Abstract
We calculated a self-thinning exponent of 1.05 for tree mass using the 3/2 power equation in 93 Cunninghamia lanceolata plots. According to Weller’s allometric model, the self-thinning exponent for tree mass was calculated as 1.28 from the allometric exponents θ and δ. The both self-thinning exponents were significantly lower than 3/2. The self-thinning exponent of organs was estimated to be 1.42 for stems, 0.93 for branches, 0.96 for leaves, 1.35 for roots and 1.28 for shoots, respectively. The self-thinning exponent of stem mass was not significantly different from 3/2, whereas thinning exponents of trees, branches, leaves and roots were significantly lower than 3/2. The stand leaf mass and stand branch mass were constant regardless of the stand density. The scaling relations among branch, leaf, stem, root and shoot mass (\( \overline{M}_{B} \), \( \overline{M}_{L} \), \( \overline{M}_{S} \), \( \overline{M}_{R} \) and \( \overline{M}_{A} \), respectively) showed that \( \overline{M}_{B} \) and \( \overline{M}_{L} \) scaled as the 3/4 power of \( \overline{M}_{S} \), whereas \( \overline{M}_{S} \) or \( \overline{M}_{A} \) scaled isometrically with respect to \( \overline{M}_{R} \).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Competition among individuals has a major impact on plant populations (Fréchette et al. 2005; Gascoigne et al. 2005), which is intensified as the plants grow in size and their resource requirements increase, and finally results in density-dependent mortality, or self-thinning (Reynolds and Ford 2005). Self-thinning is considered as one of the most important plant demographic processes and has important implications for the ecology and evolution of crowded plant populations (Analuddin et al. 2009). Reineke (1933) first derived an allometric equation between average tree diameter and maximum stand density in self-thinning stands. In this equation, the allometric coefficient was −1.605 for all species. Yoda et al. (1963) studied various soil fertility treatments in a classical self-thinning experiment and plotted the log of mean plant biomass against the log of plant density in one graph with a single subjectively placed boundary line. This described the relationship between mean plant biomass and density in overcrowded even-aged monocultures as:
where \( \overline{M} \) is mean plant biomass, N is population density, logK is a species-specific intercept, and the exponent α is close to 3/2, regardless of species, age, or site conditions. This type of boundary line was later known generally as the self-thinning line for even-aged plant populations (Westoby 1984; Bi 2004).
The seminal works of Reineke (1933) and Yoda et al. (1963) were later followed by many studies on self-thinning in terrestrial plant populations (e.g., Harper 1977; Xue et al. 1999, 2010; Hagihara 2000; Roderick and Barnes 2004; Coomes and Allen 2007; McCarthy and Weetman 2007; Zhang et al. 2007; Chen et al. 2008). Weller (1987a) demonstrated that many data sets on terrestrial vascular plants published to support the self-thinning law in fact led to exponents that diverged from 3/2. Subsequently, it was confirmed that Reineke’s allometric coefficient or Yoda et al.’s self-thinning exponent could differ as a function of plant characteristics (e.g., Zeide 1985, 1987; Weller 1987b, 1991), species (Pretzsch and Biber 2005; Pretzsch 2005, 2006; Weiskittel et al. 2009), soil nutrient conditions (Morris 2003; Bi 2004), site index (Bi 2001; Weiskittel et al. 2009), and stand histories (del Río et al. 2001) because some abiotic factors, such as light, water, nutrient availability, and temperature, could directly affect the self-thinning exponent in plant communities (Callaway et al. 2002; Deng et al. 2006). However, in some cases values of α close to 3/2 have been supported (e.g., Bégin et al. 2001; Ogawa 2001, 2009; Osawa and Kurachi 2004; Newton 2006) and this value continues to be used in some practical applications.
Bi (2004) argued that invariant self-thinning exponent might be the result of a lack of rigorous statistical testing. Some researchers suggested several statistical techniques to examine maximum size-density relations (Bi et al. 2000; Bi 2001, 2004; Zhang et al. 2005). After comparing these statistical techniques, Zhang et al. (2005) found that quantile regression had important advantages over ordinary least squares regression and principal components analysis that are commonly used statistical techniques for examining maximum size-density relations (Weiskittel et al. 2009). Quantile regression is a method for estimating the conditional quantiles of the distribution of a dependent variable in a linear regression model (Mäkinen et al. 2008). It has been suggested for finding boundaries in a variety of ecological settings (Cade and Noon 2003) because it does not require the subjective selection of a subset of the data based on predefined criteria (Zhang et al. 2005). This is helpful in dealing with very large data sets (Ducey and Knapp 2010).
Weller (1987a) proposed the allometric model, which quantifies “shape” by several allometric relationships of which the height-mass relationship and biomass density of trees (mass per unit occupied space) are the most relevant (Scrosati 2000; Verkerk 2005). In this model, the self-thinning exponent varies with the height-mass relationship and biomass density of trees if trees grow allometrically and it only equals 3/2 if trees grow isometrically, i.e. tree shape is truly invariant. Recent studies have demonstrated that tree shape and biomass density have an important influence on the self-thinning exponent (Weller 1987b; Osawa and Allen 1993; Kikuzawa 1999; Xue et al. 1999; Ogawa 2009). However, we still know little about the self-thinning exponent of tree organs, especially roots (Zhang et al. 2012). Compared to annual increments in stems, branches and leaves often have a small biomass increment. This is because leaves and low branches are often shed from trees, which might result in different thinning exponents in different organs. Weller’s model merits further investigation to test its applicability to different tree organs, since competition during the course of self-thinning alters biomass allocation (Weiner et al. 1990; Weiner and Thomas 1992). Knowledge of exponents among organs might improve our understanding of access to resources by individuals in response to competition in self-thinning stands.
The partitioning of above-ground mass with respect to root mass of plants influences many of the functions of plant communities (e.g. Zerihun and Montagu 2004; Niklas 2005; Hui and Jackson 2006). Allometric theory proposed by Niklas and Enquist (2002) claimed that above-ground mass scales nearly isometrically with respect to root mass, while leaf mass scales as the 3/4 power of stem mass (Niklas 2004, 2005, 2006; Niklas and Spatz 2006). Nevertheless, the scaling relationship between above-ground mass and root mass at the forest level remains controversial (Cheng and Niklas 2007).
Chinese fir (Cunninghamia lanceolata) is one of China’s most important commercial tree species. Its planted area exceeded 9.2 million ha in 2005, accounting for 29 % of the total forested area in south China (Lei 2005; Zhang et al. 2013). The typical rotation age of C. lanceolata ranges from 19 to 24 years with a diameter at breast height of 14 cm (Zhou et al. 2001). However, self-thinning exponents for its organs are unclear. In this study, we analyzed biomass data from 93 even-aged C. lanceolata plots undergoing self-thinning to demonstrate the applicability of Weller’s model as a framework for understanding the mean organ mass–density relationships of this species. The objectives of this study were: (1) to examine the self-thinning exponents for total tree mass using the 3/2 power equation and Weller’s model; (2) to check height-mass and biomass density-mass allometry for total tree mass; (3) to compare the difference in height-mass and biomass density-mass allometry for all tree organs and (4) to inspect scaling relations between branch, leaf, stem and root mass.
Materials and methods
Field investigation
Field work was conducted in 31 even-aged C. lanceolata plantations across several provinces (Hunan, Jiangxi, Guangdong and Guangxi) over a broad region in southern China at latitudes ranging from 22°00′ to 26°86′N and longitudes from 109°39′ to 117°19′E. The region has a subtropical monsoon climate characterized by long hot summers, high humidity, and mild winters. Mean annual temperature is over 22 °C. Mean annual precipitation is between 1500 and 2000 mm and falls mainly during the rainy season from April to August. The soils under the C. lanceolata stands are classified as lateritic red earth.
Thirty-one even-aged pure stands of C. lanceolata were chosen to examine the self-thinning boundary lines. The stands had large stem diameters and tree heights, were planted to the same density, and had closed canopies and high site quality. Tree mortality rates of each stand exceeded 5 %. Three sampling plots, each 20 m × 20 m in area, were established in each stand. The plots were separated by about 100 m. The living and dead trees were counted in all plots. The diameter at breast height (DBH) of each tree was calculated from the girth of the tree trunk measured at breast height (1.3 m). The tree height of each tree was measured with a telescoping leveling rod to the nearest meter.
Fifteen sample trees in each stand were felled in 2010. Tree height (h) and DBH were measured. Each of the felled trees was separated into stem, branches, leaves and roots. For each tree, the root mass down to 1 m in depth was determined by the method of excavation described by Fang et al. (2007) and Xue et al. (2011). Although we understood that a percentage of roots can grow beyond the diameter of the projected crown, roots that overlapped with other tree roots could offset remote roots outside the projected area (Xiang et al. 2011). Fresh masses of stem, branches, leaves and roots were weighed. A subsample of each organ was taken, its fresh mass measured, and brought back to the laboratory for determination of oven-dry mass. The dry-to-fresh mass ratio was used to calculate oven dry mass of each tree component.
The simple allometric equation for mass of an organ M o, such as the stem, branch, leaf, or root, in relation to DBH and h was as follows:
where a and b are coefficients. On the basis of the DBH and h of all individuals within each stand, M o was calculated using these allometric relationships established for different organs in different stands. Tree mass M was defined as the sum of the organ masses. A summary of the C. lanceolata stands is given in Table 1.
Determination of the self-thinning line
Weller (1987a) proposed the allometric model as follows:
where α = 1/(2φ) is the thinning exponent, and φ reflects changes in plant shape with size. The model recognizes that a plant can add mass by height growth, radial growth and packing biomass w in the space already occupied, and assumes that height H, area occupied A and biomass density d (=w/(AH)) vary with plant mass M according to the allometric power relationships H ∝ M θ, d ∝ M δ and R ∝ M φ, where R is the side length of the occupied area of a square A (A = 10,000/N with A in m2 and N in trees per ha) (the original definition of R given by Weller (1987a, b) was ‘the radius of the occupied area’, and the present definition was used for convenient calculation because the difference between the two was minor) and θ, δ and φ are real parameters. Then, Weller formulated a relationship among the parameters for self-thinning stands as:
Inserting Eq. 4 into Eq. 3 gives the following equation:
Equation 5 allows the self-thinning boundary line with the slope −α to be estimated from the allometric exponents θ and δ. It is apparent from Eq. 5 that α in Eq. 1 equals 3/2 only if 1 − (θ + δ) is equal to 2/3. Mean organ biomass density \( \bar{d}_{o} \) and mean tree biomass density \( \bar{d} \) were calculated for each stand by dividing the total organ biomass or stand biomass by the sum of the product of each tree height and its occupied area of a square A (Xue et al. 1999).
The data points of \( \bar{M} - N \) and \( \overline{M}_{o} - N \) were used to formulate the self-thinning line. The estimated thinning exponents were calculated from exponents θ and δ in Eq. 5.
Quantile regression was used to estimate the exponent of the self-thinning boundary line given by Eq. 1.
Results
Self-thinning line of tree mass based on the 3/2 power law of self-thinning
The relationship between mean tree mass and stand density for the C. lanceolata stands is shown in Fig. 1. Data points for 93 plots are shown for this species. The estimating quantile regression exponents α for the 90th, 92.5th, 95th 97.5th and 99th percentile ranged from 1.12 ± 0.04 to 1.05 ± 0.03 (Table 2). The self-thinning estimated by the 99th percentile plotted on the self-thinning boundary (the secondary line from the upper right in Fig. 1), whereas self-thinning estimated by the 97.5th percentile and other percentiles were over and below the self-thinning boundary, respectively (Fig. 1). Therefore, the exponent of the self-thinning boundary line was assumed to be 1.05, which was significantly lower than 3/2 (P < 0.001).
Scatterplots of mean tree mass \( \overline{M}_{T} \) to density N in the C. lanceolata stands. The self-thinning line is given by Eq. 1
Height-mass and biomass density-mass allometry for total tree mass
The allometric relationships of mean tree height \( \overline{H} \) to tree mass \( \overline{M}_{\text{T}} \) are shown in Fig. 2a. Height significantly increased with increasing mean tree mass on log–log coordinates (P = 2.08 × 10−12). The allometric relationship was formulated as:
Allometric relationship for total tree mass. a Allometric relationship between mean tree height H and mean tree mass \( \overline{M}_{T} \). The straight lines are based on the equation: \( \overline{H} = a\overline{M}_{T}^{\theta } \), a is a coefficient; b allometric relationship between mean tree biomass density \( \overline{d}_{T} \) and mean tree mass \( \overline{M}_{T} \). The straight lines are based on the equation: \( \overline{d}_{T} = b \), where b is a coefficient
The value of the allometric exponent θ was estimated to be 0.1956 ± 0.019 (SE) for the tree (Table 3).
The resulting mean biomass density of the tree ranged from 1.77 to 3.64 kg m−3 (Fig. 2b). The exponent δ of the tree did not differ significantly from zero (P = 0.578). The allometric relationship was formulated as:
The value of the allometric exponent δ was 0.0243 ± 0.0251 (Table 3).
The estimated thinning exponent from Eq. 5 using θ calculated with Eq. 6 and δ calculated with Eq. 7 was 1.28, which was significantly less than 3/2 (P < 0.001) (Table 3).
Height-mass and biomass density-mass allometry for all organs
Height significantly increased with increasing mean organ mass on log–log coordinates (8.54 × 10−37 < P < 2.14 × 10−21). The allometric relationship was formulated as:
The value of the allometric exponent θ was estimated at 0.1789 ± 0.0364 (SE), 0.2320 ± 0.0107 (SE), 0.2260 ± 0.0364 (SE), 0.1856 ± 0.0204 (SE) and 0.1927 ± 0.0121 (SE) for the stem, branches, leaves, roots and shoot (stem + branches + leaves), respectively (Table 3).
The exponent δ of organs was significantly different from zero (3.02 × 10−19 < P < 0.015). The allometric relationship was formulated as:
The value of the allometric exponent δ was 0.1171 ± 0.0231, −0.3094 ± 0.0235, −0.2653 ± 0.0251, 0.0722 ± 0.0480 and 0.0233 ± 0.0189 for the stem, branches, leaves, roots and shoot, respectively (Table 3).
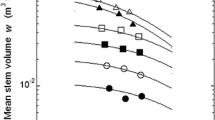
Figures 3a–d show scatter plots of mean organ mass in relation to stand density. The estimated thinning exponents using θ calculated with Eq. 8 and δ calculated with Eq. 9 were 1.42 for stem, 0.93 for branches, 0.96 for leaves, 1.35 for roots, and 1.28 for shoot, respectively (Table 3). The thinning exponent of stem was not significantly different from 3/2, whereas thinning exponents of branches, leaves, roots and shoot were significantly lower than 3/2 (P < 0.005).
Scatterplots of mean stem mass \( \overline{M}_{o} \) to density N in the C. lanceolata stands. The self-thinning line given by Eq. 5. a Stem; b branches; c leaves; d roots
Biomass density was high in the stem and low in other organs (Fig. 4a–d). Generally, the boundary stands that are stands assumed to represent the uppermost limit of the mean tree mass-density relationship (solid symbols) had higher biomass density than other stands (open symbols). Significant positive correlations existed between branch biomass density and stand density (P = 3.52 × 10−11) as well as between leaf biomass density and stand density (P = 1.10 × 10−5), whereas stem and root biomass densities were not significantly related to stand density (P > 0.269).
Scaling relations among branch, leaf, stem and root mass
The scaling relations among branch, leaf, stem, shoot and root mass (\( \overline{M}_{B} \), \( \overline{M}_{L} \), \( \overline{M}_{S} \), \( \overline{M}_{A} \) and \( \overline{M}_{R} \), respectively) are shown in Figs. 5a–d. The \( \overline{M}_{B} \) versus \( \overline{M}_{S} \), \( \overline{M}_{L} \) versus \( \overline{M}_{S} \), \( \overline{M}_{A} \) versus \( \overline{M}_{R} \) and \( \overline{M}_{R} \) versus \( \bar{M}_{S} \) regression slopes were significantly correlated (P < 0.001). All observed scaling exponents (α) complied remarkably well with those predicted by the model (Table 4). \( \overline{M}_{B} \) and \( \overline{M}_{L} \) scaled as the 0.66 and 0.67 power of \( \overline{M}_{S} \), respectively, which were not significantly different from 3/4 (P < 0.05). \( \overline{M}_{R} \) and \( \overline{M}_{A} \) scaled as the 0.94 and 1.04 power of \( \overline{M}_{S} \), respectively, which were not significantly different from 1 (P < 0.01). These results indicate that \( \overline{M}_{B} \) and \( \overline{M}_{L} \) scale as the 3/4 power of \( \overline{M}_{S} \) (\( \overline{M}_{B} \) ∝ \( \overline{M}_{L} \) ∝ \( \overline{M}_{S}^{3/4} \)) and \( \overline{M}_{R} \) and \( \overline{M}_{S} \) as well as \( \overline{M}_{A} \) and \( \overline{M}_{R} \) scale isometrically with respect to each other (\( \overline{M}_{R} \) ∝ \( \overline{M}_{S} \), \( \overline{M}_{A} \) ∝ \( \overline{M}_{R} \)) in the self-thinning C. lanceolata stands.
Scaling relations among branch, leaf, stem, root and shoot mass. a \( \overline{M}_{B} \) versus \( \overline{M}_{S} \); b \( \overline{M}_{L} \) versus \( \overline{M}_{S} \); c \( \overline{M}_{R} \) versus \( \overline{M}_{S} \); d \( \overline{M}_{A} \) versus \( \overline{M}_{R} \). The straight lines are based on the equation: \( \overline{M}_{B} \) or \( \overline{M}_{L} = {{\upbeta }}_{1} \overline{M}_{S}^{{a_{1} }} \), \( \overline{M}_{R} = {{\upbeta }}_{ 2} \overline{M}_{S}^{{a_{2} }} \) and \( \overline{M}_{A} = {{\upbeta }}_{3} \overline{M}_{R}^{a3} \), where α1, α2 and α3 are scaling exponents, β1, β2 and β3 are coefficients
Discussion
Self-thinning boundary line of trees and organs
Weller’s allometric theory and quantile regression yielded differing estimates for the thinning exponent of C. lanceolata stands. But both exponents were significantly lower than 3/2, which suggests that this exponent is not constant. Some researchers reached a similar conclusion (e.g., Bi 2001, 2004; Pretzsch and Biber 2005; Pretzsch 2005, 2006; Weiskittel et al. 2009). As a method of data analysis, quantile regression proved to be an effective mean for estimating the self-thinning boundary line (Zhang et al. 2005). This method showed no significant departures from ordinary least squares regression or the reduced major axis method (Sun et al. 2010). Therefore, further studies are needed on other species using this method of analysis.
The means by which space is filled with organ mass differ considerably. An equivalent space contains greater stem mass and smaller masses of branches, leaves and roots in C. lanceolata stands. Because of limited light availability, the area occupied by individual trees (A) is difficult to increase, but the space occupied by the tree (HA) increases as long as H increases, with the result that biomass densities of leaves and branches rapidly decrease with increasing tree height. Stem mass increases with increasing tree height and DBH, and root mass increases owing to wood accumulation in the rootstock and thick roots; consequently, the stem and root densities increase steadily, which leads to a steeper slope of the self-thinning lines for stems and roots.
The self-thinning line exponent of the stem is close to 3/2, which supports the 3/2 power law of self-thinning. In contrast, the slopes of self-thinning lines of branches, leaves, roots and tree deviates from −3/2, which can be regarded as evidence in favor of Weller’s allometric theory (Weller 1987a). Mohler et al. (1978) suggested that the slope of the self-thinning line of tree organs varies from −0.95 to −1.30 in Abies balsamea and −0.81 to −1.90 in Prunus pensylvanica, respectively. The present study also demonstrated variation of −0.93 to −1.42 in the slope of self-thinning lines among C. lanceolata organs and tree. The 3/2 power law of self-thinning is derived on the basis of a simple geometric model of space occupation by growing trees. The growth patterns of individual branches, leaves and trees may change from isometric to simple allometric with increased competition intensity in dense stands, which leads to deviation in the slope of the self-thinning line from −3/2.
The self-thinning exponents concerning leaf mass and branch mass per tree approach 1 (Table 3), which indicates that the stand leaf biomass \( y_{\text{L}} = \overline{M}_{L} N = k_{L} N^{ - 1} N = K_{L} \) and stand branch biomass \( y_{B} = \overline{M}_{B} N = k_{B} N^{ - 1} N = K_{B} \) are constant regardless of stand density N. The hypothesis of constant leaf biomass is consistent with the observations in self-thinning of Nothofagus solandri (Osawa and Allen 1993), Pinus banksiana and Populus tremuloides (Osawa and Kurachi 2004). Moreover, Xue and Hagihara (2008) reported that the leaf biomass per ground area of developing P. densiflora stands under self-thinning process reached a constant value at 33 years. It seems that the constant leaf biomass of self-thinning stands has been well established. Westoby (1977) found that self-thinning could be expressed as L = KN −3/2, where L is leaf area per plant. Therefore, future work on self-thinning should measure leaf area, which is useful for finding the relationship between optimum leaf area and density in self-thinning populations.
Correlation between organ biomass density and stand density
Stem biomass density (P = 0.275) and root biomass density (P = 0.269) remain relatively steady regardless of density change (Fig. 4a, d). As trees grow taller the proportion of the structures (stem and large roots) that support and supply the productive tissues (foliage and fine roots) increases. As a result, trees have to allocate an increasing portion of their resources to the stem and large roots (Kozlowski et al. 1991). Dead wood (dead cells) continuously accumulates in the stem and roots with stand growth and the stem and roots themselves are largely composed of dead wood, so that their biomass densities show little change. Begonia et al. (1988) found that competition among spruce trees induced a significant increase in biomass density over time for stems and a significant decrease for branches, which probably is the result of hormonal inhibition of lateral bud development (Jobidon 2000).
Branch biomass density declines significantly with decreasing stand density on log–log coordinates (P = 3.52 × 10−11) (Fig. 4b). Dense stands can restrict branch development and potentially reduce the time for natural branch shedding, because they result in reduced maximum (Garber and Maguire 2005) and average branch size (Pinkard and Neilsen 2003). In addition, low branches often die because of light deprivation in C. lanceolata trees, and in a developing stand with a completely closed canopy, smaller trees are shaded out by neighboring larger trees, which results in the smaller individuals usually being thinned out (Xue and Hagihara 1999), so that lateral growth of the branches for the larger trees is difficult. Consequently, the increase in branch biomass accumulation is slow compared to the rate of increase in tree growth space, leading to a decrease in branch biomass density with decreasing stand density. Chiba (2001) found that live branch numbers declined exponentially and dead branch numbers increased exponentially with decreasing crown height of young hinoki (Chamaecyparis obtuse) trees in Japan.
Leaf biomass density declines significantly with decreasing stand density on log–log coordinates (Fig. 4c) (P = 1.10 × 10−5). Stand density is known to be a strong determinant of leaf biomass. Intense competition between neighboring trees restricts the potential for optimal expansion of crowns with regard to light interception (Longuetaud et al. 2008). At the onset of competition, lower branches die early because mutual shading decreases crown height (Reynolds and Ford 2005), which can result in reduced leaf biomass. Low leaf biomass is also caused either by shade intolerance, because low light inside the crown results in shedding of shade-intolerant leaves at the base of a canopy in dense stands, or by branching patterns, as the whorled structure of a conifer crown constrains the distribution of needles (Osawa and Allen 1993).
Enquist and Niklas (2002) proposed a general allometric model to predict scaling relationships among leaf mass \( \overline{M}_{L} \), stem mass \( \overline{M}_{S} \), and root mass \( \overline{M}_{R} \), which suggests that the scaling relations among \( \overline{M}_{L} \), \( \bar{M}_{S} \) and \( \overline{M}_{R} \) can be derived from the amount of resource used per individual plant (R 0), approximates metabolic demand and gross photosynthesis (B) (Enquist et al. 1998, 1999; West et al. 1999), and the model predicts R 0 ∝ B ∝ \( \overline{M}_{L} \) ∝ \( \overline{M}_{S}^{3/4} \) ∝ \( \overline{M}_{R}^{3/4} \). Although this metabolic theory has been challenged (Kozłowski and Konarzewski 2004, 2005; Glazier 2006) due to different physiological and morphological factors (Reich et al. 2006), environmental variability (Deng et al. 2008) and species specificity (Isaac and Carbone 2010), our results indicate that branch mass \( \overline{M}_{B} \) and leaf mass \( \overline{M}_{L} \) nearly scale as the 3/4 power of stem mass \( \overline{M}_{S} \) (\( \overline{M}_{B} \) ∝ \( \overline{M}_{L} \) ∝ \( \overline{M}_{S}^{3/4} \)), whereas \( \overline{M}_{S} \) or shoot mass \( \overline{M}_{A} \) scales nearly isometric with respect to root mass \( \overline{M}_{R} \) (\( \overline{M}_{S} \) ∝ \( \overline{M}_{R} \) and \( \overline{M}_{A} \) ∝ \( \overline{M}_{R} \)) in self-thinning C. lanceolata stands. These results are consistent with the expectations of Enquist and Niklas’ model and some empirical studies (Enquist et al. 1998, 1999; Niklas and Enquist 2001, 2002; Enquist and Niklas 2002). The results of our study support the allometric model based on metabolic theory and provide evidence for the existence of nearly a constant scaling exponent for tree organ mass in self-thinning stands.
References
Analuddin K, Suwa R, Hagihara A (2009) The self-thinning process in mangrove Kandelia obovata stands. J Plant Res 122:53–59
Bégin E, Bégin J, Bélanger L, Rivest L-P, Tremblay S (2001) Balsam fir self-thinning relationship and its constancy among different ecological regions. Can J For Res 31:950–959
Begonia GB, Aldrich RJ, Nelson CJ (1988) Effects of simulated weed shade on soybean photosynthesis, biomass partitioning and axillary bud development. Photosynthetica 22:309–319
Bi H (2001) The self-thinning surface. For Sci 47:361–370
Bi H (2004) Stochastic frontier analysis of a classic self-thinning experiment. Austral Ecol 29:408–417
Bi H, Wan G, Turvey ND (2000) Estimating the self-thinning boundary line as a density-dependent stochastic biomass frontier. Ecology 81:1477–1483
Cade BS, Noon BR (2003) A gentle introduction to quantile regression for ecologists. Front Ecol Environ 1:412–420
Callaway RM, Brooker RW, Choler P, Kikvidze Z, Lortie CJ, Michalet R, Paolini L, Pugnaire FI, Newingham B, Aschehoug ET, Armas C, Kikodze D, Cook BJ (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848
Chen K, Kang H-M, Bai J, Fang X-W, Wang G (2008) Relationship between the virtual dynamic thinning line and the self-thinning boundary line in simulated plant populations. J Integr Plant Biol 50:280–290
Cheng D-L, Niklas KJ (2007) Above- and below-ground biomass relationships across 1534 forested communities. Ann Bot 99:95–102
Chiba Y (2001) The dynamics of the hinoki crown and production of dead branches. Abstracts of the 48th Japanese Ecological Society. (in Japanese)
Coomes DA, Allen RB (2007) Mortality and tree-size distributions in natural mixed-age forests. J Ecol 95:27–40
del Río M, Montero G, Bravo F (2001) Analysis of diameter-density relationships and self-thinning in non-thinned even-aged scots pine stands. For Ecol Manag 142:79–87
Deng JM, Wang GX, Morris EC, Wei XP, Li DX, Chen BM, Zhao CM, Liu J, Wang Y (2006) Plant mass-density relationship along a moisture gradient in north-west China. J Ecol 94:953–958
Deng JM, Li T, Wang GX, Liu J, Yu ZL et al (2008) Trade-offs between the metabolic rate and population density of plants. PLoS One 3:e1799
Ducey MJ, Knapp RA (2010) A stand density index for complex mixed species forests in the northeastern United States. For Ecol Manag 260:1613–1622
Enquis BJ, Brown JH, West GB (1998) Allometric scaling of plant energetics and population density. Nature 395:163–165
Enquist BJ, Niklas KJ (2002) Global allocation rules for patterns of biomass partitioning across seed plants. Science 295:1517–1520
Enquist BJ, West GB, Charnov EL, Brown JH (1999) Allometric scaling of production and life history variation in vascular plants. Nature 401:907–911
Fang S, Xue J, Tang L (2007) Biomass production and carbon sequestration potential in poplar plantations with different management patterns. J Environ Manag 85:672–679
Fréchette M, Alunno-Bruscia M, Dumais J-F, Daigle G, Sirois R (2005) Incompleteness and statistical uncertainty in competition/stocking experiments. Aquaculture 246:209–225
Garber SM, Maguire DA (2005) Vertical trends in maximum branch diameter in two mixed-species spacing trials in the central Oregon Cascades. Can J For Res 35:295–307
Gascoigne JC, Beadman HA, Saurel C, Kaiser MJ (2005) Density dependence, spatial scale and patterning in sessile biota. Oecologia (Berl.) 145:371–381
Glazier DS (2006) The 3/4-power law is not universal: evolution of isometric, ontogenetic metabolic scaling in pelagic animals. Bioscience 56:325–332
Hagihara A (2000) Time-trajectory of mean phytomass and density in self-thinning plant populations. Bull Fac Sci Univ Ryukyus 70:99–112
Harper JL (1977) Population biology of plants. Academic Press Inc, London, p 892
Hui DF, Jackson RB (2006) Geographical and interannual variability in biomass partitioning in grassland ecosystems: a synthesis of field data. New Phytol 169:85–93
Isaac NJ, Carbone C (2010) Why are metabolic scaling exponents so controversial? Quantifying variance and testing hypotheses. Ecol Lett 13:728–735
Jobidon R (2000) Density-dependent effects of northern hardwood competition on selected environmental resources and young white spruce (Picea glauca) plantation growth, mineral nutrition, and stand structural development—a 5-year study. For Ecol Manag 130:77–97
Khan MNS, Suwa R, Hagihara A (2005) Allometric relationships for estimating the aboveground phytomass and leaf area of mangrove Kandelia candel (L.) Druce trees in the Manko Wetland, Okinawa Island, Japan. Trees 19:266–272
Kikuzawa K (1999) Theoretical relationships between mean plant size, size distribution and self-thinning under one-sided competition. Ann Bot (Lond) 83:11–18
Kozłowski J, Konarzewski M (2004) Is West, Brown and Enquist’s model of allometric scaling mathematically correct and biologically relevant? Funct Ecol 18:283–289
Kozłowski J, Konarzewski M (2005) West, Brown and Enquist’s model of allometric scaling again: the same questions remain. Funct Ecol 19:739–743
Kozlowski TT, Kramer PJ, Pallardy SG (1991) The physiological ecology of woody plants. Academic, New York
Lei J (2005) Forest resource in China. China Forestry Publishing House, Beijing, p 172 (in Chinese)
Longuetaud F, Seifert T, Leban JM, Pretzsch H (2008) Analysis of long-term dynamics of crowns of sessile oaks at the stand level by means of spatial statistics. For Ecol Manag 255:2007–2019
Mäkinen A, Kangas A, Kalliovirta J, Rasinmäki J, Välimäki E (2008) Comparison of treewise and standwise forest simulators by means of quantile regression. For Ecol Manag 255:2709–2717
McCarthy JW, Weetman G (2007) Self-thinning dynamics in a balsam fir (Abies balsamea (L.) Mill.) insect-mediated boreal forest chronosequence. For Ecol Manag 241:295–309
Mohler CL, Marks PL, Sprugel DG (1978) Stand structure and allometry of trees during self-thinning of pure stands. J Ecol 66:599–614
Morris EC (2003) How does fertility of the substrate affect intraspecific competition? Evidence and synthesis from self-thinning. J Ecol 18:287–305
Newton PE (2006) Asymptotic size–density relationships within self-thinning black spruce and jack pine stand-types: parameter estimation and model reformulations. For Ecol Manag 22:49–59
Niklas KJ (2004) Plant allometry: is there a grand unifying theory? Biol Rev 79:871–889
Niklas KJ (2005) Modelling below- and above-ground biomass for nonwoody and woody plants. Ann Bot 95:315–321
Niklas KJ (2006) A phyletic perspective on the allometry of plant biomass and functional organ-categories. A tansley review. New Phytol 171:27–40
Niklas KJ, Enquist BJ (2001) Invariant scaling relationships for interspecific plant biomass production rates and body size. Proc Natl Acad Sci USA 98:2922–2927
Niklas KJ, Enquist BJ (2002) On the vegetative biomass partitioning of seed plant leaves, stems, and roots. Am Nat 159:482–497
Niklas KJ, Spatz H-C (2006) Allometric theory and the mechanical stability of large trees: proof and conjecture. Am J Bot 93:824–828
Ogawa K (2001) Time trajectories of mass and density in a Chamaecyparis obtusa seedling population. For Ecol Manag 142:291–296
Ogawa K (2009) Theoretical analysis of the interrelationships between self-thinning, biomass density, and plant form in dense populations of hinoki cypress (Chamaecyparis obtusa) seedlings. Eur J For Res 128:447–453
Osawa A, Allen RB (1993) Allometric theory explains self-thinning relationships of mountain beech and red pine. Ecology 74:1020–1032
Osawa A, Kurachi N (2004) Spatial leaf distribution and self-thinning exponent of Pinus banksiana and Populus tremuloides. Trees 18:327–338
Pinkard EA, Neilsen WA (2003) Crown and stand characteristics of Eucalyptus nitens in response to initial spacing: implications for thinning. For Ecol Manag 172:215–227
Pretzsch H (2005) Link between the self-thinning rules for herbaceous and woody plants. Sci Agric Bohem 36:98–107
Pretzsch H (2006) Species-specific allometric scaling under selfthinning. Evidence from long-term plots in forest stands. Oecologia 146:572–583
Pretzsch H, Biber P (2005) A re-evaluation of Reineke’s rule and stand density index. For Sci 51:304–320
Reich PB, Tjoelker MG, Machado J-L, Oleksyn J (2006) Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature 439:457–461
Reynolds JH, Ford ED (2005) Improving competition representation in theoretical models of self-thinning: a critical review. J Ecol 93:362–372
Roderick ML, Barnes B (2004) Self-thinning plant populations from a dynamic viewpoint. Funct Ecol 18:197–203
Scrosati R (2000) The interspecific biomass-density relationship for terrestrial plant: where do clonal red seaweeds stand and why? Ecol Lett 3:191–197
Sun HG, Zhang JG, Duan AG (2010) A comparison of selecting data points and fitting coefficients methods for estimating self-thinning boundary line. Chin J Plant Ecol 34:409–417 (in Chinese with English summary)
Tadaki Y, Takeuchi I, Kawahara T, Sato A, Hatiya K (1979) Growth analysis on the natural stands of Japanese red pine (Pinus densiflora Sieb. et Zucc.). III. Results of experiment Research note). Bull For For Prod Res Inst 305:125–144 (in Japanese)
Verkerk PJ (2005) The role of crown packing on the ability of trees to compete for light in a temperate mixed natural regeneration. Master’s thesis, Wageningen University, The Netherland
Weiner J, Thomas SC (1992) Competition and allometry in three species of annual plants. Ecology 73:648–656
Weiner J, Berntson GM, Thomas SC (1990) Competition and growth form in a woodland annual. J Ecol 78:459–469
Weiskittel A, Gould P, Temesgen H (2009) Sources of variation in the self-thinning boundary line for three species with varying levels of shade tolerance. For Sci 55:84–93
Weller DE (1987a) Self-thinning exponent correlated with allometric measures of plant geometry. Ecology 68:813–821
Weller DE (1987b) A reevaluation of the −3/2 power rule of plant self-thinning. Ecol Monogr 57:23–43
Weller DE (1991) The self-thinning rule, dead or unsupported?—a reply to Lonsdale. Ecology 72:747–750
West GB, Brown JH, Enquist BJ (1999) A general model for the structure, and allometry of plant vascular systems. Nature 400:664–667
Westoby M (1977) Self-thinning driven by leaf area not by weight. Nature 265:330–331
Westoby M (1984) The self-thinning rule. Adv Ecol Res 14:167–226
Xiang WH, Liu SH, Deng XW, Shen AH, Lei XD, Tian DL, Zhao MF, Peng CH (2011) General allometric equations and biomass allocation of Pinus massoniana trees on a regional scale in southern China. Ecol Res 26:697–711
Xue L, Hagihara A (1999) Density effect, self-thinning and size distribution in Pinus densiflora Sieb. et Zucc. stands. Ecol Res 14:49–58
Xue L, Hagihara A (2008) Density effects on organs in self-thinning Pinus densiflora Sieb. et Zucc. Stands. Ecol Res 23:689–695
Xue L, Ogawa K, Hagihara A, Liang S, Bai J (1999) Self-thinning exponents based on the allometric model in Chinese pine (Pinus tabulaeformis Carr.) and Prince Rupprecht’s larch (larix principis-rupprechtii Mayr) stands. For Ecol Manag 117:87–93
Xue L, Chen FX, Feng HF (2010) Time-trajectory of mean component weight and density in self-thinning Pinus densiflora stands. Eur J For Res 129:1027–1035
Xue L, Pan L, Zhang R, Xu PB (2011) Density effects on organs in self-thinning Eucalyptus urophylla stands. Trees 25:1021–1031
Yoda K (1971) Ecology of Forests. Tsukiji Shokan, Tokyo, Japan, 331p. (in Japanese)
Yoda K, Kira T, Ogawa H, Hozumi H (1963) Self-thinning in overcrowded pure stands under cultivated and natural conditions. (Intraspecific competition among higher plants. XI.). J Biol Osaka City Univ 14:107–129
Zeide B (1985) Tolerance and self-tolerance of trees. For Ecol Manag 13:149–166
Zeide B (1987) Analysis of the 3/2 power law of self-thinning. For Sci 33:517–537
Zeide B (1995) A relationship between size of trees and their number. For Ecol Manag 72:265–272
Zerihun A, Montagu KD (2004) Belowground to aboveground biomass ratio and vertical root distribution responses of mature Pinus radiata stands to phosphorus fertilization at planting. Can J For Res 34:1883–1894
Zhang L, Bi H, Gove JH, Heath LS (2005) A comparison of alternative methods for estimating the self-thinning boundary line. Can J For Res 35:1507–1514
Zhang J, Oliver WW, Ritchie MW (2007) Effect of stand densities on stand dynamics in white fir (Abies concolor) forests in northeast California, USA. For Ecol Manag 244:50–59
Zhang WP, Jia X, Morris EC, Bai YY, Wang GX (2012) Stem, branch and leaf biomass-density relationships in forest communities. Ecol Res 27:819–825
Zhang X, Duan A, Zhang J (2013) Tree biomass estimation of Chinese fir (Cunninghamia lanceolata) based on Bayesian Method. PLoS One 8:e79868
Zhou GM, Guo RJ, Wei XL, Wang XJ (2001) Growth model and cutting age of Chinese fir planted forest in Zhejiang Province. J Zhejiang For Coll 18:219–222 (in Chinese with English summary)
Acknowledgments
This study was partially supported by Foundation of Guangdong Forestry Bureau (Nos. 4400-F11031, 4400-F11055).
Author information
Authors and Affiliations
Corresponding author
Additional information
The online version is available at http://www.springerlink.com
Corresponding editor: Chai Ruihai
Rights and permissions
About this article
Cite this article
Xue, L., Hou, X., Li, Q. et al. Self-thinning lines and allometric relation in Chinese fir (Cunninghamia lanceolata) stands. J. For. Res. 26, 281–290 (2015). https://doi.org/10.1007/s11676-015-0059-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-015-0059-3