Abstract
Density effects on the growth of self-thinning Eucalyptus urophylla stands were examined for 7 years. Tree height and stem diameter at breast height were measured during the experimental period. Stems, branches, leaves, bark and roots of 45 E. urophylla trees were sampled in three different density stands in order to establish their biomass equations. Change trends of the biological time τ and density ρ were described used corresponding equations. The stem weight ratio increased and leaf weight ratio decreased, whereas those of branch, bark and root were relatively steady from 2 years after the planting. The competition-density (C-D) effect equation of mean organ weight w o was derived by combining the allometric power relationship between mean tree weight w and w o with the C-D effect equation of self-thinning stands. The equations of the C-D effect for w and ρ and for w o and ρ were used to describe the C-D effects in tree and organs during course of self-thinning, respectively, and showed a good fit to the data. Leaf biomass of different density stands reached a more or less constant level with time elapse. High density produced the greatest biomass and stem biomass, so that it is the best choice in silvicultural practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Competition among individual plants occurs as plants in a stand grow larger (Xue et al. 2010). Numerous studies have focused on plant competition (e.g. Fetene 2003; Hunt et al. 2006; Berger et al. 2008; Manning et al. 2009), because it is a key process affecting plant populations and communities (Berger et al. 2008). Competition-density (C-D) is an effect explaining the common finding that average plant size decreases when stand density increases. The relationship between plant weight and density is the reciprocal of the C-D effect (Shinozaki and Kira 1956), which can be examined by equations developed by authors such as Watkinson (1980, 1984) and Vandermeer (1984). This relationship is known as the logistic theory of the C-D effect and was initially developed for non-self-thinning stands (Stankova and Shibuya 2003).
Self-thinning or density-dependent mortality begins when competition becomes more severe. This can result in increases in mean plant size and a decrease in plant density. The logistic theory of the C-D effect can be applied to self-thinning stands, but there would be a theoretical limit in reconciling the C-D effect (Minowa 1982; Naito 1992). Hagihara (1999) constructed a model for describing the C-D effect in self-thinning stands in line with the logistic theory of the C-D effect, which predicts the C-D effect in stands of Pinus densiflora and Pinus massoniana (Xue and Hagihara 1998, 2002).
Quantifying the amount and distribution of biomass is important in understanding the structure and function of the ecosystem (Grove and Malajczuk 1985). Understanding the relationship between plant density and weight of particular tree organs (stem, branches, leaves, bark and roots) at all stages of tree growth would help to estimate biomass production and quantify stand development patterns over time. In most studies on C-D effect, only stem volume or tree weight has been measured (e.g. Xue and Hagihara 1998, 2002; Stankova and Shibuya 2003), little is known about the C-D effect on individual tree organs. Although Xue and Hagihara (2008) demonstrated the relationship between organ biomass and stand density in P. densiflora stands, their results were not related to root biomass. Since competition alters the biomass allocation patterns of tree organs (Xue et al. 2010), and the mechanisms of competition differ between the above- and below-ground parts of plants (Weiner 1990), it is important to examine the competitive effects on tree organs separately. Organ biomass can be estimated using standard forest inventory data and allometric relationships (e.g. Montagu et al. 2005; Xue and Hagihara 2008), although root weight is difficult and more expensive to obtain compared with above-ground components.
The increasing demand for wood has meant that plantations of fast-growing exotic trees have become an increasingly important source of wood products in China. Eucalyptus urophylla is a fast-growing species and good paper pulp as well as board species. This species originates in Indonesia and was introduced to China in the 1960s (Yang and Zhong 1997). It is one of the major silvicultural tree species planted for wood in South China. However, the C-D effect on E. urophylla tree organs has not been analyzed. Such information would help to estimate organ biomass production and quantify stand development patterns over time.
In this study, density effect on tree growth and organ biomass was examined in E. urophylla stands. The objectives of this study are (1) to examine growth characteristics of self-thinning E. urophylla stands; (2) to fit the C-D equation of organs to organ biomass; (3) to compare the difference in the C-D effect among organs; (4) to check whether the leaf biomass per unit land area remains constant in self-thinning stands with different densities.
Materials and methods
Study site
The experimental site is located in the central area of Leizhou Peninsula, Guangdong Province, China. This region has a tropical monsoon climate. Mean annual temperature, temperatures in the hottest month (July) and in the coldest month (January) are 22.9, 28.9 and 15.2°C, respectively. Mean annual rainfall and evaporation are 1,700 and 1,763 mm, respectively. The slope of experimental site is gentle having a slope of 5°. Soil is orthox resulting from shallow sea deposit, with a soil depth over 100 cm. Chemical characteristics of the soil at 0–40 cm depth were as follows: pH value was 5.1, contents of organic matter, total N, P and K were 11.44, 0.51, 0.18 and 2.03 g kg−1, respectively, and available N, P and K contents were 38.06, 2.75 and 13.16 mg kg−1, respectively. The main understory was Eriachne pallescens R. Br. with a cover of 60%.
Sampling methods
The E. urophylla S. T. Blake stands of 6.0 ha were established in 2000, which consisted of three-stand densities of 1,111 trees ha−1 (low density, 3 m × 3 m spacing), 1,667 trees ha−1 (middle density, 3 m × 2 m spacing) and 2,500 trees ha−1 (high density, 2 m × 2 m spacing). The investigated plot area was 30 m × 20 m. Three replicates of the plot were randomly located in each density stand. Tree height (h) and stem diameter at breast height of 1.3 m above the ground (d) (over bark diameter) for each tree were measured annually from age 1 to 7 years.
The h and d for all trees were measured at each plot, divided into five-diameter classes at 2-cm intervals, and then 15 sample trees proportionally distributed over the range of diameter in each density stand were selected for destructive harvesting in 2007. The root biomass (>0.2 cm in diameter) downwards to 1 m depth was estimated by excavating the soil around the roots according to the planting spacings (Fang et al. 2007). Roots were divided into root stock and three-diameter classes: 0.2–0.5, 0.5–2 and >2 cm. The stratified clipping method was followed at a 0.5-m interval (from the ground to the top) to estimate the weight of stem and bark of each sample tree at different strata, and fresh weight of leaves and branches contained in each 0.5 m thick horizontal layers was measured (Khan et al. 2005). Subsamples of stem, branches, leaves, bark and roots from each root class were taken for estimating the ratio of dry/fresh weight.
The following simple allometric equation for weight W o of an organ, such as stem, branch, leaf, bark or root, to d and h was examined for each of different density stands:
where a and b are coefficients. Separate equations for each plant organ and plant density were fitted using ordinary least-squares estimates (Table 1).
On the basis of the d and h of all individuals within each plot recorded at the annual measurements, it was possible to calculate mean organ weight, w o, using these allometric relationships established for different organs in different density stands. Mean tree weight, w, was defined as the sum of mean weights of organs.
Model fitting
The equation
where w o is mean organ weight, w is mean tree weight, and g the coefficient and k the allometric constant specific to growth stages (Kira et al. 1956; Xue and Hagihara 2008), was fitted to the data. The g and k were obtained by the method of least squares (Table 2).
Equation 2 was used to estimate relationship between mean tree weight w and mean organ weight w o. Dividing both sides of Eq. 2 by w results in
where w o/w stands for the dry weight ratio of each organ.
Hagihara (1999) developed the reciprocal equation of the C-D effect in self-thinning stands as follows:
where w is the mean tree weight and ρ is stand density, and A t and B are coefficients specific to growth stages.
The mean organ weight w o is derived by considering Eqs. 2 and 4 as follows:
The biological time τ is defined as the integral of the coefficient of growth λ(t) with respect to physical time t (Shinozaki 1961):
The τ can be derived by the following equation (Shinozaki and Kira 1956):
where w 0 is initial mean plant weight.
The τ–t relationship can be expressed by a hyperbolic equation (Hozumi 1977) as
where the reciprocal of F denotes the intrinsic growth rate at the initial growth stage, the reciprocal of I denotes the maximum value of τ as t tends to infinity and L denotes a lag time.
The relationship between stand density and biological time τ can be expressed by the following equation (Shinozaki 1961; Hozumi 1977):
where ρ i is initial density, and q and μ are coefficients.
Relative growth rate (RGR), defined as the rate of size increase per unit of time per unit of size (i.e. RGR = (dsize/dtime)(1/size); Hunt 1990), is a sensitive measure of competitive ability, so that it and the above equations of the C-D effect in self-thinning stands were employed to examine various growth characteristics of E. urophylla stands.
Results
The E. urophylla growth
Figure 1 shows the mean diameter at breast height D of E. urophylla in each density stand from 1 to 7 years after the planting. Stem diameter increased rapidly in the first 3 years and the RGRs of low-, middle- and high-density stand were above 0.36, 0.32 and 0.22 cm cm−1 year−1, respectively, and then trees gradually decreased their growth rate. Increasing density led to a significant decrease in the diameter growth pattern of the E. urophylla trees from 3 years after the planting.
Height growth was less sensitive to the density effect than diameter growth. Although the mean tree height H was also affected by stand density, the trees in all density stands kept a relatively high RGR from 1 to 6 years after the planting (Fig. 2). In 6 years, the height growth of the high density stand became slow compared with other density stands.
Biological time τ
With increasing physical time t, the biological time τ increased rapidly during early growth stages and became slow gradually during later growth stages (Fig. 3). The τ–t curve given by Eq. 8 was steep from 1 to 5 years and became less steep from 6 to 7 years. The constants F, I and L were calculated to be 0.6182 year, 0.1155 and 0.001 year, respectively.
Relationship between biological time τ and physical time t. The regression was given by Eq. 8 (R 2 = 0.990)
Tree density
Figure 4 shows changes in tree density in each stand during the stand development from 1 to 7 years. The mortality was caused by a crowding effect, competition in the high-density stand was more intense than the low-density stand, so that the mortality was increased with increasing density. The relationships were well fitted with Eq. 9 (R 2 > 0.895), and different stand densities tend to converge to the same density level after a sufficient lapse of time.
Change trend of density ρ with biological time τ. Filled circles low-density stand, filled squares middle-density stand, filled triangles high-density stand. The data were fitted using Eq. 9 (R 2 = 0.895 for low-density stand stem, R 2 = 0.966 for middle-density, R 2 = 0.925 for high-density stand)
Allometric relationships between the weights of tree organs and the tree weight
The w o/w stands for the dry weight ratio of each organ, which is shown in Fig. 5a–c. The stem weight ratio increased after the planting, which changed from 0.18 to 0.58, from 0.19 to 0.58 and from 0.21 to 0.59, in the low-, middle- and high-density stands, respectively. However, leaf weight ratio decreased from 0.61 to 0.05, from 0.60 to 0.05 and from 0.58 to 0.05 in the corresponding stands. The weight ratios of branch, bark and root remained relatively steady from 2 years after the planting.
Relationships between dry weight ratios w o/w of stems (open circles), branches (open squares), leaves (open triangles), bark (open inverted triangles) and root (open diamonds) to mean dry weight w of trees. The curves are fitted by Eq. 8. a The low-density stand (R 2 > 0.908 for each organ), b the medium-density stand (R 2 > 0.826 for each organ), c the high-density stand (R 2 > 0.868 for each organ)
The C-D effect of self-thinning stands
Figure 6 shows the relationship of mean tree w to density ρ in the E. urophylla stands. The C-D effect was evident from 3 years after planting and was well described with the reciprocal equation given by Eq. 4 at a given time (R 2 > 0.776 for each growth stage). Difference among densities in mean tree weight was evident from 2 years after the planting, when trees in the low-density stand were already 20 and 21% larger in mean weight than those in the middle- and high-density stand (Fig. 6). The relative growth advantage of the former had increased to 23 and 39% by the 7th year. The C-D curve shifted upward with the progress of time. The changes of the coefficients A t and B in Eq. 4 were shown in Table 2. Initially, the coefficient A t increased abruptly up to a maximum value, and thereafter decreased gradually with increasing stand age, whereas the coefficient B decreased and tended to close to zero.
The C-D effect between mean tree weight w and density ρ at a given time in the E. urophylla stands. The curves were fitted using Eq. 4. Filled circles 2-year-old (R 2 = 0.776), open circles 3-year-old (R 2 = 0.992), filled squares 4-year-old (R 2 = 0.998), open squares 5-year-old (R 2 = 0.958), filled triangles 6-year-old (R 2 = 0.808), open triangles 7-year-old (R 2 = 0.955)
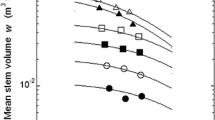
The C-D effect strongly affected mean stem weight w S from 3 to 7 years (Fig. 7a). The mean stem weight of the low-density stand increased by 60.9 kg from 2 to 7 years, which was 24 and 39% more than the middle- and high-density stands, indicating that competition in the middle- and high-density stands had become more intense with time relative to the low-density stand. The w S–ρ data was well fitted by Eq. 5 at each growth stage and the change trend of the w S–ρ curve was the same as the w–ρ curve.
The C-D effects between organ yield w o and density ρ. a Stem, b branch, c leaf, d bark, and e root. The curves show Eq. 5, where the g and k values, and the A t and B values are obtained from Eqs. 2 and 4, respectively. Filled circles 2-year-old (R 2 > 0.753 for each organ), open circles 3-year-old (R 2 > 0.845 for each organ), filled squares 4-year-old (R 2 > 0.803 for each organ), open squares 5-year-old (R 2 > 0.922 for each organ), filled triangles 6-year-old (R 2 > 0.803 for each organ), open triangles 7-year-old (R 2 > 0.884 for each organ)
During the 5 years between ages 3 and 7 increment in mean branch weight in the low-density stand was 7.2 kg, 11% and 37% more than the middle- and high- density stands. The w Br–ρ data points were well described by w Br–ρ curve given by Eq. 5 (Fig. 7b). The time trend of the w Br–ρ relationship was almost the same as that of the w S–ρ relationship.
The increment in mean leaf weight in the low-, middle- and high-density stands were 2.5, 2.4 and 2.1 kg from 2 to 7 years, respectively, which were less than those of mean stem or mean branches, so that scale of the w L–ρ curve was smaller than the two latters (Fig. 7c). The w L–ρ curve given by Eq. 5 moved up on log–log coordinates during the whole experimental period.
The relationships between mean bark weight w Ba and density ρ as well as between mean root weight w R and density ρ are shown in Fig. 7d and e, respectively. The two figures indicate that w Ba–ρ and w R–ρ relationships could be well described by Eq. 5. The w Ba–ρ and w R–ρ curves moved upwards on log–log coordinates from 3 to 7 years in the E. urophylla stands.
Discussion
The E. urophylla growth
The reduction in stem diameter with increasing density from 1,111 to 2,500 tree ha−1 in the E. urophylla stands is consistent with other density experiments in E. nitens (Pinkard and Neilsen 2003), E. grandis (Kearney 1999), E. urophylla, E. pellita, E. camaldulensis (Bernardo et al. 1998), E. pilularis and E. cloeziana (Alcorn et al. 2007). The increased competition for environmental resources (light, water and nutrients) with increased stand density can reduce average stem diameter within the stand (e.g. Kearney 1999; Neilsen and Gerrand 1999; Alcorn et al. 2007). Compared with the low-density stand, lateral growth of the crown in the high-density stand is impeded early, resulting in a small crown width. Meanwhile, natural pruning in the high-density stand occurs earlier and stronger than in the low-density stand, so that the crown length of the former is smaller than the latter. Therefore, crown size decreases with increasing density, whereas a small crown contains fewer leaves, which is unfavorable for photosynthesis and weakens the growth of tree diameter, as a result, diameter growth decreased with increasing density.
The effect of planting density on height growth was not as pronounced as the effects on diameter in this study, which is consistent with other studies (e.g. Deans and Milne 1999; Neilsen and Gerrand 1999). The relationship between height growth and density varies with tree species, site and the stage of stand growth. Some studies indicate that height growth is insensitive to density (e.g. Seidel 1984; Lanner 1985). However, a few studies show that height growth increases with increasing density in young plantations (Gilbert et al. 1995; Knowe and Hibbs 1996; Ritchie 1997). In addition, some researchers reported that height growth in intermediate densities was greater than in the high and the low densities (e.g. Cole and Newton 1987; Giordano and Hibbs 1993; Pienaar and Shiver 1993). In contrast with the above results, our study shows that height growth of the E. urophylla increases with decreasing density, which is consistent with studies in eucalypts (Opie et al. 1984; Alcorn et al. 2007) and other broadleaf (e.g. Niemistö 1995) and conifer species (e.g. Malimbwi et al. 1992). As individual trees in a stand grow, individual competition for resources in the high-density stand is more intense than in the low-density stand. Compared to the low-density stand, the leaves within and among individual trees in the high-density stand overlap with each other, and the growth of leaves located the lower crown is repressed, which reduces individual photosynthesis and affects tree growth, so that height growth in the high-density stand is slightly smaller than that in the low-density stand.
In the stands with complete crown closure, density is inversely related to the square of average crown diameter. A close relationship exists between average stem diameter and crown width, whereas the correlation between height and crown dimensions is negative when diameter is equal (Zeide 2010), and therefore the latter is a better predictor of density than average height. Stem volume is a function of tree height or diameter and a good proxy of tree biomass. Therefore, tree height or diameter is good indicators of tree biomass.
The C-D effect
Shinozaki and Kira (1956) developed the reciprocal equation of the C-D effect in populations as follows:
Here, A and B are coefficients at a given growth stage, and the coefficients A and B are, respectively, defined as
and
where Y(t) is the final yield, τ is called biological time, and initial mean plant weight w 0 is independent of density ρ (Shinozaki 1961). The population density in Eq. 10 does not decrease with plant growth, i.e. the population grows without self-thinning.
Hagihara (1999) constructed a model for describing the C-D effect in self-thinning stands in line with the logistic theory of the C-D effect as Eq. 4, and a new assumption about the relationship between the actual density ρ and initial density ρ i is newly incorporated into the logistic theory of the density effect in self-thinning populations. The coefficient A t and B in Eq. 4 is, respectively, defined as
and
where ε is a coefficient independent of both ρ and ρ i, but is a function of time. The coefficient A t in Eq. 4 is quite different from the coefficient A in Eq. 10. The difference between Eqs. 4 and 10 results from the difference in mathematics interpretation between coefficient A t in Eq. 4 and coefficient A in Eq. 10. Considering Eqs. 11, 12 and 13, it follows that A t is equal to the sum of A and −εB.
Equation 4 used simple variables (w and ρ) and well fit the observed data as shown in this study, and its validity is also supported by the data of P. densiflora (Xue and Hagihara 1998), P. massoniana (Xue and Hagihara 2002) and Betula platyphylla var. japonica and Betula ermanii (Stankova and Shibuya 2003). This suggests that Eq. 4 can be applied to analyze the growth characteristics and dynamics of stands with different densities under self-thinning. The analysis of the plant growth during the course of self-thinning using Hagihara’s model would contribute towards solving the problems because of its wide applicability. The results show that Eq. 5 derived from allometric equation and the C-D theories is validated on 7-year-old E. urophylla data, and the observed w o value was highly correlated with simulated w o value (Fig. 7a–e). Equation 5 provides a high degree of accuracy in predicting organ biomass allocation patterns for different density stands, and it can be applied to stands where previous information of organ biomass and density is available. Moreover, this equation can predict organ biomass over a wider density range (Fig. 7a–e), which is comprehensive and convenient for the growth analysis of self-thinning stands. Equation 9 can be well fitted using simple variables (ρ, ρ i, τ) and shows the relationship between density of the E. urophylla stands and biological time τ during the self-thinning process, which is important for understanding the growth behavior of the plant population. Although there are many tree species being used for reforestation in China, their C-D effect during the course of self-thinning has been poorly reported. Therefore, Eqs. 5 and 9 will be a useful tool in comparing the C-D effect among different species and densities.
Equation 5 can be used to analyze the organ growth of 7-year-old E. urophylla stands with different densities under self-thinning. However, the model application should be age-dependent, since the theory of density effect is based on the logistic growth of plant. As trees grow older and taller, they spend more energy to supply leaves with the same amount of water than do younger and shorter trees. Taller stems entail greater expenditures for their construction and maintenance (Zeide 1995). As a result, trees have to allocate a decreasing portion of their resources to leaves, and their closure decreases (Zeide 1991). Since growth of tree organs may not obey a logistic growth law in older stands, Eq. 5 should be applied only to the self-thinning young stands.
In the evaluation of stand growth stage, the physical time is not a good indicator of stand growth, because it does not reflect physiological condition and the site quality on which the stand growth is dependent (Stankova and Shibuya 2003), whereas value of biological time τ relates to physiological and environmental conditions of stand growth (Shinozaki 1961). Therefore, coefficients A t and B in Eq. 5 and density ρ in Eq. 9 are expressed as functions of biological time. Although biological time τ cannot be estimated from the stand data directly, it can be derived based on coefficient B and initial mean plant weight (Xue and Hagihara 2002). In this study, the C-D effect equation of organ (Eq. 5) and density-biological time equation (Eq. 9) are successfully used to the E. urophylla stands, indicating that biological time is a applicable and practical indicator of stand development stage.
Organ biomass change
There is a slight increment in mean leaf weight w L from 6 to 7 years in E. urophylla stands, and their values are 0.11, 0.05 and 0.05 kg kg−1 year−1 in the low-, middle- and high-density stands, respectively, and corresponding leaf biomass decreases by 1 and 2% and increases by 2% due to decreasing density, which indicates that leaf biomass reaches a more or less constant level. Ogawa (2008) also demonstrated constant leaf biomass in mature forest stands after canopy closure. As self-thinning occurs, unoccupied space is produced. Therefore, the survivors can extend more branches and develop more leaves, and result in increment in the mean leaf mass per tree, which may cause that the leaf biomass per unit land area will remain more-or-less constant in self-thinning stands (Ogawa et al. 2010). Therefore, constant leaf biomass might be a common feature of forest stands.
The E. urophylla biomass distribution by organs shows a significant increase over time for stems and a significant decrease for leaves. The stem weight proportion increased from 36–38% at 2 years to 57–59% at 7 years, whereas the leaf weight proportion decreased from 29–31 to 4–5% in all stands. Stem weight proportions at 7 years in the present results are in close proximity with estimates obtained from Eucalyptus stands in Australia (Cromer 1996; Zewdie et al. 2009), but which are higher than E. urophylla stands in Leizhou Peninsula, China (Xue 2009) and lower than E. urophylla stands in Fujian Province and Guangxi Province, China (Lin et al. 2003; Ye et al. 2010). Leaf weight proportions are lower than the data of Cromer (1996) and Zewdie et al. (2009), but they are the same as estimates obtained from E. urophylla stands by Xue (2009) and higher than data coming from Lin et al. (2003) and Ye et al. (2010). Therefore, E. urophylla is characterized by growth having an high stem biomass proportion and a low leaf biomass proportion. Beadle and Mummery (1989) reported a similar decrease to that reported here in the ratio of leaf area to sapwood area in two provenances of E. nitens between ages 1 and 4 years. This decrease is matched with a change in carbon allocation from leaf mass to stem. Zeide (1985) and Zewdie et al. (2009) also concluded that the percent contribution of stem increased whereas that of leaves decreased with increasing stand age. The period of time leading up to canopy closure is characterized by changes in the relative partitioning of biomass between leaves and stem (Beadle and Inions 1990). After canopy closure, the proportion of the stem continues to increases, whereas the proportion of leaves decreases as trees grow older and taller. The reason is that older and taller trees entail greater expenditures for their construction and maintenance, and spend more energy to supply leaves with the same amount of water than do younger and shorter trees (Zeide 1995). Competing vegetation induces a shift in carbon allocation from the leaves to the stem, probably the result of a hormonal inhibition in lateral bud development (Xue and Hagihara 2008). Moreover, the rapidly changing light conditions experienced by individual crowns within the stand may cause this change (Medhurst et al. 1999).
Stand density determines the timing and intensity of resource competition among trees (Harrington et al. 2009) and influences forest productivity and diameter (Zeide 2005). It allows a forester to estimate the patterns of stand development (Sprintsin et al. 2009). Results of this research indicate that biomass of individual trees is maximized when stands are grown at the low density that promote crown development and thereby accelerate individual tree growth. However, total biomass is maximized at the high density, which was 51 and 29% greater than those of low- and middle-density stands when the E. urophylla stands were 7 years old. Since the main objective for E. urophylla stands is the production of pulpwood, high density produces the greatest biomass, especially the greatest stem biomass, which is 52 and 32% greater than those of low- and middle-density stands 7 years after planting, so that it is the best choice in silvicultural practice. Stand density has an important effect on total stem volume of stands. High density leads to a relatively long period of intense competition among trees, and therefore release treatments from the competition should be carried out early in the stands. Light thinning is recommended, which can produce the most desirable stand-level volume growth.
References
Alcorn PJ, Pyttel P, Bauhus J, Smith RGB, Thomas D, James R, Nicotra A (2007) Effects of initial planting density on branch development in 4-year-old plantation grown Eucalyptus pilularis and Eucalyptus cloeziana trees. For Ecol Manag 252:41–51
Beadle CL, Inions G (1990) Limits to growth of eucalypts and their biology of production. In: Dargavel J, Semple N (eds) Prospects for Australian forest plantations. Centre for Resource and Environmental Studies, ANU, Canberra, pp 183–193
Beadle CL, Mummery DC (1989) Stand growth and development of leaf area index in young plantations of Eucalyptus nitens at 2 × 2 m spacings. N Z For Res Inst Bull 151:254–258
Berger U, Piou C, Schiffers K, Grimm V (2008) Competition among plants: concepts, individual-based modeling approaches, and a proposal for a future research strategy. Perspect Plant Ecol Evol Syst 9:121–135
Bernardo AL, Reis MGF, Reis GG, Harrison RB, Firme DJ (1998) Effect of spacing on growth and biomass distribution in Eucalyptus camaldulensis, E. pellita and E. urophylla plantations in southeastern Brazil. For Ecol Manag 104:1–13
Cole E, Newton M (1987) Fifth-year response of Douglas-fir to crowding and non-coniferous competition. Can J For Res 17:181–186
Cromer RN (1996) Silviculture of Eucalyptus plantations in Australia. In: Attiwill PM, Adams PM (eds) Nutrition of eucalyptus. CSIRO, Canberra, Australia, pp 259–273
Deans JD, Milne R (1999) Effects of respacing on young Sitka spruce crops. Forestry 72:47–57
Fang S, Xue J, Tang L (2007) Biomass production and carbon sequestration potential in poplar plantations with different management patterns. J Environ Manag 85:672–679
Fetene M (2003) Intra- and inter-specific competition between seedlings of Acacia etbaica and a perennial grass (Hyparrenia hirta). J Arid Environ 55:441–451
Gilbert IR, Seavers GP, Jarvis PG, Smith H (1995) Photomorphogenesis and canopy dynamics. Phytochrome-mediated proximity perception accounts for the growth dynamics of canopies of Populus trichocarpa × deltoids ‘Beaupre’. Plant Cell Environ 18:475–497
Giordano PA, Hibbs DE (1993) Morphological response to competition in red alder: the role of water. Funct Ecol 7:462–468
Grove TS, Malajczuk N (1985) Biomass accumulation by trees and under storey shrubs in an age series of Eucalyptus diversicolor F. Muell. stands. For Ecol Manag 11:59–74
Hagihara A (1999) Theoretical considerations on the C-D effect in self-thinning plant populations. Res Popul Ecol 41:151–159
Harrington TB, Harrington CA, DeBell DS (2009) Effects of planting spacing and site quality on 25-year growth and mortality relationships of Douglas-fir (Pseudotsuga menziesii var. menziesii). For Ecol Manag 258:18–25
Hozumi K (1977) Ecological and mathematical considerations on self-thinning in even-aged pure stands. I. Mean plant weight-density trajectory during the course of self-thinning. Bot Mag Tokyo 90:165–179
Hunt R (1990) Basic growth analysis: plant growth analysis for beginners. Unwin Hyman, London
Hunt MA, Battaglia M, Davidson NJ, Unwin GL (2006) Competition between plantation Eucalyptus nitens and Acacia dealbata weeds in northeastern Tasmania. For Ecol Manag 233:260–274
Kearney D (1999) Characterisation of branching patterns, changes caused by variations in initial stocking and implications for silviculture, for E. grandis and E. pilularis plantations in the North Coast region of NSW. Honours Thesis. Department of Forestry. The Australian National University, Canberra, p 89
Khan MNS, Suwa R, Hagihara A (2005) Allometric relationships for estimating the aboveground phytomass and leaf area of mangrove Kandelia candel (L.) Druce trees in the Manko Wetland, Okinawa Island, Japan. Trees 19:266–272
Kira T, Ogawa H, Hozumi K (1956) Intraspecific competition among higher plant. V. Supplementary notes on the C-D effect. J Inst Polytech Osaka City Univ Ser D 7:1–14
Knowe SA, Hibbs DE (1996) Stand structure of young red alder as affected by plant density. For Ecol Manag 82:69–85
Lanner RM (1985) On the insensitivity of height growth to spacing. For Ecol Manag 13:143–148
Lin D, Luo SF, Gao XK (2003) Study on growth situation of Eucalyptus urophylla S. T. Blake after planting one circle six years. J Fujian Coll For 23(3):261–265 (in Chinese with English summary)
Malimbwi RE, Persson A, Iddi S, Chamshama SAO, Mwihomeke ST (1992) Effects of spacing on yield and some wood properties of Pinus patula at Rongai, northern Tanzania. For Ecol Manag 53:297–306
Manning P, Houston K, Evans T (2009) Shifts in seed size across experimental nitrogen enrichment and plant density gradients. Basic Appl Ecol 10:300–308
Medhurst JL, Battaglia M, Cherry ML, Hunt MA, White DA, Beadle CL (1999) Allometric relationships for Eucalyptus nitens (Deane and Maiden) Maiden plantations. Trees 14:91–101
Minowa M (1982) A theoretical approach to forest growth model. I. The log-Mitscherlich theory. J Jpn For Soc 64:461–467 (in Japanese with English summary)
Montagu K, Duttmer K, Barton C, Cowie A (2005) Developing general allometric relationships for regional estimates of carbon sequestration—an example using Eucalyptus pilularis from seven contrasting sites. For Ecol Manag 204:113–127
Naito K (1992) Studies on forest growth modeling. Bull Utsunomiya Univ For 28:1–95 (in Japanese with English summary)
Neilsen WA, Gerrand AM (1999) Growth and branching habit of Eucalyptus nitens at different spacing and the effect on final crop selection. For Ecol Manag 123:217–229
Niemistö P (1995) Influence of initial spacing and row-to-row distance on the growth and yield of silver birch (Betula pendula). Scand J For Res 10:245–255
Ogawa K (2008) The leaf mass/number trade-off of Kleiman and Aarssen implies constancy of leaf biomass, its density and carbon uptake in forest stands: scaling up from shoot to stand level. J Ecol 96:188–191
Ogawa K, Adu-Bredu S, Yokota T, Hagihara A (2010) Leaf biomass changes with stand development in hinoki cypress (Chamaecyparis obtusa [Sieb. et Zucc.] Endl.). Plant Ecol 211:79–88
Opie JE, Curtin RA, Incoll WD (1984) Stand management. In: Hillis WE, Brown AG (eds) Eucalypts for wood production. CSIRO, Melbourne, pp 179–200
Pienaar LV, Shiver BD (1993) Early results from an old-field loblolly pine spacing study in the Georgia Piedmont with competition control. South J Appl For 17:193–196
Pinkard EA, Neilsen WA (2003) Crown and stand characteristics of Eucalyptus nitens in response to initial spacing: implications for thinning. For Ecol Manag 172:215–227
Ritchie GA (1997) Evidence for red: far red signaling and photomorphogenic response in Douglas-fir (Pseudotsuga menziesii) seedlings. Tree Physiol 17:161–168
Seidel KW (1984) A western larch-Engelmann spruce spacing study in eastern Oregon: results after 10 years. USDA For Serv Res Note PNW-409
Shinozaki K (1961) Logistic theory of plant growth. Doctoral Thesis. Kyoto University, Kyoto (in Japanese)
Shinozaki K, Kira T (1956) Intraspecific competition among higher plants. VII. Logistic theory of the C-D effect. J Inst Polytech Osaka City Univ Ser D 7:35–72
Sprintsin M, Karnieli A, Sprintsin S, Cohen S, Berliner P (2009) Relationships between stand density and canopy structure in a dryland forest as estimated by ground-based measurements and multi-spectral spaceborne images. J Arid Environ 73:955–962
Stankova T, Shibuya M (2003) Adaptation of Hagihara’s competition-density theory for practical application to natural birch stands. For Ecol Manag 186:7–20
Vandermeer J (1984) Plant competition and the yield–density relationship. J Theor Biol 109:393–399
Watkinson AR (1980) Density-dependence in single-species populations of plants. J Theor Biol 83:345–357
Watkinson AR (1984) Yield–density relationships: the influence of resource availability on growth and self-thinning in populations of Vulpia fasciculate. Ann Bot 53:469–482
Weiner J (1990) Asymmetric competition in plant populations. Trees 5:360–364
Xue P (2009) Growth and biomass of six-year-old Eucalyptus urophylla plantation in Leizhou Forestry Bureau. Eucalypt Sci Technol 26:18–21 (in Chinese with English summary)
Xue L, Hagihara A (1998) Growth analysis of the self-thinning stands of Pinus densiflora Sieb. et Zucc. Ecol Res 13:183–191
Xue L, Hagihara A (2002) Growth analysis on the C-D effect in self-thinning Masson pine (Pinus massoniana) stands. For Ecol Manag 165:249–256
Xue L, Hagihara A (2008) Density effects of tree organs in self-thinning Pinus densiflora Sieb. et Zucc. stands. Ecol Res 23:689–695
Xue L, Chen FX, Feng HF (2010) Time-trajectory of mean component weight and density in self-thinning Pinus densiflora stands. Eur J For Res 129:1027–1035
Yang WD, Zhong LS (1997) Researches on the experiment of planting densities for new introduction Eucalyptus urophylla. Eucalypt Sci Technol 14(2):37–41 (in Chinese)
Ye SM, Tan LH, Long T, Lan JX (2010) Research on biomass-density effect of Eucalyptus urophylla plantation. J Anhui Agric Sci 38:11594–11596, 11607 (in Chinese with English summary)
Zeide B (1985) Tolerance and self-tolerance of trees. For Ecol Manag 13:149–166
Zeide B (1991) Self-thinning and stand density. For Sci 37:517–523
Zeide B (1995) A relationship between size of trees and their number. For Ecol Manag 72:265–272
Zeide B (2005) How to measure stand density. Trees 19:1–14
Zeide B (2010) Comparison of self-thinning models: an exercise in reasoning. Trees 24:1117–1126
Zewdie M, Olsson M, Verwijst T (2009) Above-ground biomass production and allometric relations of Eucalyptus globulus Labill. coppice plantations along a chronosequence in the central highlands of Ethiopia. Biomass Bioenergy 33:421–428
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Chen.
Rights and permissions
About this article
Cite this article
Xue, L., Pan, L., Zhang, R. et al. Density effects on the growth of self-thinning Eucalyptus urophylla stands. Trees 25, 1021–1031 (2011). https://doi.org/10.1007/s00468-011-0576-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-011-0576-4