Abstract
Sessile biota can compete with or facilitate each other, and the interaction of facilitation and competition at different spatial scales is key to developing spatial patchiness and patterning. We examined density and scale dependence in a patterned, soft sediment mussel bed. We followed mussel growth and density at two spatial scales separated by four orders of magnitude. In summer, competition was important at both scales. In winter, there was net facilitation at the small scale with no evidence of density dependence at the large scale. The mechanism for facilitation is probably density dependent protection from wave dislodgement. Intraspecific interactions in soft sediment mussel beds thus vary both temporally and spatially. Our data support the idea that pattern formation in ecological systems arises from competition at large scales and facilitation at smaller scales, so far only shown in vegetation systems. The data, and a simple, heuristic model, also suggest that facilitative interactions in sessile biota are mediated by physical stress, and that interactions change in strength and sign along a spatial or temporal gradient of physical stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To understand processes that regulate community structure, population dynamics and stability it is necessary to understand the spatial scale at which ecological interactions occur (Levin 1992; Schneider 1994). Finding the appropriate spatial scale at which to gather data is a difficult problem that often only becomes apparent a posteriori. Data gathered at an inappropriate spatial scale may fail to show patterns that exist at a smaller or larger scale (Ray and Hastings 1996; Hamer and Hill 2000; Williams and Liebhold 2000; Kaiser 2003), or may show different patterns (Bertness and Leonard 1997; Morgan et al. 1997; Chase and Leibold 2002). This is particularly problematic for mobile organisms, for which it is difficult to define the scale over which individuals interact with each other regularly (Frank and Brickman 2000). However, in sessile organisms, the scale of interaction and patchiness in population density is much more readily defined. These systems provide a useful model in which to examine the importance of spatial scale in interactions among individuals.
Sessile, attached organisms that occur in physically perturbed or resource limited environments are often highly patchy, even in an apparently uniform environment. Patchiness can take the form of patterns such as banding or regular patches, for example, in semiarid and peatland vegetation (Klausmeier 1999; Couteron and Lejeune 2001; Rietkerk et al. 2002, 2004a) or in mussel beds (van de Koppel et al. 2005). (We draw a distinction between pattern formation which occurs in a uniform environment, and zonation which occurs along an environmental gradient.) Vegetation patterns are created by a combination of positive and negative interactions at different spatial scales–small-scale facilitation through soil shading and root enhancement of soil permeability, and larger scale competition for water (Klausmeier 1999; Rietkerk et al. 2002). Similar patterns in mussel beds can be modelled by assuming facilitation at small scales and competition at larger scales, in analogy to vegetation (van de Koppel et al. 2005). No field data exist to demonstrate whether different types of interactions do occur at different spatial scales in patchy mussel beds, however.
Mussels are often dominant species in the intertidal, and a clear understanding of their ecology is important for our knowledge of coastal systems. In this study, we examined a patterned bed of blue mussels Mytilus edulis L. as a model system to test the hypothesis that scale and density dependent effects on growth explain the patterning of mussel beds, similar to terrestrial systems with regular patterns: competition and facilitation occurring at different spatial scales. We show that patterned mussels do indeed show small-scale facilitation and large-scale competition, as with patterned vegetation systems, although competition and facilitation are separated temporally as well as by spatial scale.
Methods
Site
The experiment was set up on a mudflat in the Menai Strait, North Wales, UK. The site is at approximately low water springs and has strong tidal currents (up to 60 cm−1) and a short slack tide period (20–60 min) (JG and CS unpublished data). The site is usually used by the commercial mussel industry for ongrowing mussels prior to moving them to subtidal fattening areas. We used two experimental mussel beds which were created with the help of the commercial mussel growers to look at mussel growth rates and density at two spatial scales that spanned ~4 orders of magnitude, from 0.0625 m2 quadrats to 400 m2 squares (Beadman 2003; Beadman et al. 2004). Mussels were by far the dominant species in these beds, thus interspecific competition was not likely to play an important role in mussel growth (Beadman et al. 2004).
Experimental design and layout
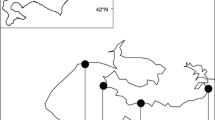
Mussels were laid in two replicate 80×80 m beds, separated by 20 m. Each bed was divided into sixteen 20×20 m squares containing one of four density treatments: High (H; 7.5 kg m−2), Medium High (MH; 5 kg m−2), Medium Low (ML; 3 kg m−2) and Low (L; 2 kg m−2) in a Latin squares design (Fig. 1). The slope from the water to land side of the beds was negligible (Caldow et al. 2003). Mussels at this site are relatively mobile on a small scale, and naturally form up into patches on a scale of 2–3 m (see below). This means that there was no way to reliably manipulate densities on a scale smaller than this without interfering significantly with the environment and the natural process of pattern formation (e.g. by using enclosures or artificial substrates). Density variation on the smaller scale was thus allowed to occur naturally, and this factor was used as a covariate in the analysis.
The two beds were seeded in April 2000, using seed from the same cohort collected from wild subtidal beds. The seed were hosed over the side of a boat at high tide. It was not possible to lay the mussels in precise squares using this method, but a posteriori examination of the site showed that the boundaries between squares of different densities were clear. To make sure that seed was suitably randomised at the start of the experiment, mussel length was sampled from each square on 21 April 2000 and no difference in length was found by square density or bed (ANOVA: square density P=0.19, bed P=0.754).
Sampling
The beds were sampled on seven occasions from June 2000 to April 2001 (Fig. 5), by taking four 0.25×0.25 m quadrats from each square at random while avoiding large gaps in mussel cover. The mussels in each quadrat were counted, and 30 mussels selected at random were measured for shell length (nearest mm from the umbo to the edge of the posterior margin of the shell) and flesh dry weight (DW) by drying in an oven at 90°C for 12 h. Five mussels from each quadrat were burnt in a muffle furnace for 2 h at 550°C to determine their ash free dry weight (AFDW). Analysis showed that AFDW and DW were closely correlated (DW=0.0316+1.18AFDW, P<0.0005, R 2=0.93; Beadman 2003). Accordingly, we have used DW in our analyses because it allows for larger sample sizes.
We measured seasonal trends in mussel food availability by taking water samples from the Menai Strait two or three times weekly over the course of the experiment and analysing for chlorophyll a concentration by filtering on GFF 45-μm filter paper, extracting chlorophyll with acetone and measuring concentration on a Turner laboratory fluorometer.
The most important mussel predator at this site is the green crab Carcinus maenas, which targets smaller mussels. The oystercatcher Haematopus ostralegus, which targets larger mussels, can also be present in large numbers in winter, but recent data from winter 1999–2000 and 2000–2001 showed that oystercatchers consume less than 2% of biomass at this site (Caldow et al. 2003).
We measured relative seasonal densities of C. maenas by sampling with a net from a boat at high tide. We used an unbaited 1-m2 flat net left on the bottom for 10 min. On each sampling occasion (Fig. 5) each square in Bed 1 was sampled three times.
Patterning
The existence of regular patterns (as opposed to irregular patchiness) was tested post hoc in April 2005, in another mussel bed, since the experimental mussel bed had been harvested. This mussel bed was located close to the site of the experimental mussel bed in the Menai Strait (~2 km away) on the same type of substrate (soft mud), at the same tidal height (1.5 m above chart datum) and very similar flow conditions (JG unpublished data), commercial stocking densities and mussels of the same size (mean length ~25 mm) and from the same source as in the original experiment.
We tested for regular patterns by sampling along two 25 m transects, measuring the density of mussels every half metre using an 0.1 m2 quadrat. Densities were similar to the original experiment (0–4,000 mussels m−2). The data were analysed using a Fast Fourier Transform, using Matlab.
Data analysis
Inspection of the data and previous model fitting (Beadman 2003) demonstrated that mussel growth can be divided into two approximately linear periods. There is a period of rapid growth (June–September; “summer”, four sampling periods), followed by a period of little or no growth, or even some weight loss (November–April; “winter”, three sampling periods). Summer data and winter data were therefore analysed separately.
To assess the effect of density and spatial scale in summer data, we used a General Linear Model (GLM), with factors as follows: (1) square density treatment as a fixed factor with four levels (H, MH, ML and L); (2) bed as a fixed factor with two levels; (3) square as a fixed factor with 32 levels, nested within square density and bed, (4) date as a fixed factor with four levels; and (5) quadrat density as a covariate. Where there were interaction effects, data were analysed within each level of interacting factors (Underwood 1997).
We examined winter growth independent of summer growth by generating regression equations for shell length and DW in September (end of the summer period) as a function of quadrat density, for each square density treatment. We then used the September regression fits to correct March length and DW data and calculate shell and meat weight increments from the winter months. A drawback of this approach is the assumption that quadrat densities measured in March were valid throughout the winter. There was no significant change in quadrat density over the winter months for any square density treatment (ANOVA with date as random factor and square density treatment as fixed factor: date P=0.074, square density P=0.611, interaction P=0.613), so we concluded that this would not affect the final results significantly. Corrected length and DW data for March were analysed using a GLM with square density treatment and bed as fixed factors, square as a fixed factor nested in square density and bed and quadrat density as a covariate. We used March rather than April data since analysis showed that the mussels had started a new period of spring growth between the March and April sampling periods.
Both summer data and March corrected data showed homogeneity of variance between each replicate (square) and each date (Bartlett’s test and Levene’s test, P>0.05 for length and DW). March corrected data was normally distributed (Anderson–Darling test, P>0.05 for length and DW) but Summer data was not (P< 0.0005 for length and DW, even under transformation). However, GLM is robust for validity (does not give false rejections of the null hypothesis) under deviations from normality as long as variance is homogeneous and sample sizes are not unbalanced (Underwood 1997, Zar 1999). Since our sample sizes are balanced, we considered transformation to be unnecessary.
Density related variation in mussel size could arise through size and density specific mortality as well as through density dependent growth. If predators are an important source of mortality, predation can result in mussels in dense patches having, on average, a greater shell length if predators such as C. maenas, which target smaller mussels, also target denser patches.
This process would show up in the length frequency distributions. Selective predation on smaller mussels in denser quadrats would result in the denser quadrats having truncated length frequency distribution with a smaller standard deviation and positive skew.
We therefore compared length frequency distributions for the 64 densest quadrats and the 64 sparsest quadrats in the March data. We measured skew and compared standard deviations using resampling (100 runs with n=20 samples with replacement) to generate a frequency distribution for the standard deviation for low-density quadrats and high-density quadrats. These were compared using a t test.
We compared chlorophyll data for summer and winter using a t test. Summer data was taken as the period covering the four summer sampling dates (June 15–September 30). Winter data covered the November and March sampling dates but was cut off before the spring bloom, which started around March 20, so we did not cover the April 8 sampling date (October 26–March 20).
Wave and wind data
In order to assess seasonal variation in significant wave height, we acquired wave data from the British Oceanographic Data Centre for the closest source for which a time series of wave data was available—St. Gowan Lightvessel on the southwest coast of Wales. Absolute values of wave height will not be comparable with the Menai Strait, but gross seasonal trends will be similar.
More local data was obtained from hourly wind averages from February 2003 to January 2004 from a weather station on the roof of the School of Ocean Sciences, University of Wales Bangor, in Menai Bridge (about 2 km from the field site). From these we calculated the mean number of (1) days per month with gusts >15 ms−1 from any direction, (2) days with gusts >15 ms−1 from the quadrant from north to east, and (3) days with mean wind >5 ms−1 from the northeast quadrant. We focused on the northeast quadrant because the site has a long fetch in this direction.
Modelling
To assess the combined effect of competition and mechanical stress on small-scale density dependence, we produced a simple heuristic model of mussel energetics as a function of local mussel density. In the model, energy available to the mussels for metabolism, growth and reproduction (E) is a function of food availability in the water column (F), competition from surrounding mussels (C) and energy expended to resist mechanical stress from wave action (M).
C, the competition parameter, was modelled as a hyperbolic function of local mussel density y, with a coefficient c (0<c<1), which sets the maximum proportional depletion of food due to local competition.
M, the wave stress parameter, was modelled as an exponentially decreasing function of y, with a coefficient m (0<m<1) determining the maximum proportion of energy expended to resist mechanical stress.
The energy available to mussels (E) as a function of the local mussel density was determined for different ratios of c to m. This allowed us to see, at least in the simplest terms, whether both negative density dependence and positive density dependence of E could be obtained as the balance between competition for food and mechanical wave stress varied. The model was run using Matlab.
Results
Patterning
The Fast Fourier Transform analysis of both mussel transects showed clear peaks at frequencies of 0.314 and 0.431 waves per metre, corresponding to patterning with a wavelength of 2.3–3.1 m (Fig. 2).
Mussel growth
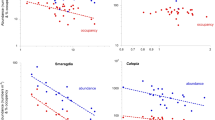
Mussel growth during the summer period was affected by the density of the mussels in the squares (large spatial scale) and in the quadrats (small spatial scale). Square density treatment had a significant negative effect on shell length and DW (Table 1). For quadrat density, we found a significant interaction between the effects of quadrat density and date on length and DW (Table 1). An analysis of quadrat density within each date demonstrated that quadrat density had a significant negative effect on length and DW for all dates (P<0.0005 for length and DW for all four dates; Fig. 3). Mussel growth also varied between individual squares (Table 1). Overall, competition between mussels was strong across both spatial scales in the summer.
Regressions of September length and DW against September quadrat density for each square treatment were all significant and negative (R 2=0.36–0.56, P<0.0005 for all treatments). These data were used to correct March data to obtain estimates of winter growth increments.
During the winter period, the relationship between growth and density was the reverse of that in the summer at the small scale. For both winter length increment and winter DW increment, quadrat density had a significant positive effect (Table 2), showing that winter growth increment of both length and DW was higher in quadrats with high density (Fig. 4). Square density treatment had no effect on mussel winter growth, although there were still significant differences in mussel growth between individual squares (Table 2).
Predation
Crabs were dense and active on Bed 1 in summer, but no crabs were found between mid November and mid March (Fig. 5). It seems unlikely, therefore, that apparent density dependence in winter is caused by size and density dependent predation due to crabs. As a check, we compared length frequency distributions for high-density and low-density quadrats for March. We found no evidence of predation-driven differences in size specific mortality from green crabs. The bootstrap analysis showed no significant difference between standard deviations of high and low density quadrats (high-density standard deviation=2.758, low-density standard deviation=2.855, t=−1.46, P=0.145). High density quadrats had a length frequency distribution with negative skew (a distribution skewed towards small mussels) rather than positive skew, while length frequency for low-density quadrats had little skew (high-density skew=−0.5, low-density skew=0.09).
Chlorophyll a concentration in the Menai Strait over the course of the experiment (black line) and crabs Carcinus maenas sampled on Bed 1 (grey bars). Crab data is mean number of crabs caught per square per sampling date. Black circles indicate mussel sampling dates, white circles indicate crab sampling dates (dates with crab sampling but no grey bar indicate zero crabs caught)
Physical environment
Chlorophyll a concentrations in the Menai Strait were lowest in late January and early February and highest from late March to late July (Fig. 5). A comparison of summer and winter (excluding the spring bloom) showed that chlorophyll levels were significantly higher from the first to last summer sampling period (June 15–September 27) than between the first two winter sampling periods (November 24–March 7) (t=4.38, P<0.0005). (Last winter sampling period, April 7, excluded because of onset of spring bloom.) Significant wave height at St. Gowan Lightvessel was greater in winter than in summer (Fig. 6). There was also a greater mean number of days per month in which the site is exposed to strong winds, both from the northeast quadrant and in any direction (Fig. 6).
Seasonal variation in significant wave height at St. Gowans Head (lines data from the British Oceanographic Data Centre) and mean days per month with gusts >15 ms−1 from any direction or from the northeast quadrant and mean wind >5 ms−1 from the northeast quadrant, February 2003 to January 2004 (bars data from School of Ocean Sciences, University of Wales Bangor)
Modelling
In the model, the energy available to mussels (E) always depends on the local density of mussels (y) (density dependence). The sign of density dependence depends on the ratio of the competition coefficient c to the wave stress coefficient m. For c>m, (energy loss from competition more important than energy loss from resisting wave stress) density dependence is negative (=competition). If m>c, (energy loss from resisting wave stress more important than energy loss from competition) density dependence is positive (=facilitation) (Fig. 7).
Discussion
In this study, we show that the sign (positive or negative) of intraspecific interactions in mussel beds varies with spatial scale, as well as temporally, by season. We found that competition at both large and small scales is important in determining mussel growth in summer, when growth rates, feeding rates and metabolic requirements are highest. During winter, however, mussel growth was higher in quadrats with high densities of mussels, suggesting facilitation over small spatial scales. Note that our results are from a soft sediment system—interactions between mussels on hard substrates may be different.
Both competition and facilitation have been demonstrated separately in mussels, on hard, soft and artificial substrates (e.g. hard substrate: facilitation—Hunt and Schiebling 2001a, competition—McQuaid and Lindsay 2000; soft substrate: facilitation—Bertness and Grosholz 1985, competition—Newell 1990, Smaal et al. 2001; artificial: facilitation—Coté and Jelnikar 1999, competition—Okamura 1986, Fréchette et al. 1992). These studies generally focused on a limited range of spatial scales, and on either competition or facilitation, and thus the interaction of competition and facilitation at different spatial scales has been difficult to assess. In mussel beds, at least on soft sediments, both types of interaction are likely to be important albeit under different circumstances.
Our spectral analysis shows regular patterning in Menai Strait mussel beds on a spatial scale of a similar magnitude (wavelength=2.3–3.1 m) to that found for patterned mussel beds in the Waddensea (wavelength=~6 m, van de Koppel et al. 2005), and vegetation systems (10–50 m, Klausmeier 1999; 31–50 m, Couteron and Lejeune 2001; 5–20 m, Rietkerk 2002). In a recent paper, it was proposed that self-organised patterning in mussel beds results from scale-dependent interaction of competition and facilitation between the mussels (van de Koppel et al. 2005), as already shown in patterned vegetation systems (Klausmeier 1999; Couteron and Lejeune 2001; Rietkerk et al. 2002, 2004a). The current study supports this hypothesis, although we found competition and facilitation to be separated in time as well as in space, with competition dominating during the summer and facilitation during the winter. Theoretical models of self-organisation in biological systems have not so far been time structured, but presumably seasonal variation in physical stress and resource availability also exists in other environments where patterning occurs (see Table 3). It remains to be seen what influence this may have over the process of self-organisation.
Our data do not provide any information about the mechanism for facilitation in this mussel bed. Possible mechanisms for facilitation in mussels include mechanical stress from waves and currents (Hunt and Schiebling 2001a), heating during exposure (Bertness and Grosholz 1985; Bertness and Leonard 1997) and predation (Bertness and Grosholz 1985; Okamura 1986; Côté and Jelnikar 1999). Heat stress can be ruled out since facilitation was observed only during the winter when mean air temperatures are rather low, although not low enough to cause physiological problems for M. edulis (February (coldest month) mean daily min. 2.6°C, max. 8.2°C at Colwyn Bay, North Wales; UK Meterological Office). Field observations show no evidence of a mussel feeding response to predators (CS unpublished data). The most probable mechanism for facilitation is thus reduction in mechanical stress from wave energy, which can be a major factor in structuring mussel populations (Hunt and Schiebling 2001a), particularly since accelerating flow, such as arises from wave action, is more likely to result in removal of mussels from the substrate than constant flow (Denny et al. 1985).
Facilitation in mussels has been examined mainly in the context of density dependent mortality, rather than growth, although juvenile ribbed mussels (Geukensia demissa) grow better in the presence of adults in physically stressful high intertidal environments (Bertness and Grosholz 1985). There is evidence that mechanical stress reduces growth in acorn barnacles (Semibalanus balanoides), which show a trade-off between meat growth and resistance to physical stress at low density. Solitary barnacles have a faster rate of particle capture than barnacles at the edge of dense hummocks, but have slower rates of meat growth due to a greater need to invest in protection from mechanical forces (shells are 2–5 times as thick) (Bertness et al. 1998). Mechanical stress could cause reduced growth in mussels through a trade-off between growth and byssus production. Mussels track wave stress closely in the strength of their byssal attachment, which can vary by a factor of 2.5 between summer and winter (Carrington 2002; Hunt and Schiebling 2001b), and solitary mussels require stronger byssal attachments than mussels in large beds (Bell and Gosline 1997). Byssus threads make up a significant proportion of the carbon allocation for Mytilus edulis during growth (8% of carbon and nitrogen allocation, Hawkins and Bayne 1985), and there is evidence of a trade-off between byssus production during periods of strong wave stress and gonad production for autumn spawning (Rhode Island, Carrington 2002) or spring spawning (S. Wales, Price 1982). Overall, mechanical stress from enhanced wave action in winter provides a possible mechanical explanation for the observed facilitation of growth in high-density quadrats during the winter.
We show, by means of a simple, heuristic modelling exercise, that in situations where mussels lose resources though local competition and mechanical stress, both positive and negative density dependence in overall energy availability can be generated by a trade-off between the two (Fig. 7). Where local competition for resources is more important, energy availability will be negative density dependent. Where mechanical stress is more important, overall energy availability will be positively density dependent if high local density is assumed to mitigate energy loss from wave stress (e.g. Bell and Gosline 1997). This kind of modelling exercise, although obviously a highly simplified version of reality, is valuable in this case in clarifying the overall outcome of two conflicting density dependent processes.
Facilitative interactions in sessile biota have been relatively neglected until recent work in terrestrial vegetation (reviewed in Bertness and Callaway 1994; Callaway 1995; Rietkerk et al. 2002) and the intertidal (reviewed in Bertness and Callaway 1994; Bertness and Leonard 1997). Facilitation of itself need not always lead to patterning (e.g. see list of plant facilitative interactions in Callaway 1995), but where regular patterns have been reported, facilitation, particularly intraspecific facilitation, also seems to be important (barnacles—Bertness et al. 1998, see Table 3). This study extends this result to beds of Mytilus edulis, providing support for the idea that the competition–facilitation theory of pattern formation, first proposed by Turing (1952), is a general rule in sessile biota.
Our results also provide evidence in support of a general rule that interactions in sessile biota may switch from negative to positive up a gradient of physical stress (Bertness and Callaway 1994; Bertness and Hacker 1994; Callaway 1995; Bertness and Leonard 1997; Callaway et al. 2002; Table 3). At our field site, significant wave height is greater in winter than summer. Wave action from gales from northerly to easterly is particularly important because (1) there is a long fetch and (2) waves are exacerbated by opposing flow on flood tide currents, which reach a maximum just before low water, creating strong bottom shear stress. Storms can distribute mussels many metres above the high tide line (personal observation). Generally, interactions have been studied up a gradient in space (e.g. intertidal, altitudinal and latitudinal—see Table 3), but in our case the gradient in physical stress is likely to occur in time (seasonal changes in wave stress).
In conclusion, our study adds to a growing body of evidence for a common ‘Turing’ mechanism for pattern formation in biological systems from embryos to ecosystems. This is interesting in the ecological context, since experimental work on self-organised patterning has been so far limited to terrestrial vegetation systems. General rules applicable across diverse ecosystems (terrestrial vs marine, plant vs animal) are rare. We also provide support for the idea that facilitative interactions in sessile biota are mediated by physical stress, and that interactions are flexible, and can change in strength and sign along a spatial or temporal gradient of physical stress. Finally, it is clear that studies of density dependence in mussel beds (and perhaps other systems of sessile organisms) need to consider spatial and temporal scale carefully if conclusions are to be meaningful.
References
Beadman HA (2003) The sustainability of mussel culture. PhD Thesis, School of Ocean Sciences, University of Wales Bangor, Menai Bridge, Anglesey, UK
Beadman HA, Kaiser MJ, Galanidi M, Shucksmith R, Willows RI (2004) Changes in species richness with stocking density of marine bivalves. J Appl Ecol 41:464–475
Bell EC, Gosline JM (1997) Strategies for life in flow: tenacity, morphometry and probability of dislodgement of two Mytilus species. Mar Ecol Prog Ser 159:197–208
Bertness MD (1989) Intraspecific competition and facilitation in a northern acorn barnacle population. Ecology 70:257–268
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol 9:191–193
Bertness MD, Ewanchuk PJ (2002) Latitudinal and climate-driven variation in the strength and nature of biological interactions in New England salt marshes. Oecologia 132:392–401
Bertness MD, Grosholz E (1985) Population dynamics of the ribbed mussel, Geukensia demissa: the costs and benefits of a clumped distribution. Oecologia 67:192–204
Bertness MD, Hacker SD (1994) Physical stress and positive associations among marsh plants. Am Nat 144:363–372
Bertness MD, Leonard GH (1997) The role of positive interactions in communities: lessons from intertidal habitats. Ecology 78:1976–1989
Bertness MD, Gaines SD, Yeh SM (1998) Making mountains out of barnacles: the dynamics of acorn barnacle hummocking. Ecology 79:1382–1394
Côté IM, Jelnikar E (1999) Predator-induced clumping behaviour in mussels (Mytilus edulis Linneaus). J Exp Mar Biol Ecol 235:201–211
Caldow RWG, Beadman HA, McGrorty S, Kaiser MJ, Goss-Custard JD, Mould K, Wilson A (2003) Effects of intertidal mussel cultivation on bird assemblages. Mar Ecol Prog Ser 259:173–183
Callaway R (1995) Positive interactions among plants. Bot Rev 61:306–349
Callaway RM, Brooker RW, Choler P, Kikvidzes Z, Lortie CJ, Michalet R, Paolini L, Pugnaire FI, Newingham B, Aschehoug ET, Armas C, Kikodze D, Cook B (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848
Carrington E (2002) The ecomechanics of mussel attachment: from molecules to ecosystems. Int Comp Biol 42:846–852
Chase J, Leibold M (2002) Spatial scale dictates the productivity-biodiversity relationship. Nature 416:427–430
Couteron P, Lejeune O (2001) Periodic spotted patterns in semi-arid vegetation explained by a propagation-inhibition model. J Ecol 89:616–628
Denny MW, Daniel TL, Koehl MAR (1985) Mechanical limits to size in wave-swept organisms. Ecol Monogr 55:69–102
Frank K, Brickman D (2000) Allee effects and compensatory population dynamics within a stock complex. Can J Fish Aquat Sci 57:513–517
Fréchette M, Aitken A, Page L (1992) Interdependence of food and space limitation of a benthic suspension feeder: consequences for self-thinning. Mar Ecol Prog Ser 83:56–62
Hamer KC, Hill JK (2000) Scale-dependent effects of habitat disturbance on species richness in tropical forests. Conserv Biol 14:1435–1440
Hawkins AJS, Bayne BL (1985) Seasonal variation in the relative utilisation of carbon and nitrogen by the mussel Mytilus edulis: budgets, conversion efficiencies and maintenance requirements. Mar Ecol Prog Ser 25:181–188
Hunt HL, Schiebling RE (2001a) Patch dynamics of mussels on rocky shores: integrating process to understand pattern. Ecology 82:3213–3231
Hunt HL, Schiebling RE (2001b) Predicting wave dislodgement of mussels: variation in attachment strength with body size, habitat and season. Mar Ecol Prog Ser 25:181–188
Kaiser MJ (2003) Detecting the effects of fishing on seabed community diversity: importance of scale and sample size. Conserv Biol 17:512–520
Klausmeier CA (1999) Regular and irregular patterns in semiarid vegetation. Science 284:1826–1828
van de Koppel J, Herman PMJ, Thoolen P, Heip CHR (2001) Do alternate stable states occur in natural ecosystems? Evidence from a tidal flat. Ecology 82:3449–3461
van de Koppel J, Rietkerk M, Dankers M, Herman PMJ (2005) Scale-dependent feedback and regular spatial patterns in young mussel beds. Am Nat 165 (in press)
Levin SA (1992) The problem of pattern and scale in ecology. Ecology 73:1943–1967
Lively CM, Raimondi PT (1987) Desiccation, predation and mussel-barnacle interactions in the northern Gulf of California. Oecologia 74:304–309
McQuaid C, Lindsay T (2000) Effect of wave exposure on growth and mortality rates of the mussel Perna perna: bottom-up regulation of intertidal populations. Mar Ecol Prog Ser 206:147–154
Morgan R, Brown J, Thorson J (1997) The effects of spatial scale on the functional response of fox squirrels. Ecology 78:1087–1097
Newell CR (1990) The effects of mussel (Mytilus edulis Linnaeus 1758) position in seeded bottom patches on growth at subtidal lease sites in Maine. J Shellfish Res 9:113–118
Okamura B (1986) Group living and the effects of spatial position in aggregations of Mytilus edulis. Oecologia 69:341–347
Price HA (1982) An analysis of factors determining seasonal variation in the byssal attachment strength of Mytilus edulis. J Mar Biol Assoc UK 62:147–155
Ray C, Hastings A (1996) Density dependence: are we searching at the wrong spatial scale? J Anim Ecol 65:556–566
Rietkerk M, Boerlijst MC, van Langevelde F, HilleRisLambers R, van de Koppel J, Kumar L, Prins HHT, de Roos AM (2002) Self-organisation of vegetation in arid ecosystems. Am Nat 160:524–530
Rietkerk M, Dekker SC, de Ruiter PC, van de Koppel J (2004a) Self-organised patchiness and catastrophic shifts in ecosystems. Science 305:1929–1929
Rietkerk M, Dekker SC, Wassen MJ, Verkroost AWM, Bierkens MFP (2004b) A putative mechanism for bog patterning. Am Nat 163:699–708
Schneider DC (1994) Quantitive ecology: spatial and temporal scaling. San Diego University Press, San Diego
Smaal A, van Stralen M, Schuiling E (2001) The interaction between shellfish culture and ecosystem processes. Can J Fish Aquat Sci 58:991–1002
Tewksbury JJ, Lloyd JD (2001) Positive interactions under nurse plants: spatial scale, stress gradient and benefactor size. Oecologia 127:425–434
Turing AM (1952) The chemical basis of morphogenesis. Phil Trans Roy Soc Lond B 237:37–72
Underwood AJ (1997) Experiments in Ecology. Cambridge University Press, Cambridge
Williams D, Liebhold A (2000) Spatial scale and the detection of density dependence in spruce budworm outbreaks in eastern North America. Oecologia 124:544–552
Zar JH (1999) Biostatistical analysis. 4th edn. Prentice Hall, Upper Saddle River, New Jersey
Acknowledgements
Thanks to Kim Mould of Myti Mussels for use of the field site and the setting up of the experiment, to Gwynne Parry-Jones and members of CREAM for field work, and to Johan van de Koppel for helpful comments on several drafts. JG and CS are supported by grants from BBSRC and DEFRA and HB was supported by a grant from NERC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Martin Attrill
Rights and permissions
About this article
Cite this article
Gascoigne, J.C., Beadman, H.A., Saurel, C. et al. Density dependence, spatial scale and patterning in sessile biota. Oecologia 145, 371–381 (2005). https://doi.org/10.1007/s00442-005-0137-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0137-x