We provide the most up-to-date summary addressing the evidence comparing efficacy and safety of gastrointestinal bleeding prophylaxis with different pharmacological interventions. Rather than mechanically pooling the included trials together (i.e., pooling number of events and total number of patients), we considered the unit-of-analysis issues and adjusted our statistical analysis approach accordingly. For the PEPTIC trial, we extracted the RRs or ratio of medians with 95% confidence intervals—measures of effect in which the authors accounted for clustering unit and crossover design. We then pooled these results with RRs or ratio of medians of other included trials. This approach makes it possible to incorporate, in a single NMA, the PEPTIC trial with other trials that randomized individual patients.
In addition, we considered the substantial, and unequal contamination in each group of the PEPTIC trial (during the PPI periods, 4.1% of the patients received H2RAs; during the H2RA periods, 20.1% of the patients received PPIs), and, therefore, conducted a sensitivity analysis restricted to the high compliance PEPTIC results reported by the authors.
We considered whether risk of bias would influence the results, and if that was the case, we focused on low risk of bias studies. We presented absolute effects and used the GRADE approach to assess the certainty of evidence, and in doing so considered the stability of results based on statistical approach and different analyses/models. When results were vulnerable to the statistical approach (this occurred for pneumonia), or the results from different analyses/models differed (occurred for CIB and overt bleeding), or we were uncertain of the baseline risk (for CIB and overt bleeding) and thus the absolute estimates, we rated down the certainty of the evidence.
Including the PEPTIC trial results in limitations and challenges. Pooling RRs or ratio of medians for other trials required omission of trials in which there were zero event in both groups. Limitations of the PEPTIC trial include the open-label design and the high levels of contamination such that clinicians sometimes chose whether or not to prescribe a study medication subjectively rather than based on randomization period [6]. In a pairwise sensitivity analysis suggested by a reviewer that assumes no mortality difference between H2RAs and placebo and, therefore, combines H2RAs with placebo, including all PEPTIC data suggested a possible mortality increase with PPIs, while an analysis focusing on the high compliance PEPTIC centres did not.
Other limitations include the clinical heterogeneity across the eligible trials. Most trials did not report the duration of follow-up. For trials that did not report on overt bleeding, we regarded the results for CIB as representing overt bleeding. Although network meta-regression detected several possible subgroup effects, the credibility of these subgroup effects are low [76].
We are aware of the uncertainty regarding baseline risk estimates on which we relied for estimates of absolute effect. The potential predictors employed in our risk stratification approach are based primarily on low or moderate certainty evidence [13]. Our approach does not represent a validated clinical prediction rule [97].
Finally, our pooled subgroup analysis addressing the hypothesis that there is a mortality increase with PPIs restricted to sicker patients that included both the SUP-ICU and the PEPTIC trials, as well as the pairwise meta-analyses of PPIs versus placebo/H2RAs, assumes that PPIs may increase mortality, rather that H2RAs reduce mortality. This assumption is, however, consistent with both the evidence and the biology offered by those who find this a credible subgroup hypothesis. The PEPTIC trial did not report the subgroup results for APACHE II scores of 0–23, so we combined the three strata of illness severity (0–13, 14–17 and 18–23) into a single category by ourselves which may induce bias.
Relation to prior work
Our updated search included two more trials than our previous NMA, of which one is the large PEPTIC trial. Clinicians should be aware of the entirety of the evidence rather than making clinical decision based on any single trial.
Both the updated and prior NMA support the hypothesis that PPIs and H2RAs probably have little or no impact on mortality. Subgroup analyses of the SUP-ICU trial [12] and the PEPTIC trial [6] raised the possibility that PPIs may increase mortality relative to placebo or H2RAs in the sicker patients. However, our assessment is that, based on current evidence, the credibility of these subgroup effects, remains low credibility (ESM 7) [76]. This does not, however, mean that one can dismiss the subgroup effect. Ideally, additional evidence from future RCTs should address the issue.
Both the updated and the prior NMA suggest PPIs and H2RAs probably reduce CIB and overt bleeding, and for higher bleeding risk patients the reductions in CIB are probably important (Tables 3 and 4). The prior NMA suggested PPIs and H2RAs may increase the risk of pneumonia, while the current NMA suggested no important difference between PPIs, H2RAs and placebo. Results for other outcomes were similar.
The different results between this updated NMA and the prior one relate to not only the inclusion of new eligible trials, but also the differences in the statistical approach necessitated by the sophisticated adjusted analysis of the PEPTIC data (see strengths of our approach above). For CIB and overt bleeding, in this updated NMA we did not find counterintuitive results which were found in the prior one, so the results for CIB and overt bleeding came from NMA rather than direct pairwise meta-analysis, and we further considered the influence of risk of bias. For pneumonia, no new eligible trial was included in the updated NMA; however, the results changed significantly, attributable to the different statistical approach used and a series of methods judgments. These differences reflect the remaining uncertainty regarding the impact of prophylaxis on pneumonia.
Conclusions
This updated NMA confirmed that PPIs and H2RAs are most likely to have a similar effect on mortality compared to each other and compared to no prophylaxis; however, the possibility that PPIs may slightly increase mortality cannot be excluded (low certainty evidence). PPIs and H2RAs probably reduce bleeding; PPIs may be more effective, and for higher bleeding risk patients the reductions are important. Our results supported a prior clinical practice guideline that made a weak recommendation for using gastrointestinal bleeding prophylaxis in critically ill patients at higher risk (> 4%) of CIB and for using PPIs rather than H2RAs [5]. The weak recommendation reflects several concerns including the absence of high quality evidence for key outcomes, the limitations of the risk stratification suggested in that guideline and reproduced here, and uncertainty how much we should value reductions in CIB without an impact on mortality. These concerns highlight the need for further RCTs.
References
Cook D, Guyatt G (2018) Prophylaxis against upper gastrointestinal bleeding in hospitalized patients. N Engl J Med 378:2506–2516
Barletta JF, Kanji S, MacLaren R, Lat I, Erstad BL (2014) Pharmacoepidemiology of stress ulcer prophylaxis in the United States and Canada. J Crit Care 29:955–960
Krag M, Perner A, Wetterslev J et al (2015) Stress ulcer prophylaxis in the intensive care unit: an international survey of 97 units in 11 countries. Acta Anaesthesiol Scand 59:576–585
Wang Y, Ye Z, Ge L et al (2020) Efficacy and safety of gastrointestinal bleeding prophylaxis in critically ill patients: systematic review and network meta-analysis. BMJ 368:l6744
Ye Z, Reintam Blaser A, Lytvyn L et al (2020) Gastrointestinal bleeding prophylaxis for critically ill patients: a clinical practice guideline. BMJ 368:l6722
Young PJ, Bagshaw SM, Forbes AB et al (2020) Effect of stress ulcer prophylaxis with proton pump inhibitors vs histamine-2 receptor blockers on in-hospital mortality among ICU patients receiving invasive mechanical ventilation: the PEPTIC randomized clinical trial. JAMA 323:616–626
Hutton B, Salanti G, Caldwell DM et al (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162:777–784
Guyatt GH, Busse JW. Modification of cochrane tool to assess risk of bias in randomized trials. https://www.evidencepartners.com/resources/methodological-resources/
Ades AE, Sculpher M, Sutton A et al (2006) Bayesian methods for evidence synthesis in cost-effectiveness analysis. PharmacoEconomics 24:1–19
Lumley T (2002) Network meta-analysis for indirect treatment comparisons. Stat Med 21:2313–2324
van Valkenhoef G, Dias S, Ades AE, Welton NJ (2016) Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods 7:80–93
Krag M, Marker S, Perner A et al (2018) Pantoprazole in patients at risk for gastrointestinal bleeding in the ICU. N Engl J Med 379:2199–2208
Granholm A, Zeng L, Dionne JC et al (2019) Predictors of gastrointestinal bleeding in adult ICU patients: a systematic review and meta-analysis. Intensive Care Med 45:1347–1359
Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G (2013) Graphical tools for network meta-analysis in STATA. PLoS ONE 8:e76654
Balshem H, Helfand M, Schunemann HJ et al (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64:401–406
Brignardello-Petersen R, Bonner A, Alexander PE et al (2018) Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol 93:36–44
Guyatt GH, Oxman AD, Kunz R et al (2011) GRADE guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol 64:1283–1293
Guyatt GH, Oxman AD, Kunz R et al (2011) GRADE guidelines: 8. Rating the quality of evidence–indirectness. J Clin Epidemiol 64:1303–1310
Guyatt GH, Oxman AD, Kunz R et al (2011) GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol 64:1294–1302
Guyatt GH, Oxman AD, Montori V et al (2011) GRADE guidelines: 5. Rating the quality of evidence–publication bias. J Clin Epidemiol 64:1277–1282
Guyatt GH, Oxman AD, Vist G et al (2011) GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol 64:407–415
Guyatt GH, Oxman AD, Vist GE et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926
Puhan MA, Schunemann HJ, Murad MH et al (2014) A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 349:g5630
Muzlovic I, Stubljar D (2019) Stress ulcer prophylaxis as a risk factor for tracheal colonization and hospital-acquired pneumonia in intensive care patients: impact on latency time for pneumonia. Acta Clinica Croatica 58:72–86
Alhazzani W, Guyatt G, Alshahrani M et al (2017) Withholding pantoprazole for stress ulcer prophylaxis in critically Ill patients: a pilot randomized clinical trial and meta-analysis. Crit Care Med 45:1121–1129
Apte NM, Karnad DR, Medhekar TP, Tilve GH, Morye S, Bhave GG (1992) Gastric colonization and pneumonia in intubated critically ill patients receiving stress ulcer prophylaxis: a randomized, controlled trial. Crit Care Med 20:590–593
Bashar FR, Manuchehrian N, Mahmoudabadi M, Hajiesmaeili MR, Torabian S (2013) Effects of ranitidine and pantoprazole on ventilator-associated pneumonia: a randomized double-blind clinical trial. Tanaffos 12:16–21
Ben-Menachem T, Fogel R, Patel RV et al (1994) Prophylaxis for stress-related gastric hemorrhage in the medical intensive care unit. A randomized, controlled, single-blind study. Ann Intern Med 121:568–575
Burgess P, Larson GM, Davidson P, Brown J, Metz CA (1995) Effect of ranitidine on intragastric pH and stress-related upper gastrointestinal bleeding in patients with severe head injury. Dig Dis Sci 40:645–650
Cannon LA, Heiselman D, Gardner W, Jones J (1987) Prophylaxis of upper gastrointestinal tract bleeding in mechanically ventilated patients. A randomized study comparing the efficacy of sucralfate, cimetidine, and antacids. Arch Intern Med 147:2101–2106
Chan KH, Lai EC, Tuen H et al (1995) Prospective double-blind placebo-controlled randomized trial on the use of ranitidine in preventing postoperative gastroduodenal complications in high-risk neurosurgical patients. J Neurosurg 82:413–417
Conrad SA, Gabrielli A, Margolis B et al (2005) Randomized, double-blind comparison of immediate-release omeprazole oral suspension versus intravenous cimetidine for the prevention of upper gastrointestinal bleeding in critically ill patients. Crit Care Med 33:760–765
Cook D, Guyatt G, Marshall J et al (1998) A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med 338:791–797
De Azevedo JRA, Soares MDGA, Silva GAE, De Lima PG (2000) Prevention of stress ulcer bleeding in high risk patients. Comparison of three drugs. GED Gastrenterologia Endoscopia Digestiva 19:239–244
Eddleston JM, Pearson RC, Holland J, Tooth JA, Vohra A, Doran BH (1994) Prospective endoscopic study of stress erosions and ulcers in critically ill adult patients treated with either sucralfate or placebo. Crit Care Med 22:1949–1954
El-Kersh K, Jalil B, McClave SA et al (2018) Enteral nutrition as stress ulcer prophylaxis in critically ill patients: a randomized controlled exploratory study. J Crit Care 43:108–113
Fink M, Karlstadt RG, Maroko RT, Field B (2003) Intravenous pantoprazole (IVP) and continuous infusion cimetidine (C) prevent upper gastrointestinal bleeding (UGIB) regardless of APSII score (APACHE II) in high risk intensive care unit (ICU) patients. Gastroenterology 124:A625–A626
Groll A, Simon JB, Wigle RD, Taguchi K, Todd RJ, Depew WT (1986) Cimetidine prophylaxis for gastrointestinal bleeding in an intensive care unit. Gut 27:135–140
Gursoy O, Memis D, Sut N (2008) Effect of proton pump inhibitors on gastric juice volume, gastric pH and gastric intramucosal pH in critically ill patients : a randomized, double-blind, placebo-controlled study. Clin Drug Investig 28:777–782
Halloran LG, Zfass AM, Gayle WE, Wheeler CB, Miller JD (1980) Prevention of acute gastrointestinal complications after severe head injury: a controlled trial of cimetidine prophylaxis. Am J Surg 139:44–48
Hanisch EW, Encke A, Naujoks F, Windolf J (1998) A randomized, double-blind trial for stress ulcer prophylaxis shows no evidence of increased pneumonia. Am J Surg 176:453–457
Harlaftis N, Basdanis G, Papapolychroniadis C et al (1997) Nosocomial pneumonia in mechanically ventilated patients during stress ulcer prophylaxis with sucralfate and ranitidine. Hell J Gastroenterol 10:230–235
Jakob SM, Parviainen I, Ruokonen E, Uusaro A, Takala J (2005) Lack of effect of ranitidine on gastric luminal pH and mucosal PCO2 during the first day in the ICU. Acta Anaesthesiol Scand 49:390–396
Kantorova I, Svoboda P, Scheer P et al (2004) Stress ulcer prophylaxis in critically ill patients: a randomized controlled trial. Hepatogastroenterology 51:757–761
Karlstadt RG, Iberti TJ, Silverstein J et al (1990) Comparison of cimetidine and placebo for the prophylaxis of upper gastrointestinal bleeding due to stress-related gastric mucosal damage in the intensive care unit. J Intensive Care Med 5:26–32
Kaushal S, Midha V, Sood A, Chopra SC, Gupta C (2000) A comparative study of the effects of famotidine and sucralfate in prevention of upper gastro-intestinal bleeding in patients of head injury. Indian J Pharmacol 32:246–249
Khorvash F, Abbasi S, Meidani M, Dehdashti F, Ataei B (2014) The comparison between proton pump inhibitors and sucralfate in incidence of ventilator associated pneumonia in critically ill patients. Adv Biomed Res 3:52
Labattut AG, Santolalla PM, De Andres AP et al (1992) Efficacy of sucralfate in the prevention of upper gastrointestinal stress bleeding in intensive care patients: comparison vs a control group. Clin Intensive Care 3:S19–S25
Laggner AN, Lenz K, Base W, Druml W, Schneeweiss B, Grimm G (1989) Prevention of upper gastrointestinal bleeding in long-term ventilated patients. Sucralfate versus ranitidine. Am J Med 86:81–84
Laggner AN, Lenz K, Graninger W et al (1988) Stress ulcer prophylaxis in a general intensive care unit: sucralfate versus ranitidine. Der Anaesthesist 37:704–710
Lee TH, Hung FM, Yang LH (2014) Comparison of the efficacy of esomeprazole and famotidine against stress ulcers in a neurosurgical intensive care unit. Adv Digest Med 1:50–53
Levy MJ, Seelig CB, Robinson NJ, Ranney JE (1997) Comparison of omeprazole and ranitidine for stress ulcer prophylaxis. Dig Dis Sci 42:1255–1259
Lin CC, Hsu YL, Chung CS, Lee TH (2016) Stress ulcer prophylaxis in patients being weaned from the ventilator in a respiratory care center: a randomized control trial. J Formos Med Assoc 115:19–24
Liu BL, Li B, Zhang X et al (2013) A randomized controlled study comparing omeprazole and cimetidine for the prophylaxis of stress-related upper gastrointestinal bleeding in patients with intracerebral hemorrhage. J Neurosurg 118:115–120
Lou W, Xia Y, Xiang P et al (2018) Prevention of upper gastrointestinal bleeding in critically ill Chinese patients: a randomized, double-blind study evaluating esomeprazole and cimetidine. Curr Med Res Opin 34:1449–1455
Macdougall BR, Bailey RJ, Williams R (1977) H2-receptor antagonists and antacids in the prevention of acute gastrointestinal haemorrhage in fulminant hepatic failure. Two controlled trials. Lancet (London, England) 1:617–619
Martin LF, Booth FV, Karlstadt RG et al (1993) Continuous intravenous cimetidine decreases stress-related upper gastrointestinal hemorrhage without promoting pneumonia. Crit Care Med 21:19–30
Nielsen HJ, Witt K, Moesgaard F, Kehlet H (1989) Ranitidine for improvement of delayed hypersensitivity response in patients with sepsis. Acta Chir Scand 155:445–449
Nourian A, Mohammadi M, Beigmohammadi MT et al (2018) Comparing efficacy of enteral nutrition plus ranitidine and enteral nutrition alone as stress ulcer prophylaxis. J Comp Effect Res 7:493–501
Peura DA, Johnson LF (1985) Cimetidine for prevention and treatment of gastroduodenal mucosal lesions in patients in an intensive care unit. Ann Intern Med 103:173–177
Pickworth KK, Falcone RE, Hoogeboom JE, Santanello SA (1993) Occurrence of nosocomial pneumonia in mechanically ventilated trauma patients: a comparison of sucralfate and ranitidine. Crit Care Med 21:1856–1862
Powell H, Morgan M, Li SK, Baron JH (1993) Inhibition of gastric acid secretion in the intensive care unit after coronary artery bypass graft. A pilot control study of intravenous omeprazole by bolus and infusion, ranitidine and placebo. Theor Surg 8:125–130
Prakash S, Rai A, Gogia AR, Prakash S (2008) Nosocomial pneumonia in mechanically ventilated patients receiving ranitidine or sucralfate as stress ulcer prophylaxis. Indian J Anaesth 52:179–184
Prod'hom G, Leuenberger P, Koerfer J et al (1994) Nosocomial pneumonia in mechanically ventilated patients receiving antacid, ranitidine, or sucralfate as prophylaxis for stress ulcer. A randomized controlled trial. Ann Intern Med 120:653–662
Reusser P, Gyr K, Scheidegger D, Buchmann B, Buser M, Zimmerli W (1990) Prospective endoscopic study of stress erosions and ulcers in critically ill neurosurgical patients: current incidence and effect of acid-reducing prophylaxis. Crit Care Med 18:270–274
Ruiz-Santana S, Ortiz E, Gonzalez B, Bolanos J, Ruiz-Santana AJ, Manzano JL (1991) Stress-induced gastroduodenal lesions and total parenteral nutrition in critically ill patients: frequency, complications, and the value of prophylactic treatment. A prospective, randomized study. Crit Care Med 19:887–891
Ryan P, Dawson J, Teres D, Celoria G, Navab F (1993) Nosocomial pneumonia during stress ulcer prophylaxis with cimetidine and sucralfate. Arch Surg 128:1353–1357
Selvanderan SP, Summers MJ, Finnis ME et al (2016) Pantoprazole or placebo for stress ulcer prophylaxis (POP-UP): randomized double-blind exploratory study. Crit Care Med 44:1842–1850
Solouki M, Mar'ashian SM, Koochak M, Nasiri A, Mokhtari M, Amirpour A (2009) Ventilator-associated pneumonia among ICU patients receiving mechanical ventilation and prophylaxis of gastrointestinal bleeding. Iran J Clin Infect Dis 4:177–180
Solouki M, Marashian SM, Kouchak M, Mokhtari M, Nasiri E (2009) Comparison between the preventive effects of ranitidine and omeprazole on upper gastrointestinal bleeding among ICU patients. Tanaffos 8:37–42
Somberg L, Morris J, Fantus R et al (2008) Intermittent intravenous pantoprazole and continuous cimetidine infusion: effect on gastric pH control in critically ill patients at risk of developing stress-related mucosal disease. J Trauma 64:1202–1210
Spapen H, Diltoer M, Nguyen DN, Ingels G, Ramet J, Huyghens L (1995) One week treatment with cimetidine does not attenuate the cortisol response to a short corticotropin test in stable intensive care patients: a prospective, randomized, and controlled study. Acta Anaesthesiol Belg 46:133–140
Thomason MH, Payseur ES, Hakenewerth AM et al (1996) Nosocomial pneumonia in ventilated trauma patients during stress ulcer prophylaxis with sucralfate, antacid, and ranitidine. J Trauma 41:503–508
Tryba M, Zevounou F, Torok M, Zenz M (1985) Prevention of acute stress bleeding with sucralfate, antacids, or cimetidine. A controlled study with pirenzepine as a basic medication. Am J Med 79:55–61
Zinner MJ, Zuidema GD, Smith P, Mignosa M (1981) The prevention of upper gastrointestinal tract bleeding in patients in an intensive care unit. Surg Gynecol Obstet 153:214–220
Schandelmaier S, Briel M, Varadhan R, Schmid CH, Devasenapathy N, Hayward RA. A new instrument to assess the credibility of effect modification analyses (ICEMAN) in randomized controlled trials and meta-analyses (in submission)
Darlong V, Jayalakhsmi TS, Kaul HL, Tandon R (2003) Stress ulcer prophylaxis in patients on ventilator. Trop Gastroenterol 24:124–128
Fabian TC, Boucher BA, Croce MA et al (1993) Pneumonia and stress ulceration in severely injured patients. A prospective evaluation of the effects of stress ulcer prophylaxis. Arch Surg 128:185–191
Fogas JF, Kiss KK, Gyura FG, Tóbiás ZT, Molnár ZM (2013) Effects of proton pump inhibitor versus H2-receptor antagonist stress ulcer prophylaxis on ventilator-associated pneumonia: a pilot study. Crit Care 17:S150–S151
Gundogan K, Karakoc E, Teke T, Zerman A, Coruh A, Sungur M (2017) Effects of enteral nutrition on stress ulcer hemorrhage in critically ill patients: multicenter randomized controlled trial. Intensive Care Medicine Experimental Conference:30th Annual Congress of the European Society of Intensive Care Medicine, ESICM 2017. Austria. 2015 (2012 Supplement 2011) (no pagination)
Hummer-Sigiel M, Jacquier A, Girard A, Garric J, Laxenaire MC, Mandorla JY (1986) Ranitidine pour la prophylaxie de l'ulcére de stress chexz les traumatisés crâniens graves. Ann Med Nancy l'Est 25:101–103
Kotlyanskaya A, Luka B, Mukherji R, Shukla M, Vajinder V, Cohen H (2007) A comparison trial of lansoprazole disintegrating tablet, lansoprazole suspension or ranitidine for stress ulcer prophylaxis in critically ill patients. Crit Care Med 35:A194–A194
Phillips J, Metzler M, Huckfeldt R, Olsen K (1998) A multicenter, prospective, randomized clinical trial of continuous infusion i.v. ranitidine vs omeprazole suspension in the prophylaxis of stress ulcers. Crit Care Med 26:101A
Simms HH, DeMaria E, McDonald L, Peterson D, Robinson A, Burchard KW (1991) Role of gastric colonization in the development of pneumonia in critically ill trauma patients: results of a prospective randomized trial. J Trauma 31:531–536
Wee B, Liu CH, Cohen H, Kravchuk S, Reddy K, Mukherji R (2013) IV famotidine vs IV pantoprazole for stress ulcer prevention in the ICU: a prospective study. Crit Care Med 41:A181
Bhanot RD (2010) Nosocomial pneumonia in mechanically ventilated patients receiving ranitidine, omeprazole or sucralfate as stress ulcer prophylaxis. Am J Respir Crit Care Med 181:A6039
Metz CA, Livingston DH, Smith JS, Larson GM, Wilson TH (1993) Impact of multiple risk factors and ranitidine prophylaxis on the development of stress-related upper gastrointestinal bleeding: a prospective, multicenter, double-blind, randomized trial. The Ranitidine Head Injury Study Group. Crit Care Med 21:1844–1849
Mustafa NA, Akturk G, Ozen I, Koksal I, Erciyes N, Solak M (1995) Acute stress bleeding prophylaxis with sucralfate versus ranitidine and incidence of secondary pneumonia in intensive care unit patients. Intensive Care Med 21:287
Basso N, Bagarani M, Materia A, Fiorani S, Lunardi P, Speranza V (1981) Cimetidine and antacid prophylaxis of acute upper gastrointestinal bleeding in high risk patients. Controlled, randomized trial. Am J Surg 141:339–341
Brophy GM, Brackbill ML, Bidwell KL, Brophy DF (2010) Prospective, randomized comparison of lansoprazole suspension, and intermittent intravenous famotidine on gastric pH and acid production in critically ill neurosurgical patients. Neurocrit Care 13:176–181
Cartier F, Gauthier-Lafaye P, Lareng L, Mottin J, Cara M, Passelecq J (1980) Cimetideine in patients at risk of stress ulcers: a multi-centre controlled trial. Intensive Care Med 6:54
Friedman CJ, Oblinger MJ, Suratt PM et al (1982) Prophylaxis of upper gastrointestinal hemorrhage in patients requiring mechanical ventilation. Crit Care Med 10:316–319
Larson GM, Davidson P, Brown J, Wilson T, Bishop A (1989) Comparison of ranitidine versus placebo on 24-hour gastric Ph and upper gastrointestinal (UGI) bleeding in head injury patients. Am J Gastroenterol 84:1165
Luk GD, Summer WR, Messersmith JF (1982) Cimetidine and antacid in prophylaxis of acute gastrointestinal bleeding: a randomized, doubleblind, controlled study. Gastroenterology 82:1121
Pan X, Zhang W, Li Z et al (2004) The preventive effects of rabeprazole on upper gastrointestinal tract hemorrhage in patients with severe acute pancreatitis. Chin J Gastroenterol 9:30–32
van den Berg B, van Blankenstein M (1985) Prevention of stress-induced upper gastrointestinal bleeding by cimetidine in patients on assisted ventilation. Digestion 31:1–8
McGinn T, Wyer P, McCullagh P, Wisnivesky J, Devereaux PJ, Stiell I, Richardson S, Agoritsas T, Guyatt G. Clinical Prediction Rules. In: Users' Guides to the Medical Literature. Chapt. 19.4
Acknowledgements
We thank Rachel Couban for her help with the search strategy and running the search. We thank Diane Heels-Ansdell for the support and suggestions for the statistical analysis for subgroup analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
YW, ZKY, RAS, ARB and GHG are the co-authors of the BMJ rapid recommendation for gastrointestinal bleeding prophylaxis (https://doi.org/10.1136/bmj.l6722). AP and MHM are the co-authors of the SUP-ICU trial (https://doi.org/10.1056/NEJMoa1714919); WA, DC and GHG are the investigators of the REVISE trial (NCT03374800).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Ge, L., Ye, Z. et al. Efficacy and safety of gastrointestinal bleeding prophylaxis in critically ill patients: an updated systematic review and network meta-analysis of randomized trials. Intensive Care Med 46, 1987–2000 (2020). https://doi.org/10.1007/s00134-020-06209-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-020-06209-w