Abstract
Although much research on the density effect in nonself-thinning populations has been conducted, there has been very little research on density effects in self-thinning populations. Furthermore, the density effect of plant organs in self-thinning populations is little reported. The present study analyzed the yield–density (Y–D) effects on organs, such as stem, branch and leaf, together with that on stands of self-thinning Pinus densiflora Sieb. et Zucc.. The stand yield- and organ Y–D effects were well described by reciprocal and parabolic equations, respectively, throughout the experiment. The value of coefficient B in the reciprocal equation decreased monotonically with increasing stand age and became significantly closer to zero at the end of experiment (33-year-old stand), indicating that the constant final stand yield was established regardless of the density realized. The value of the relative growth coefficient h in the allometric equation between mean organ weight and mean aboveground weight was significantly smaller than 1.0 for stem, indicating that stem yield increases monotonically with increasing realized density. The h-value was significantly larger than 1.0 for branch throughout the experiment, and for leaf except at 33 years old, indicating that optimum densities exist. The h-value for leaf was not significantly different from 1.0 at 33 years old, indicating that the leaf yield reached a constant level regardless of realized density. The constant final leaf yield was established at almost the same growth stage as the establishment of constant final stand yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As plants in a population develop, plant growth becomes limited by the rate of available resources, so that competition among plants occurs. During the early growth stage, such competition causes a decrease in mean plant weight with increasing density without mortality. However, as plants grow larger, density-dependent mortality begins, and an increase in mean plant weight is accompanied by a decrease in density. The phenomenon that mean plant weight decreases [competition–density (C–D) effect], whereas yield (mean plant weight times density) increases with increasing density [yield–density (Y–D) effect] is called the density effect (Kira et al. 1953).
Although some mathematical models have been proposed to describe the density effect (e.g. Shinozaki and Kira 1956; Bleasdale and Nelder 1960; Nelder 1962; Bleasdale 1967; Farazdaghi and Harris 1968; Watkinson 1980, 1984; Vandermeer 1984), the reciprocal equations of the C–D effect and Y–D effect proposed by Shinozaki and Kira (1956) are widely used. These reciprocal equations are based on the logistic theory of plant growth and explain the density effect fairly well over a wide range of density. Since the reciprocal equations were derived for nonself-thinning populations, Hagihara (1999) reconstructed a new model to describe the density effect in self-thinning populations in line with the Shinozaki–Kira theory. Hagihara's model was confirmed as being applicable to the density effect in self-thinning populations (Xue and Hagihara 1998, 1999, 2002; Stankova and Shibuya 2003).
The Y–D effect, especially the Y–D effects on organs, is more important than the C–D effect from both theoretical and practical viewpoints. For some herbaceous plants, the relationships between yields of plant organs and density have been reported (e.g. Bleasdale and Nelder 1960; Bleasdale 1967; Watkinson 1984). Concerning woody species, little is known about the relationships between the yields of organs and density.
The present study employs data collected by Ando (1962) and Tadaki et al. (1979) in self-thinning Pinus densiflora Sieb. et Zucc. stands with different densities. The aims of this study were to examine (1) whether the Y–D effect of the stand can be explained by the reciprocal equation of the Y–D effect in self-thinning populations; (2) whether the Y–D effects on organs, such as stem, branch and leaf, can be explained by the parabolic equation derived from the reciprocal equation of the C–D effect in self-thinning populations and the allometric relationship of mean organ weight to mean aboveground weight; and (3) the difference in the Y–D effect among organs.
Materials and methods
Data source
Ando (1962) established three 0.01 ha plots with different densities in naturally regenerated 9-year-old stands of Pinus densiflora Sieb. et Zucc. When the stands were 14 years old, 20 sample trees for low- and middle-density stands, and 30 sample trees for a high-density stand were felled (Ando et al. 1962). When the stands were 33 years old, eight sample trees for each density stand were felled (Tadaki et al. 1979). The weight of organs, such as stem, branch and leaf, was measured for each sample tree.
Establishment of allometric relationships between organ weight and stem diameter at breast height
The following simple allometric equation for weight, w o, of an organ, such as stem, branch or leaf, to stem diameter at breast height (DBH), D, was examined for each of the different density stands:
where a and b are coefficients specific to 14- or 33-year-old stands. First, it was confirmed that there were no significant differences (F-test) in error variance between the curvilinear regression lines of Eq. 1 for 14- and 33-year-old stands in each organ (0.11 < P < 0.79). Second, it was confirmed that the b-values of the respective curvilinear regression lines for 14- and 33-year-old stands were not significantly different (t-test) in each organ (0.34 < P < 0.89). Finally, it was confirmed that the a-values did not show significant differences (t-test) in each organ between 14- and 33-year-old stands (0.12 < P < 0.94) on the assumption that the b-value is the same between the two curvilinear regression lines. As a result, 14- and 33-year-old data could be lumped together for establishing the allometric relationship of Eq. 1 for each organ of each density, as shown in Fig. 1.
Allometric relationships between organ weight, w o, and diameter at breast height, D. The straight lines are based on Eq. 1. a Low-density stand (R 2 = 0.98 for stem, R 2 = 0.95 for branch, R 2 = 0.94 for leaf); b middle-density stand (R 2 = 0.96 for stem, R 2 = 0.85 for branch, R 2 = 0.82 for leaf); c high-density stand (R 2 = 0.96 for stem, R 2 = 0.85 for branch, R 2 = 0.82 for leaf). Open circles 14-year-old stem, filled circles 33-year-old stem, open squares 14-year-old branch, filled squares 33-year-old branch; open triangles 14-year-old leaf, filled triangles 33-year-old leaf
Estimate of mean organ weight
On the basis of the DBHs of all individuals recorded at 2-year intervals from 14 to 30 years old and at the age of 33 years (Tadaki et al. 1979), mean organ weight, w x , was calculated using the allometric relationships established for different organs in different density stands (Fig. 1). Mean aboveground weight, w, was defined as the sum of the mean weights of organs.
Results
Allometric relationship between mean organ weight and mean aboveground weight
As shown in Fig. 2, the allometric relationship between mean organ weight, w x , and mean aboveground weight, w, followed the equation (Kira et al. 1956):
where g and h are a coefficient and the relative growth coefficient specific to organs, respectively. As shown in Fig. 3, the value of h for stem was significantly less than 1.0 (P < 0.031), and showed an almost constant trend throughout the experiment (P > 0.39), ranging from 0.791 to 0.883. The h-value for branch was significantly larger than 1.0 (P < 0.037) and kept a more or less steady trend (P > 0.16) throughout the experiment between 1.41 and 1.66. The h-value for leaf was significantly larger than 1.0 (P < 0.023) except for 33-year-old stand, and the h-values ranged from 1.39 to 1.51 without showing a significant difference (P > 0.93) within the first half of the experiment and then decreased from 1.51 at the middle, reaching a significantly smaller value of 1.02 (t = 3.44, P = 0.038) at the end of experiment.
Relationships of mean stem weight, w S, branch weight, w B, and leaf weight, w L to mean aboveground weight, w. The straight lines were based on Eq. 2. Circles Stem (R 2 > 0.97); squares branch (R 2 > 0.93); triangles leaf (R 2 > 0.94)
Time trends of relative growth coefficient h in Eq. 2. Circles stem; squares branch; triangles leaf. The h-value for leaf at 33 years old was not significantly different from 1.0 (t = 0.26, P = 0.42)
Density effect of self-thinning stands
Hagihara (1999) developed the reciprocal equation of the competition–density (C–D) effect in self-thinning stands on the basis of the logistic theory of the C–D effect in nonself-thinning populations (Shinozaki and Kira 1956) as follows:
where w is the mean tree weight and ρ is the realized density, and A t and B are coefficients specific to growth stages. It was confirmed that Eq. 3 well explained the C–D effect in stands of Pinus densiflora Sieb. et Zucc. (Xue and Hagihara 1998, 1999), Pinus massoniana Lamb. (Xue and Hagihara 2002), Etula platyphylla Sukatchev var. japonica Hara and B. ermanii Cham. (Stankova and Shibuya 2003).
From Eq. 3, yield per area y, which is the product of w and realized density ρ, can be expressed as the reciprocal equation of the yield–density (Y–D) effect in self-thinning stands,
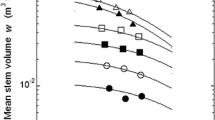
The relationship between stand yield y and realized density ρ is shown in Fig. 4. Stand yield y (aboveground biomass) increased with increasing realized density ρ. The y–ρ relationships fitted well for all growth stages using the reciprocal equation of the Y–D effect given by Eq. 4. The stand Y–D curve on log–log coordinates shifted upward and leftward with increasing stand age. As shown in Fig. 5, the value of A t decreased gradually from 0.0250 ha Mg−1 at 14 years old to 0.0119 ha Mg−1 at 33 years old, and the value of B decreased from 649 Mg−1 at 14 years old to 0.100 Mg−1 at 33 years old. When the stands were 14 years old, the number of trees in low-density stand was about 30% and 9%, respectively, of those of middle- and high-density stands, which resulted in low yield in low-density stand, though mean aboveground weight was greater in the low-density stand than in middle- and high-density stands. Differences in stand yield among three different density stands decreased with increasing stand age, and the stand yield finally became constant irrespective of realized density at 33 years old.
Yield–density (Y–D) effect between stand yield y and realized density ρ. The data were fitted using Eq. 4. Filled circles 14-year-old (R 2 = 0.94), open circles 18-year-old (R 2 = 0.92), filled squares 22-year-old (R 2 = 0.88), open squares 26-year-old (R 2 = 0.92), filled triangles 30-year-old (R 2 = 0.97), open triangles 33-year-old
Time trends of coefficients A t and B in the reciprocal equation of the Y–D effect given by Eq. 4. a Coefficient A t , b coefficient B. The value of B at 33 years old was not significantly different from zero (t = 0.03, P = 0.98)
The value of coefficient B in Eq. 4 decreased monotonically with increasing stand age (Fig. 5b) and became closer to zero at 33 years old (t = 0.029, P = 0.98). As the B-value approaches to zero, Eq. 4 can be written in the form:
Therefore, final stand yield Y reaches a constant with a value of 1/A t irrespective of realized density ρ. Figure 4 shows that the law of constant final yield (Kira et al. 1953; Hozumi et al. 1956) was established in P. densiflora stands.
Yield–density effect with respect to organs
Yield of an organ per area y x , which is the product of mean organ weight w x and realized density ρ, is derived as follows by considering Eqs. 2 and 3 (cf. Hozumi and Shinozaki 1960):
When h = 1, Eq. 6 is synonymous with the reciprocal equation of Eq. 4. When h < 1, organ yield y x increases monotonically with increasing realized density ρ, whereas when h > 1, y x has its maximum at optimum realized density ρ opt (Bleasdale and Thompson 1966; Hozumi 1973).
Figure 6a shows the relationship between stem yield, y S, and realized density, ρ. The stem yield increased monotonically with increasing realized density, because the value of h was less than 1.0 (Fig. 3). The difference in stem yield among different density stands decreased with increasing stand age. The y S–ρ relationships were well fitted by Eq. 6 throughout the experiment.
Y–D effects between organ yield, y x , and realized density ρ. a Stem, b branch, c leaf. The curves show Eq. 6, where the g- and h-value, and the A t - and B-value are estimates, respectively, obtained in Eqs. 2 and 4. Filled circles 14-year-old (R 2 = 0.98 for stem, R 2 = 0.86 for branch, R 2 = 0.90 for leaf); open circles, 18-year-old (R 2 = 0.98 for stem, R 2 = 0.85 for branch, R 2 = 0.88 for leaf); filled squares, 22-year-old (R 2 = 0.99 for stem, R 2 = 0.71 for branch, R 2 = 0.81 for leaf); open squares, 26-year-old (R 2 = 0.99 for stem, R 2 = 0.71 for branch, R 2 = 0.72 for leaf); filled triangles, 30-year-old (R 2 = 0.71 for stem, R 2 = 0.58 for branch, R 2 = 0.90 for leaf); open triangles, 33-year-old (R 2 = 0.98 for stem, R 2 = 0.93 for branch)
The parabolic relationship between branch yield, y B, and realized density, ρ, is shown in Fig. 6b. This figure indicates that an optimum density exists at each growth stage except at 33 years old. The y B–ρ curve given by Eq. 6, where the value of h was larger than 1.0 (Fig. 3), well explained the data, and the curve moved upwards and leftwards on log–log coordinates with the passage of time.
As shown in Fig. 6c, a parabolic relationship also exists between leaf yield, y L, and realized density, ρ, where the h-value was larger than 1.0. The y L–ρ relationship could be well described by Eq. 6. The time trend of the y L–ρ relationship was almost the same as that of the y B–ρ relationship (Fig. 6b).
Time trends of optimum density and maximum yield
By differentiating both sides of Eq. 6 with realized density, ρ, the optimum density, ρ opt, of an organ in self-thinning stands can be obtained as follows (cf. Hozumi 1973):
The value of the relative growth coefficient h in Eq. 2 was less than 1.0 for mean stem weight, w S, whereas the value was larger than 1.0 for mean branch weight, w B, and for mean leaf weight, w L, except for the 33-year-old stand (Fig. 3). Therefore, from Eq. 7, optimum densities exist for branch and leaf yields.
Figure 7 depicts the time trends of optimum densities for branch and leaf yields calculated by Eq. 7. The optimum density was 60,607 ha−1 for branch and 55,111 ha−1 for leaf at 14 years old, and decreased gradually to 7,694 ha−1 for branch and 22,559 ha−1 for leaf at 30 years old. The optimum density for branch was greater than that for leaf from 14 to 18 years old, with the former became smaller than the latter thereafter.
Inserting Eq. 7 into Eq. 6, maximum yield \( y_{{x_{{\max }} }} \) of an organ is given by the following equation (cf. Hozumi 1973):
As shown in Fig. 8, the maximum yield was 2.81 Mg ha−1 for branch and 2.76 Mg ha−1 for leaf at 14 years old and increased gradually to 13.0 Mg ha−1 for branch and 9.16 Mg ha−1 for leaf at 30 years old. The difference between maximum yields of branch and leaf became larger with increasing stand age.
Discussion
Stand yield- and organ yield-realized density relationships were described well by Eqs. 4 and 6, respectively, at each growth stage (Figs. 3, 6), indicating that the analysis of the Y–D effect based on the two equations is useful.
The existence of optimum density for the leaf yield may be ascribed to leaf shading at the bottom of a canopy in high-density stands of P. densiflora having a shade-intolerant nature. As stands grow, however, the leaf yield reached a constant level regardless of realized density. Hozumi et al. (1962) pointed out that leaf yield is several times more strongly inhibited by increasing density than stand yield. As a result, the ceiling of leaf yield is observed to proceed towards that of stand yield. It seems that our results support their conclusion (cf. Figs. 4, 6c). However, our results make it clear that an optimum density for leaf yield before its actual ceiling exists.
The branch yield was also maximized at a certain density. However, the density that maximizes the branch yield appeared throughout the experiment—a different trend from the case of leaf yield. With increasing stand density, the number of lateral branches gradually decreases and their length becomes shorter. Weiner et al. (1990) and Weiner and Fishman (1994) pointed out that branches are fewer in highly crowded stands than in low-density stands. Therefore, an optimum density for the branch yield may exist.
In case of the stem yield, no particular density maximizing stem yield existed throughout the experiment. Begonia et al. (1988) and Jobidon (2000) suggested that competing vegetation induces a shift in carbon allocation from branch to stem, probably resulting from hormonal inhibition of lateral bud development. In a study on Scots pine trees, Nilsson and Albrektson (1993) found that the allocation of carbon to stem has high priority for trees under high competitive stress. Therefore, the stem yield probably increased monotonically with increasing realized density.
It is apparent from Fig. 5 that the value of coefficient B in Eq. 4 tended to be zero with the passage of time. As a result, Eq. 6 takes the form (cf. Hozumi 1973):
When the value of relative growth coefficient h is smaller than 1.0, final organ yield Y x increases with increasing realized density, ρ, which corresponds to the final stem yield (Fig. 6a). When the h-value is larger than 1.0, Y x decreases with increasing realized density, which corresponds to final branch yield (Fig. 6b). When the h-value is equal to 1.0, Y x reaches a constant with a value of g/A t, which corresponds to final leaf yield (Fig. 6c), where the h-value was not significantly different from 1.0 (t = 0.102, P = 0.42) (Fig. 3). Therefore, it is concluded that the density effect on organ yield differs among stem, branch and leaf.
References
Ando T (1962) Growth analysis on the natural stands of Japanese red pine (Pinus densiflora Sieb. et Zucc.) II. Analysis of stand density and growth (in Japanese with English summary). Bull For For Prod Res Inst 147:45–77
Ando T, Sakaguchi K, Narita T, Satoo S (1962) Growth analysis on the natural stands of Japanese red pine (Pinus densiflora Sieb. et ZUCC.) I. Effects of improvement cutting and relative growth (in Japanese with English summary). Bull For For Prod Res Inst 144:1–30
Begonia GB, Aldrich RJ, Nelson CJ (1988) Effects of simulated weed shade on soybean photosynthesis, biomass partitioning and axillary bud development. Photosynthetica 22:309–319
Bleasdale JKA (1967) The relationship between the weight of a plant part and total weight as affected by plant density. J Hortic Sci 42:51–58
Bleasdale JKA, Nelder JA (1960) Plant population and crop yield. Nature 188:342
Bleasdale JKA, Thompson R (1966) The effects of plant density and the pattern of plant arrangement on the yield of parsnips. J Hortic Sci 41:371–378
Farazdaghi H, Harris PM (1968) Plant competition and crop yield. Nature 217:289–290
Hagihara A (1999) Theoretical considerations on the C–D effect in self-thinning plant populations. Res Popul Ecol 41:151–159
Hozumi K (1973) Interactions among higher plants (in Japanese). Kyouritsu-Shuppan, Tokyo
Hozumi K, Shinozaki K (1960) Logistic theory of plant growth (in Japanese). In: Kira T (ed) Plant ecology, vol 2. Kokinn-Shoinn, Tokyo, pp 272–304
Hozumi K, Asahira T, Kira T (1956) Intraspecific competition among higher plants V. Effect of some growth factors on the process of competition. J Inst Polytech Osaka City Univ Ser D7:15–34
Hozumi K, Shinozaki K, Kira T (1962) Effect of light intensity and planting density on the growth of Hibiscus Moscheutos Linn. III. Analysis of leaf growth based on the logistic theory (in Japanese with English summary). Physiol Ecol 11:62–77
Jobidon R (2000) Density-dependent effects of northern hardwood competition on selected environmental resources and young white spruce (Picea glauca) plantation growth, mineral nutrition, and stand structural development—a 5-year study. For Ecol Manage 130:77–97
Kira T, Ogawa H, Sakazaki N (1953) Intraspecific competition among higher plants I. Competition–yield–density interrelationship in regularly dispersed populations. J Inst Polytech Osaka City Univ Ser D4:1–16
Kira T, Ogawa H, Hozumi K (1956) Intraspecific competition among higher plant V. Supplementary notes on the C–D effect. J Inst Polytech Osaka City Univ Ser D7:1–14
Nelder JA (1962) New kinds of systematic designs for spacing experiments. Biometrics 18:283–307
Nilsson U, Albrektson A (1993) Productivity of needles and allocation of growth in young Scots pine trees of different competitive status. For Ecol Manage 62:173–187
Shinozaki K, Kira T (1956) Intraspecific competition among higher plants VII. Logistic theory of the C–D effect. J Inst Polytech Osaka City Univ Ser D7:35–72
Stankova T, Shibuya M (2003) Adaptation of Hagihara’s competition–density theory for practical application to natural birch stands. For Ecol Manage 186:7–20
Tadaki Y, Takeuchi I, Kawahara T, Sato A, Hatiya K (1979) Growth analysis on the natural stands of Japanese red pine (Pinus densiflora Sieb. et Zucc.) III. Results of experiment (research note in Japanese). Bull For For Prod Res Inst 305:125–144
Vandermeer J (1984) Plant competition and the yield-density relationship. J Theor Biol 109:393–399
Watkinson AR (1980) Density-dependence in single-species populations of plants. J Theor Biol 83:345–357
Watkinson AR (1984) Yield–density relationships: the influence of resource availability on growth and self-thinning in populations of Vulpia fasciculata. Ann Bot 53:469–482
Weiner J, Fishman L (1994) Competition and allomery in Kochia scoparia. Ann Bot 73:263–271
Weiner J, Berntson GM, Thomas SC (1990) Competition and growth form in a woodland annual. J Ecol 78:459–469
Xue L, Hagihara A (1998) Growth analysis of the self-thinning stands of Pinus densiflora Sieb. et Zucc. Ecol Res 13:183–191
Xue L, Hagihara A (1999) Density effect, self-thinning and size distribution in Pinus densiflora Sieb et Zucc. Stand. Ecol Res 14:49–58
Xue L, Hagihara A (2002) Growth analysis on the C–D effect in self-thinning Masson pine (Pinus massoniana) stands. For Ecol Manage 165:249–256
Acknowledgments
This study was partially supported by the Knowledge Innovation Project of Chinese Academy of Sciences (KZCX3-SW-418) and by a Grant-in-Aid for Scientific Research (no. 16651009) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Xue, L., Hagihara, A. Density effects on organs in self-thinning Pinus densiflora Sieb. et Zucc. stands. Ecol Res 23, 689–695 (2008). https://doi.org/10.1007/s11284-007-0427-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-007-0427-3