Abstract
Biomass pretreatment for the production of second-generation (2G) ethanol and biochemical products is a challenging process. The present study investigated the synergistic efficiency of purified carboxymethyl cellulase (CMCase), β-glucosidase, and xylanase from Aspergillus fumigatus JCM 10253 in the hydrolysis of alkaline-pretreated sugarcane bagasse (SCB). The saccharification of pretreated SCB was optimised using a combination of CMCase and β-glucosidase (C + β; 1:1) and addition of xylanase (C + β + xyl; 1:1:1). Independent and dependent variables influencing enzymatic hydrolysis were investigated using response surface methodology (RSM). Hydrolysis using purified CMCase and β-glucosidase achieved yields of 18.72 mg/mL glucose and 6.98 mg/mL xylose. Incorporation of xylanase in saccharification increased the titres of glucose (22.83 mg/mL) and xylose (9.54 mg/mL). Furthermore, characterisation of SCB biomass by scanning electron microscopy, X-ray diffraction, and Fourier transform infrared spectroscopy respectively confirmed efficient structural disintegration and revealed the degree of crystallinity and spectral characteristics. Therefore, depolymerisation of lignin to produce high-value chemicals is essential for sustainable and competitive biorefinery development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic biomass is a polymer that has attracted worldwide attention due to its economic potential for renewable feedstock sources for energy production, especially given the rapid depletion of oil reserves and other environmental issues. Lignocellulosic waste materials such as those from agricultural, agro-industrial, and forestry processes are comparatively less expensive than other sources of feedstock (Wang et al. 2018; Velvizhi et al. 2023). Therefore, methods to manipulate the chemical composition of lignocelluloses to combat energy resource depletion are in great demand (Dharmaraja et al. 2023). Lignocellulose biomass-derived biofuels can replace fossil fuels due to greater efficiency and yield. The quantity of lignocellulose dry matter available is believed to be 85 to 125 billion tonnes (Zhang et al. 2016). However, chemical complexity makes it difficult to utilise the sugar molecules in these substances. Few processes are able to efficiently convert complex sugars of lignocelluloses into simple fermentable sugars. Complexity is contributed by lignin, hemicellulose, and cellulose fibrils, which are abundant in plant cell walls (Velvizhi et al. 2022). Thus, there is a need for an effective pretreatment procedure that can break down the composite cellulose molecules and allow enzymes to access and hydrolyse these substances (Chaudhary et al. 2023). Enzymatic hydrolysis fragments the cross-links between lignin and hemicellulose adjoining the cellulose fibres (Hans et al. 2023). This is followed by breaking the hydrogen bonds of the crystalline structure of cellulose, increasing the surface area of the fibres and enhancing permeability (Devi et al. 2022; Kukreti et al. 2023).
The present work focused on developing more cost-effective and viable sources of biofuel generation. Sugarcane bagasse (SCB) is a type of biomass that contributes to meeting the world’s energy needs and environmental sustainability. Worldwide, sugarcane production is ~ 1.6 billion tonnes per year, which generates ~ 279 million metric tons of SCB (Chandel et al. 2012; Jugwanth et al. 2020). Brazil is reportedly the largest sugarcane producer, followed by India and China (Khoo et al. 2018). SCB is a waste material from the sugarcane industry that has gained the attention of scientists as a prospective source for second-generation biofuel production (Ajala et al. 2021). The chemical composition of SCB is well studied in the literature (Batalha et al. 2015; Santo et al. 2018; Ávila et al. 2018; Hans et al. 2023). Another advantage is the availability of sugarcane by-products from sugar industries and alcohol-producing plants, and the resultant decrease in logistic expenses if biofuel production is established in the same unit (Ferreira-Leitão et al. 2010). Converting lignocellulosic materials into ethanol involves initial pretreatment, enzymatic hydrolysis to yield sugars, fermentation, and fractionation (Aziz et al. 2023). Pretreatment procedures break down the complex organic matter, decrease the quantity of hemicellulose and lignin, and alter the crystalline configuration of cellulose to enhance its susceptibility to enzymatic attack (Chauhan et al. 2023). Without the pretreatment step, the efficiency of converting glucose and xylose into ethanol by enzyme hydrolysis is very low, but high concentrations of xylose and glucose can be liberated with the help of a pretreatment procedure (Guilherme et al. 2015).
There are several investigations on SCB as a biomass source. However, there is a need to assess the use of sugarcane straw, alone or with bagasse, as raw material to produce fermentable sugars for ethanol production. Additionally, developing more efficient enzyme combinations requires detailed information on the explicit enzymes engaged in the breakdown of sugarcane-based organic matter (Huang et al. 2011; Adav et al. 2012; Ávila et al. 2018). Disruption of the complex structure of cellulose-hemicellulose-lignin is an essential step in biorefining biomass. Alkaline pretreatment acts directly on this structure by promoting delignification by breaking down α- and β-alkyl- and aryl-ether bonds, deprotonating phenolic groups liberated from lignin and depolymerising cellulose to eliminate some uronic acids and acetyl groups of xylan chains, consequently enhancing the susceptibility of the substrate to enzymatic breakdown. Moreover, research on the life cycle assessment of second-generation biofuel production processes using alkaline pretreatment as an initial step has demonstrated that an alkaline solution can be retrieved and used again, making the process more economically feasible (Rocha et al. 2014).
In recent years, research on the efficient and economically viable enzymatic transformation of lignocellulosic biomass has been the focus of many international studies to overcome constraints associated with the high cost of commercial enzymes. Filamentous fungi mainly produce hydrolytic enzymes, and many studies have sought superior strains with high enzyme activity, especially cellulases and hemicellulases (Santos et al. 2015; de Oliveira et al. 2017; Prajapati et al. 2020). These enzymes play significant roles during the enzymatic saccharification of biomass; the characteristic biospecificity of hydrolytic enzymes for a particular chemical bond is used to produce the corresponding products. In this regard, cellulases and cellobiases (or β-glucosidases) are responsible for breaking down the glycosidic bonds of cellulose to produce glucose while xylanases break down the glycosidic bonds of xylan to release xylose (Ibarra-Díaz et al. 2020).
This study explored the synergistic efficiency of hydrolytic enzymes produced from Aspergillus fumigatus JCM 10253 (Paramjeet et al. 2021; Saroj et al. 2022) in the hydrolysis of alkaline-pretreated SCB. Specifically, we focused on optimising and evaluating the saccharification yield using carboxymethyl cellulase (CMCase) and β-glucosidase, including supplementation of xylanase, on alkaline-pretreated SCB. To our knowledge, this is the first report on blending hydrolytic enzymes obtained from a single strain of A. fumigatus in SCB hydrolysis. Enzymatic hydrolysis was optimised using a response surface methodology (RSM), and five independent variables (temperature, pH, enzyme loading, substrate concentration, and incubation time) were evaluated. This approach can determine the enzymatic production potential and the appropriate proportions of enzymes. Furthermore, untreated, pretreated and enzymatically hydrolysed biomass were characterised using scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FTIR).
Materials and methods
Enzymes
Purified enzymes CMCase (19.5 IU/mL), β-glucosidase (0.083 IU/mL), and xylanase (26.8 IU/mL) from A. fumigatus JCM 10253 were used for enzymatic hydrolysis. Their activities were measured and defined as described in previous reports (Paramjeet et al. 2021; Saroj et al. 2022).
Raw material processing
SCB was procured from a local jaggery manufacturing unit in Warangal, Telangana, India. SCB was used as lignocellulosic biomass after juice extraction without washing, and air-dried at room temperature to reduce the moisture content. An electric crusher was used to homogenise SCB and generate fine particles. The consistency of the particle size was maintained between 3 and 5 mm in diameter by sieving. Dried biomass was stored in plastic Ziploc bags at room temperature for further experiments.
Alkaline-based pretreatment
SCB was treated with 0.5 M sodium hydroxide (10% w/v) at 121 °C for 1 h. After treatment, the solid fraction was separated by filtration and subjected to repeated washing with distilled water until the pH was neutralised. The pretreated biomass was dried at 60 °C for 12 h and stored in a desiccator for hydrolysis and physicochemical characterisation.
Enzymatic hydrolysis
Enzymatic hydrolysis was conducted at different temperatures spanning 30 − 60 °C with an initial substrate concentration of 2.5% (w/v) pretreated dry mass (SCB) and incubated for up to 48 h in 250-mL Erlenmeyer flasks with shaking at 150 rpm. Saccharification was performed in flasks containing 100 mL of 50 mM sodium citrate buffer (pH 4.8), and sodium azide (0.04%) was added to the reaction mixture to prevent microbial contamination. Reactions were treated with different concentrations of substrates (SCB) from 1 to 3.5% (w/v) and enzyme loading from 1 to 3.5 mL (v/v). Two approaches were employed: a mixture of CMCase and β-glucosidase at a 1:1 ratio (% v/v), and a mixture of CMCase, β-glucosidase and xylanase at a 1:1:1 ratio (% v/v). The effect of pH (4, 4.2, 4.6, 4.8, 5, and 5.2) on total reducing sugar (TRS) yield was tested. Samples were obtained at 12, 24, 36, 48, and 72 h following hydrolysis and enzymes were deactivated by heating for 10 min at 100 °C. The resulting solids were separated by centrifugation for 5 min at 5000 rpm and filtered using a 0.22 μm syringe filter (Millipore, Billerica, MA, USA). Total reducing sugar was quantified as the total sum of glucose, cellobiose, arabinose, xylose, and other sugars released in each treatment.
Analytical method
After enzymatic hydrolysis, chromatographic separation of soluble sugars in hydrolysates was performed using high-performance liquid chromatography (HPLC) with a Prominence UFLC instrument (Shimadzu, Kyoto, Japan) equipped with an LC-20AD pump and a refractive index detector RID-10A. The Rezex-RPM-monosaccharide-Lead (II) ion column (Phenomenex, Torrance, CA, USA) was used to detect the sugars. HPLC-grade water was used as the mobile phase under isocratic mode. The temperature of the column oven and detector were set to 80 and 50 °C, respectively. The pump flow rate was 0.6 mL/min for 30 min run time. Chromatograms for each sample and standards were compared with respect to retention time (RT) to quantify reducing sugars. Standards used in analytical procedures were HPLC-grade chemicals from Sigma-Aldrich Co. (St. Louis, MO, USA).
Physicochemical characterisation of feedstock
SEM
SEM analysis was performed using a Carl Zeiss MA15/EVO 18 SEM (Carl Zeiss Microscopy GmbH Carl-Zeiss-Promenade 10 07745, Jena, Germany) instrument to examine the surface morphological characteristics of SCB. Untreated, pretreated and enzymatically hydrolysed SCB samples were fixed with carbon tape supporting an aluminium stub and analysed with acceleration voltage and magnification.
XRD
The cellulose crystalline structure of SCB samples was analysed using a Bruker D8 Advanced X-ray Diffractometer (Bruker AXS GmbH, Germany). Samples were dehydrated at 60 °C in an oven before analysis. Intensities were determined between 10° and 60° of a 2θ scale (scattering angle) with a scanning speed of 0.05°/scan. XRD patterns were used to calculate the cellulose crystalline index, represented as CrI, using the following equation (Segal et al. 1959):
where I002 represents the intensity of the (crystalline phase) 002 peak (2θ = 21.8°), and Iam represents the intensity of the peak corresponding to the amorphous phase (2θ = 18.2°).
FTIR
Changes in the chemical structure of SCB were determined following pretreatment and enzymatic hydrolysis by FTIR analysis using a 3000 Hyperion Microscope and a Vertex 80 FTIR System (Bruker Optics GmbH, Ettlingen, Germany). FTIR spectra of SCB were recorded at 4 cm−1 resolution over a 500 − 4000 cm−1 wavenumber range (Bala and Singh 2019).
Optimisation of enzymatic hydrolysis to generate glucose and xylose using RSM
Design Expert 7.0 software (Stat-Ease, Minneapolis, MN, USA) was used to apply the quadratic model to examine correlations between the quantity of sugars released and five variables governing the hydrolysis with the help of enzymes. The assortment criteria for elements and the range of variables depended on the condition of the operating system, which affected the hydrolysis process by enzymes. For the dominant complex strategy, variables were fixed on a five-point scale, as depicted in Tables 1 and 2. The central composite design (CCD) comprised five factors, namely temperature (A, 30, 40, 50 and 60 °C), pH (B, 4.6, 4.8 and 5), enzyme loading (C, 2, 2.5, 3 and 3.5 mL), substrate concentration (D, 2, 2.5, 3 and 3.5 g) and incubation time (E, 24, 36, 48, 60 and 72 h), for reducing sugar (glucose and xylose) production from the substrate (pretreated SCB). Two sets of enzymes were prepared to employ RSM. In the first set, only purified CMCase and β-glucosidase (C + β; 1:1 v/v) were mixed in equal proportion. The second set was prepared by mixing enzymes of the first set combined with purified xylanase (C + β + xyl; 1:1:1 v/v).
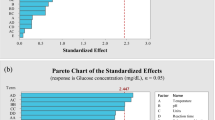
Fifty runs for each enzyme set were conducted and production of reducing sugar was considered a response activity. The results of the dominant complex strategy for the examined variables (temperature, pH, enzyme loading, substrate concentration and incubation time) are summarised in Tables 3 and 4. RSM was employed for analysis of variance (ANOVA) to obtain significant statistical parameters. The R2 value was determined to assess the significance of the model. Second-order polynomial equations were applied to determine the relationships between independent variables to evaluate the predicted response, and 3D graphs were plotted to assess the effects of significant variables on the response. All experiments were performed in triplicate to identify differences in the obtained data, and results are presented as the mean ± standard deviation.
Results and discussion
Hydrolytic performance of alkaline-pretreated SCB under different conditions
Effect of temperature
The effects of temperature on enzymatic hydrolysis using cellulases (C + β) and supplementation with xylanase (C + β + xyl) are shown in Figs. 1a and 2a, respectively. Optimal enzymatic hydrolysis under both conditions was obtained at 50 °C with total reducing sugar of 27.59 mg/mL and 34.65 mg/mL, respectively. At 30 °C and 40 °C, the degree of enzymatic hydrolysis of pretreated SCB was lower than that at 50 °C, and the amount of reducing sugar was lower when the hydrolysis temperature was raised to 60 °C. This behaviour was most likely brought on by higher temperatures increasing enzyme inactivation. Saccharification using a mixture of cellulases from Fusarium incarnatum, A. niger and Trichoderma harzianum achieved maximal reducing sugar production at 50 °C (Pant et al. 2021). Huang et al. (2015) assessed alkali-pretreated bamboo residues and the optimum enzymatic saccharification yield was achieved at 50 °C. Similar studies have been conducted to maximise the enzymatic hydrolysis of lignocelluloses using endo-1,4-β-xylanase at 50 °C (Ge et al. 2014; Yang et al. 2015; Chen et al. 2020).
Effect of pH
pH is a critical factor affecting cellulose and hemicellulose degradation for reducing sugar production. Herein, the maximal yield was obtained at pH 4.8. The total reducing sugar released by hydrolysis using cellulases (C + β) and with addition of xylanase (C + β + xyl) was 32.13 mg/mL and 40.37 mg/mL, respectively (Figs. 1b and 2b). Recent studies found that pH 4.8 was optimum for hydrolysis of corn stover powder and sugarcane bagasse to produce reducing sugar (Zhang and Wu 2021; Barrameda et al. 2023). Maximum reducing sugar production is typically in the pH range of 4.8 − 5.0 (Alrumman 2016; Procentese et al. 2017; Sun et al. 2018). pH significantly affects the hydrolytic behaviour of cellulases; the hydrolytic process can only occur after the formation of an enzyme–substrate complex, and pH has a similar influence on both adsorption and hydrolysis, which typically occur at pH 4.8.
Effect of enzyme loading
The effect of enzyme loading on the hydrolysis of pretreated SCB was determined (Figs. 1c and 2c). An increase in enzyme loading from 1 to 3 mL (v/v) in the reaction mixture containing pretreated biomass resulted in an increase in total reducing sugar yield to 32.3 mg/mL with enzyme loading (C + β) at 2.5 mL (v/v). Similarly, enzyme loading (C + β + xyl) of 3 mL (v/v) achieved a 41.4 mg/mL total reducing sugar yield. Sugar production was not enhanced by an additional increase in enzyme load. Elevated quantities of cellulase have the potential to reverse saccharification by accelerating transglycosylation processes, causing hydrodynamic instability, inappropriate mixing and slurry suspension. The enzyme dose should be kept to a minimum because the expense of cellulase greatly increases the cost of the hydrolysis process (Alrumman 2016).
Effect of substrate concentration
Substrate concentration is another significant parameter affecting total reducing sugar yield during enzymatic hydrolysis. In the present study, pretreated SCB concentration achieved the maximum yield at 2.5 g (w/v %), which produced 32.9 mg/mL of total reducing sugar using cellulases (C + β). However, adding xylanase (C + β + xyl) achieved the optimum yield at a substrate concentration of 3 g (w/v %), which produced 43.87 mg/mL of total reducing sugar. In both cases, the optimum conditions for total reducing sugar production were determined after a 48-h incubation (Figs. 1d and 2d).
During saccharification, adding excess substrate may reduce sugar yield through end-product inhibition when combined with limited mass transfer within the reaction mixture, due to the high viscosity of the slurry. Other factors include a lower level of carbohydrate conversion at a high substrate loading and a decrease in the reaction of cellulose material due to a low quantity of enzyme loading. Thus, saccharification degree strongly depended on the enzyme–substrate ratio (Alrumman 2016). Several reports demonstrated an increase in substrate concentration leading to high sugar yield (Akhtar et al. 2001; Alrumman 2016; Pant et al. 2021). In enzymatic hydrolysis, converting polysaccharides to monosaccharides is a more complex process that depends on enzyme–substrate synergism (Rajak and Banerjee 2016).
Effect of incubation time
To enhance the rate of enzymatic hydrolysis, it is essential optimise critical process parameters such as temperature, pH, enzyme loading, substrate to liquid ratio and saccharification time. These process parameters contribute to the enzymatic hydrolysis of lignocelluloses into monomeric sugars (Vimala Rodhe et al. 2011; Sharma et al. 2015). Enzymatic hydrolysis using cellulases (C + β) and adding xylanase (C + β + xyl) achieved a maximum total reducing sugar yield of 34.31 mg/mL and 44.03 mg/mL, respectively, after 48 h (Figs. 1e and 2e). Furthermore, increasing the duration of saccharification did not enhance total reducing sugar production. Previous studies reported a maximum reducing sugar yield after 48 h of incubation using different pretreated biomass and enzymatic cocktails from fungal sources (Sharma et al. 2017; Pimentel et al. 2021; Shankar et al. 2022; Infanzón-Rodríguez et al. 2023). Furthermore, according to Mahamud and Gomes (2012), factors such as pretreatment of the substrate, enzyme and substrate concentrations, product inhibition and enzyme stability affect the rate and degree of saccharification, with the rate of hydrolysis decreasing rapidly over time as a result.
Physicochemical characterisation of untreated, alkaline-pretreated and enzymatically hydrolysed SCB
SEM analysis
SEM analysis was carried out to study the morphological structure of SCB biomass under various treatments (Fig. 3). The morphology of untreated SCB biomass showed a compressed and rough structure (Fig. 3a). On the other hand, the morphology of alkaline-pretreated SCB biomass showed fragmentary deconstruction of the rigid structure of plant cell walls (Fig. 3b). However, enzymatically hydrolysed SCB biomass using cellulases (C + β) and with addition of xylanase (C + β + xyl) demonstrated significant disruption and exposure of the fibres (Fig. 3c and d). Hence, unprocessed organic matter was thoroughly packed and firm. After alkaline pretreatment, the composition was altered due to the formation of cavities and the release of hemicelluloses and lignin. This made the structure more open and accessible to enzymes. Several studies have described the morphological structures of lignocellulosic biomass based on SEM analysis (Lv et al. 2013; Chandel et al. 2014; de Oliveira et al. 2017; Pereira et al. 2016). Zhang et al. (2018) and Guilherme et al. (2017) revealed an increase in the surface area after pretreatment of SCB biomass by splitting fibres due to the removal of the lignin fraction and increased biomass digestibility with cellulases.
XRD analysis
After plotting 2θ versus the intensity of the cellulose signals of SCB samples (Fig. 4), the crystallinity index (CrI) was calculated from the intensity of the cellulose crystal peak at a 2θ value of 21.8 and the amorphous intensity at a 2θ value of 18.2. The intensities of crystalline cellulose, amorphous crystal, crystallinity index, and % crystallinity index are listed in Table 5. Untreated SCB had a CrI of 38.64% while alkaline-pretreated SCB had a CrI of 58.14%. Hydrolysed pretreated SCB using cellulases (C + β) had a highest CrI of 62.15% while (C + β + xyl) hydrolysed pretreated SCB with xylanase had a CrI of 61.66%. The CrI of cellulases (C + β) hydrolysed pretreated SCB was 60% higher than that of untreated SCB and 10% higher than alkaline-pretreated SCB.
In this study, the combined effects of alkaline pretreatment and enzymatic hydrolysis contributed to increased CrI. The findings showed that pretreatment of SCB resulted in a greater removal of lignin and hemicellulose. Moreover, it has been previously documented that successful biomass pretreatment increases the amorphous nature of cellulose fibres, increasing their availability for enzymatic hydrolysis (Moura et al. 2018; Nath et al. 2021; Tavares et al. 2024). Crystallinity index measures the comparative quantity of cellulose present in organic matter (Joshi et al. 2011; Ahvenainen et al. 2016). This parameter was greater than that of untreated biomass, indicating the partial removal of lignin or hemicelluloses and increased cellulose content in treated samples (Bernardinelli et al. 2015). These results are consistent with previous reports on switchgrass (Karp et al. 2015; Wyman et al. 2011), sugarcane bagasse (Zhang et al. 2018), the upper portions of sugarcane (Sindhu et al. 2013) and sweet sorghum bagasse (Zhang et al. 2011). Thus, alkaline pretreatment of SCB can be employed to improve delignification and cellulose recovery with increased cellulose exposure for effective enzymatic hydrolysis.
FTIR analysis
FTIR spectra of lignocellulosic materials reveal the molecular vibrations associated with peaks corresponding to cellulose, hemicelluloses and lignin. The observed bands and their assignments are summarised in Supplementary Table S1. Figure 5 shows FTIR spectra of untreated, alkaline-pretreated, cellulases (C + β), and addition of xylanase (C + β + xyl) hydrolysed SCB biomass. The structural characteristics of cellulose and hemicellulose were reflected by a prominent band at 1000 − 1200 cm–1. The –C–O and C–O–C stretching vibrations of cellulose were reflected in peaks at 1053 − 1164 cm–1. The bands at 897 − 898 cm–1 corresponding to β-(1,4)-glycosidic linkage C–O–C stretching vibrations were strengthened by the transition from a crystalline to an amorphous structure of cellulose (Nath et al. 2021). However, the O–H stretching vibrations of hydrogen bonds were almost the same for untreated, pretreated and enzymatic hydrolysed residues based on 3406 − 3460 cm–1 adsorption peaks. Following pretreatment, there were some peaks at 2898 − 2917 cm–1 associated with the C–H stretching vibrations of methylene groups of cellulose (Semwal et al. 2023). In addition to the –C = O bond of the acetyl group in the hemicellulose structure, adsorption peaks at 1714 − 1731 cm–1 were associated with ester linkages between hemicellulose and lignin. Considering the above, we determined that there were very few or no acetyl groups, and that the xylan content decreased following pretreatment. The corresponding bands became less intense due to hemicellulose deacetylation (Semwal et al. 2023; Hans et al. 2023; Kapoor et al. 2015). For untreated SCB, bands at 1514 cm–1 and 1604 cm–1 were attributed to the C = C stretching vibrations of aromatic rings. The lignin content was not determined in alkaline-pretreated SCB, which indicates that lignin structures were hydrolysed during pretreatment. These results showed that cellulose and hemicellulose are more exposed following alkaline pretreatment of SCB.
Regression analysis and model fitting
The relative impact on glucose and xylose production is indicated by the regression coefficient. The equation indicates which factor had the greatest effect on glucose and xylose production. After 50 trials, optimisation approaches were made simpler by RSM. Interactions between five independent variables and dependent variables for the response surface quadratic model were assessed to determine the influence of individual and combined variables on glucose and xylose yield. Specifically, ANOVA was performed to explore the variability of each response variable for enzymatic hydrolysis (C + β) of pretreated SCB (Supplementary Tables S2 and S3). The outcomes were determined using a second-order polynomial equation and the significance of regression coefficients. The models for quadratic regression for glucose and xylose are conveyed by the second-order polynomial equations, represented in Eqs. (1) and (2), respectively.
A positive symbol placed before every factor in the abovementioned mathematical model signifies a synergistic influence, while an antagonistic effect is indicated by a negative symbol. If a coefficient represents any single factors, it reflects the impact of that particular factor on the glucose and xylose yield. These factors are temperature (A), pH (B), enzyme loading (C), concentration of substrate (D) and incubation time (E). The outcome was obtained with a coefficient representing two factors. The remaining are represented by second-order terms showing interactions between the two factors and the quadratic effect.
The results of ANOVA for enzymatic hydrolysis (C + β + xyl) of pretreated SCB are depicted in Supplementary Tables S4 and S5 with respect to the response surface quadratic model. The response functions were applied with the help of regression analysis. Comparison between the five independent variables and the respective responses were outlined by the second-order polynomial equations for glucose and xylose, represented in Eqs. (3) and (4), respectively.
Correlations between p value and respective coefficients were found to be inversely proportional. The models were significant (p < 0.0001) and consistent with the quadratic models in terms of correlation between the responses and the independent variables (Supplementary Tables S2−S5). Models were capable of predicting glucose and xylose yields based on p values and lack of fit. The higher coefficient (R2) and adjusted (R2) values show the accuracy of the models. The coefficient (R2) was used to derive the fitness of the model, giving 97.5% for glucose and 94.6% for xylose from cellulases (C + β) hydrolysed SCB biomass, and their adjusted (R2) values were 95.7% and 90.9%, respectively. In xylanase (C + β + xyl) hydrolysed SCB, the fitness of the model was determined by coefficients (R2) of 92.7% and 97.8% for glucose and xylose, respectively, and their adjusted (R2) values were 87.6% and 96.3%, respectively. For both glucose and xylose, the results indicate that the developed model fitted the experimental data very well.
Model validation
RSM yielded the required theoretical models for releasing glucose and xylose based on a derived set of input variables. To validate the model, a very efficient theoretical model was used, and to observe the interfaces between variables, plots with 3D surfaces were employed, as depicted in Figs. 6 and 7. The interactive effects of variables on the production of reducing sugar were investigated by generating 3D response surface plots against any two independent factors and their corresponding sugar production while maintaining other variables at their central (0) level. Hydrolysis of SCB using cellulases (C + β) exhibited an optimal substrate concentration at 2.54% (w/v), pH 4.6, enzyme loading 2.81 mL, temperature 45.2 °C and processing duration 47.9 h. The experimental glucose yield was 18.72 mg/mL, close to the predicted value of 19.04 mg/mL. Similarly, for xylose, the optimal value of substrate concentration was achieved at 2.54% (w/v), pH 4.7, enzyme loading 2.6 mL, temperature 44.8 °C and processing duration 48 h. The experimental yield of xylose was 6.98 mg/mL, very close to the predicted value of 7.14 mg/mL, as shown in Table 6.
Hydrolysis of SCB biomass following addition of xylanase (C + β + xyl) had an optimal substrate concentration at 2.79% (w/v), pH 4.7, enzyme loading 3.5 mL, temperature 44.5 °C and processing duration 53 h. The experimental glucose yield was 22.83 mg/mL, very close to the predicted value of 23.13 mg/mL. On the other hand, for xylose yield, the optimal substrate concentration was at 2.75% (w/v), pH 4.7, enzyme loading 2.76 mL, temperature 44.8 °C and processing duration 52.9 h. The maximum yield of xylose was 9.54 mg/mL, very close to the predicted 9.91 mg/mL (Table 7). The observed values of glucose and xylose closely matched the predicted values, and the experimental results under ideal conditions matched the predictions relatively well, providing strong validation for the RSM models. Comparison of experimental and predicted values confirmed that the experimental strategy can efficiently maximise variables in the process optimisation, and examine the significance of individual, combined and cooperative impacts of test variables in the enzyme-assisted hydrolysis process.
Saccharification of dennanath and hybrid napier grass showed an increase in total reducing sugar using cellulases from A. fumigatus (CWSF-7) and commercial xylanase (Mohapatra et al. 2018). Moretti et al. (2014) demonstrated the enzymatic hydrolysis of pretreated SCB using endoglucanase, β-glucosidase and xylanase from Myceliophthora thermophila M.7.7., with enhanced glucose and xylose yields. A previous study on hydrolysis involving enzymatic cocktails from A. fumigatus SCBM6 and A. niger SCBM1 showed greater efficiency in depolymerising hemicellulose fractions (de Oliveira Rodrigues et al. 2017). Enzymatic hydrolysis of sugarcane bagasse using filter paper units (FPU), CMCase and β-glucosidase of A. niger ITV02 achieved a higher glucose yield (Infanzón-Rodríguez et al. 2023). The study showed that alkaline pretreatment of corn stover powder and enzymatic hydrolysis were optimised using RSM to maximise glucose and xylose yields (Zhang and Wu 2021). Moreover, hydrolysates have potential applications in various fields for high-value-added products and advanced biofuels such as butanol. Specifically, xylose can produce xylitol, lactic acid, acetic acid and biodegradable plastics (de Cassia Pereira et al. 2016; de Arruda et al. 2017; Mardawati et al. 2022; Devi et al. 2022; Chauhan et al. 2023). Enzymes are always specific for their particular substrates, and incorporating xylanase increases the overall efficiency of cellulases. Supplementation of xylanases increases the solubility of xylan within substrates (De Figueiredo et al. 2017; Mohapatra et al. 2018). Thus, it is essential to understand the mechanisms within reaction mixtures containing biomass, cellulases and xylanases. The present study revealed that purified cellulases can have a significant effect alongside supplementing xylanase. This strategy can be implemented for efficient enzymatic hydrolysis of different biomasses.
Conclusion
We investigated the saccharification of alkaline-pretreated SCB using hydrolytic enzymes from A. fumigatus JCM 10253. The saccharification optimisation strategy showed that combining CMCase, β-glucosidase and xylanase increased enzymatic hydrolysis efficiency and achieved a higher total reducing sugar yield. The central composite design achieved maximum glucose (22.83 mg/mL) and xylose (9.54 mg/mL) production. Therefore, process optimisation with statistical designs can effectively recover both glucose and xylose sugars from SCB. SEM analysis confirmed the cell wall disintegration of SCB by the alkaline pretreatment, which improved enzyme accessibility and adsorption by surface area. Diffractograms of SCB following different treatments demonstrated the conspicuous expansion of crystallinity in which a combination of pretreated and enzymatic (C + β) hydrolysis SCB produced a maximum CrI of 62.15%, compared with 38.64% for untreated SCB and 58.14% for alkaline-pretreated SCB. This indicated that pretreatment of SCB with 0.5 M sodium hydroxide achieved greater removal of lignin and hemicelluloses, and was simultaneously an effective pretreatment for enhancing enzymatic saccharification. FTIR analysis of pretreated SCB revealed more prominent cellulosic peaks than for untreated SCB. Furthermore, alkaline pretreatment and combined enzyme mixtures can be effective and economically beneficial for delignification, cellulose exposure and enhanced enzymatic hydrolysis to produce fermentable sugars, which could be further exploited for biofuel production.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files.
Change history
22 July 2024
Incorrect heading used for the Data Availability text.
References
Adav SS, Chao LT, Sze SK (2012) Quantitative secretomic analysis of Trichoderma reesei strains reveals enzymatic composition for lignocellulosic biomass degradation. Mol Cell Proteomics 11(M111):012419. https://doi.org/10.1074/mcp.M111.012419
Ahvenainen P, Kontro I, Svedström K (2016) Comparison of sample crystallinity determination methods by X-ray diffraction for challenging cellulose I materials. Cellulose 23:1073–1086. https://doi.org/10.1007/s10570-016-0881-6
Ajala EO, Ighalo JO, Ajala MA, Adeniyi AG, Ayanshola AM (2021) Sugarcane bagasse: a biomass sufficiently applied for improving global energy, environment and economic sustainability. Bioresour Bioprocess 8:1–25. https://doi.org/10.1186/s40643-021-00440-z
Akhtar MS, Saleem M, Akhtar MWJIJAB (2001) Saccharification of lignocellulosic materials by the cellulases of Bacillus subtilis. 3:199–202. 1560–8530/2001/03–2–199–202
Alrumman SA (2016) Enzymatic saccharification and fermentation of cellulosic date palm wastes to glucose and lactic acid. Braz J Microbiol 47:110–119. https://doi.org/10.1016/j.bjm.2015.11.015
Ávila PF, Forte MBS, Goldbeck R (2018) Evaluation of the chemical composition of a mixture of sugarcane bagasse and straw after different pretreatments and their effects on commercial enzyme combinations for the production of fermentable sugars. Biomass Bioenerg 116:180–188. https://doi.org/10.1016/j.biombioe.2018.06.015
Aziz T, Shah Z, Sarwar A, Ullah N, Khan AA, Sameeh MY, Haiying C (2023) Production of bioethanol from pretreated rice straw, an integrated and mediated upstream fermentation process. Biomass Convers Biorefinery 1–11. https://doi.org/10.1007/s13399-023-04283-w
Bala A, Singh B (2019) Development of an environmental-benign process for efficient pretreatment and saccharification of biomasses for bioethanol production. Renew Energ 130:12–24. https://doi.org/10.1016/j.renene.2018.06.033
Barrameda HJC, Requiso PJ, Alfafara CG, Nayve FRP Jr, Ventura RLG, Ventura J-RS (2023) Hydrolysate production from sugarcane bagasse using steam explosion and sequential steam explosion-dilute acid pretreatment for polyhydroxyalkanoate fermentation. Bioact Carbohydr Diet Fibre 30:100376. https://doi.org/10.1016/j.bcdf.2023.100376
Batalha LA, Han Q, Jameel H, Chang HM, Colodette JL, Borges Gomes FJ (2015) Production of fermentable sugars from sugarcane bagasse by enzymatic hydrolysis after autohydrolysis and mechanical refining. Bioresour Technol 180:97–105. https://doi.org/10.1016/j.biortech.2014.12.060
Bernardinelli OD, Lima MA, Rezende CA, Polikarpov I, deAzevedo ER (2015) Quantitative (13)C MultiCP solid-state NMR as a tool for evaluation of cellulose crystallinity index measured directly inside sugarcane biomass. Biotechnol Biofuels 8:110. https://doi.org/10.1186/s13068-015-0292-1
Chandel AK, da Silva SS, Carvalho W, Singh OV (2012) Sugarcane bagasse and leaves: foreseeable biomass of biofuel and bio-products. J Chem Technol Biotechnol 87:11–20. https://doi.org/10.1002/jctb.2742
Chandel AK, Antunes FA, Anjos V, Bell MJ, Rodrigues LN, Polikarpov I, de Azevedo ER (2014) Multi-scale structural and chemical analysis of sugarcane bagasse in the process of sequential acid-base pretreatment and ethanol production by Scheffersomyces shehatae and Saccharomyces cerevisiae. Biotechnol Biofuels 7:63. https://doi.org/10.1186/1754-6834-7-63
Chaudhary G, Chaudhary N, Saini S, Gupta Y, Vivekanand V, Panghal A (2023) Assessment of pretreatment strategies for valorization of lignocellulosic biomass: path forwarding towards lignocellulosic biorefinery. Waste Biomass Valori 1–36. https://doi.org/10.1007/s12649-023-02219-z
Chauhan SJ, Patel B, Devliya B, Solanki H, Patel HD (2023) Recent advancement in production of bioethanol from waste biomass: a review. Clean Techn Environ Policy 1–21. https://doi.org/10.1007/s10098-023-02710-0
Chen X, Xin DL, Sun FF, Zhang JH (2020) Factors affecting the hydrolytic action of xylanase during pennisetum saccharification: role of lignin. Cellulose 27:3143–3152. https://doi.org/10.1007/s10570-020-02996-z
de Arruda PV, dos Santos JC, Rodrigues RdCLB, da Silva DDV, Yamakawa CK, de Moraes Rocha GJ, Júnior JN (2017) Scale up of xylitol production from sugarcane bagasse hemicellulosic hydrolysate by Candida guilliermondii Fti 20037. 47:297–302. https://doi.org/10.1016/j.jiec.2016.11.046
de Cassia Pereira J, Travaini R, Paganini Marques N, Bolado-Rodriguez S, Bocchini Martins DA (2016) Saccharification of ozonated sugarcane bagasse using enzymes from Myceliophthora thermophila JCP 1–4 for sugars release and ethanol production. Bioresour Technol 204:122–129. https://doi.org/10.1016/j.biortech.2015.12.064
de Figueiredo FC, Carvalho AFA, Brienzo M, Campioni TS, de Oliva-Neto P (2017) Chemical input reduction in the arabinoxylan and lignocellulose alkaline extraction and xylooligosaccharides production. Bioresour Technol 228:164–170. https://doi.org/10.1016/j.biortech.2016.12.097
de Oliveira Rodrigues P, de Cássia PJ, Santos DQ, Gurgel LVA, Pasquini D, Baffi MA (2017) Synergistic action of an aspergillus (hemi-)cellulolytic consortium on sugarcane bagasse saccharification. Ind Crops Prod 109:173–181. https://doi.org/10.1016/j.indcrop.2017.08.031
Devi A, Bajar S, Kour H, Kothari R, Pant D, Singh A (2022) Lignocellulosic biomass valorization for bioethanol production: a circular bioeconomy approach. Bioenergy Res 15:1820–1841. https://doi.org/10.1007/s12155-022-10401-9
Dharmaraja J, Shobana S, Arvindnarayan S, Francis RR, Jeyakumar RB, Saratale RG, Ashokkumar (2023) Lignocellulosic biomass conversion via greener pretreatment methods towards biorefinery applications. Bioresour Technol 369:128328. https://doi.org/10.1016/j.biortech.2022.128328
Ferreira-Leitão V, Gottschalk LMF, Ferrara MA, Nepomuceno AL, Molinari HBC, Bon EP (2010) Biomass residues in Brazil: availability and potential uses. Waste Biomass Valorization 1:65–76. https://doi.org/10.1007/s12649-010-9008-8
Ge X, Sun Z, Xin D, Zhang J (2014) Enhanced xylanase performance in the hydrolysis of lignocellulosic materials by surfactants and non-catalytic protein. Appl Biochem Biotechnol 172:2106–2118. https://doi.org/10.1007/s12010-013-0673-5
Guilherme AA, Dantas PVF, Santos ES, Fernandes FAN, Macedo GR (2015) Evaluation of composition, characterization and enzymatic hydrolysis of pretreated sugar cane bagasse. Braz J Chem Eng 32:23–33. https://doi.org/10.1590/0104-6632.20150321s00003146
Guilherme ADA, Dantas PVF, Soares JCJ, dos Santos ES, Fernandes FAN, de Macedo GR (2017) Pretreatments and enzymatic hydrolysis of sugarcane bagasse aiming at the enhancement of the yield of glucose and xylose. Braz J Chem Eng 34:937–947. https://doi.org/10.1590/0104-6632.20170344s20160225
Hans M, Pellegrini VOA, Filgueiras JG, de Azevedo ER, Guimaraes FEC, Chandel AK, Polikarpov I (2023) Optimization of dilute acid pretreatment for enhanced release of fermentable sugars from sugarcane bagasse and validation by biophysical characterization. BioEnergy Research 16:416–434. https://doi.org/10.1007/s12155-022-10474-6
Huang RL, Su RX, Qi W, He ZM (2011) Bioconversion of lignocellulose into bioethanol: process intensification and mechanism research. Bioenergy Res 4:225–245. https://doi.org/10.1007/s12155-011-9125-7
Huang C, He J, Wang Y, Min D, Yong Q (2015) Associating cooking additives with sodium hydroxide to pretreat bamboo residues for improving the enzymatic saccharification and monosaccharides production. Bioresour Technol 193:142–149. https://doi.org/10.1016/j.biortech.2015.06.073
Ibarra-Díaz N, Castañón-Rodríguez JF, Gómez-Rodríguez J, Aguilar-Uscanga MG (2020) Optimization of peroxide-alkaline pretreatment and enzymatic hydrolysis of barley straw (Hordeum vulgare L.) to produce fermentable sugars using a Box-Behnken design. Biomass Convers Biorefinery 12:2389–2398. https://doi.org/10.1007/s13399-020-00853-4
Infanzón-Rodríguez MI, del Moral S, Gómez-Rodríguez J, Faife-Pérez E, Aguilar-Uscanga MG (2024) Second-generation bioethanol production and cellulases of Aspergillus niger ITV02 using sugarcane bagasse as substrate. BioEnergy Res 17:160–172. https://doi.org/10.1007/s12155-023-10640-4
Joshi B, Bhatt MR, Sharma D, Joshi J, Malla R, Sreerama L (2011) Lignocellulosic ethanol production: Current practices and recent developments. Biotechnol Mol Biol Rev 6:172–182
Jugwanth Y, Sewsynker-Sukai Y, Kana EBG (2020) Valorization of sugarcane bagasse for bioethanol production through simultaneous saccharification and fermentation: optimization and kinetic studies. Fuel 262:116552. https://doi.org/10.1016/j.fuel.2019.116552
Kapoor M, Raj T, Vijayaraj M, Chopra A, Gupta RP, Tuli DK, Kumar R (2015) Structural features of dilute acid, steam exploded, and alkali pretreated mustard stalk and their impact on enzymatic hydrolysis. Carbohyd Polym 124:265–273. https://doi.org/10.1016/j.carbpol.2015.02.044
Karp EM, Resch MG, Donohoe BS, Ciesielski PN, O’Brien MH, Nill JE, Mittal A (2015) Alkaline pretreatment of switchgrass. Acs Sustain Chem Eng 3:1479–1491. https://doi.org/10.1021/acssuschemeng.5b00201
Khoo RZ, Chow WS, Ismail H (2018) Sugarcane bagasse fiber and its cellulose nanocrystals for polymer reinforcement and heavy metal adsorbent: a review. Cellulose 25:4303–4330. https://doi.org/10.1007/s10570-018-1879-z
Kukreti N, Kumar P, Kataria R (2023) Conversion of corn stover for microbial enzymes production by Phanerochaete chrysosporium. Appl Biochem Biotechnol 1–17. https://doi.org/10.1007/s12010-023-04811-4
Lv SL, Yu Q, Zhuang XS, Yuan ZH, Wang W, Wang Q, Qi W (2013) The influence of hemicellulose and lignin removal on the enzymatic digestibility from sugarcane bagasse. Bioenergy Res 6:1128–1134. https://doi.org/10.1007/s12155-013-9297-4
Mahamud MR, Gomes DJ (2012) Enzymatic saccharification of sugar cane bagasse by the crude enzyme from indigenous fungi. J Sci Res 4. https://doi.org/10.3329/jsr.v4i1.7745
Mardawati E, Febrianti EA, Fitriana HN, Yuliana T, Putriana NA, Suhartini S, Kasbawati. (2022) An integrated process for the xylitol and ethanol production from oil palm empty fruit bunch (OPEFB) using Debaryomyces hansenii and Saccharomyces cerevisiae. Microorganisms 10:2036. https://doi.org/10.3390/microorganisms10102036
Mohapatra S, Padhy S, Das Mohapatra PK, Thatoi HN (2018) Enhanced reducing sugar production by saccharification of lignocellulosic biomass, Pennisetum species through cellulase from a newly isolated Aspergillus fumigatus. Bioresour Technol 253:262–272. https://doi.org/10.1016/j.biortech.2018.01.023
Moretti MMdS, Bocchini-Martins DA, Nunes CdCC, Villena MA, Perrone OM, Silva Rd, Boscolo M (2014) Pretreatment of sugarcane bagasse with microwaves irradiation and its effects on the structure and on enzymatic hydrolysis. Appl Energ 122:189–195. https://doi.org/10.1016/j.apenergy.2014.02.020
Moura HOMA, Campos LMA, da Silva VL, de Andrade JCF, de Assumpção SMN, Pontes LAM, de Carvalho LS (2018) Investigating acid/peroxide-alkali pretreatment of sugarcane bagasse to isolate high accessibility cellulose applied in acetylation reactions. Cellulose 25:5669–5685. https://doi.org/10.1007/s10570-018-1991-0
Nath P, Maibam PD, Singh S, Rajulapati V, Goyal A (2021) Sequential pretreatment of sugarcane bagasse by alkali and organosolv for improved delignification and cellulose saccharification by chimera and cellobiohydrolase for bioethanol production. 3 Biotech 11:59. https://doi.org/10.1007/s13205-020-02600-y
Pant S, Ritika KA, Penteado ED, Diniz AAR, Rahman MA, Kuila A (2021) NaOH pretreatment and enzymatic hydrolysis of brassica juncea using mixture of cellulases. Environ Technol Innov 21:101324. https://doi.org/10.1016/j.eti.2020.101324
Paramjeet S, Manasa P, Korrapati N (2021) Biochemical characterization of low molecular weight thermostable xylanase from Aspergillus fumigatus JCM 10253. Appl Biochem Microbiol 57:S98–S106. https://doi.org/10.1134/s0003683821100094
Pereira SC, Maehara L, Machado CMM, Farinas CS (2016) Physical–chemical–morphological characterization of the whole sugarcane lignocellulosic biomass used for 2G ethanol production by spectroscopy and microscopy techniques. Renew Energ 87:607–617. https://doi.org/10.1016/j.renene.2015.10.054
Pimentel PSS, de Oliveira JB, Astolfi-Filho S, Pereira N Jr (2021) Enzymatic hydrolysis of lignocellulosic biomass using an optimized enzymatic cocktail prepared from secretomes of filamentous fungi isolated from Amazonian biodiversity. Appl Biochem Biotechnol 193:3915–3935. https://doi.org/10.1007/s12010-021-03642-5
Prajapati BP, Jana UK, Suryawanshi RK, Kango N (2020) Sugarcane bagasse saccharification using Aspergillus tubingensis enzymatic cocktail for 2G bio-ethanol production. Renew Energ 152:653–663. https://doi.org/10.1016/j.renene.2020.01.063
Procentese A, Raganati F, Olivieri G, Elena Russo M, Marzocchella A (2017) Pre-treatment and enzymatic hydrolysis of lettuce residues as feedstock for bio-butanol production. Biomass Bioenerg 96:172–179. https://doi.org/10.1016/j.biombioe.2016.11.015
Rajak RC, Banerjee R (2016) Enzyme mediated biomass pretreatment and hydrolysis: a biotechnological venture towards bioethanol production. Rsc Adv 6:61301–61311. https://doi.org/10.1039/c6ra09541k
Rocha GJM, Nascimento VM, da Silva VFN, Corso DLS, Gonçalves AR (2014) Contributing to the environmental sustainability of the second generation ethanol production: delignification of sugarcane bagasse with sodium hydroxide recycling. Ind Crops Prod 59:63–68. https://doi.org/10.1016/j.indcrop.2014.05.002
Santo ME, Rezende CA, Bernardinelli OD, Pereira N, Curvelo AAS, Deazevedo ER, Guimaraes FEG (2018) Structural and compositional changes in sugarcane bagasse subjected to hydrothermal and organosolv pretreatments and their impacts on enzymatic hydrolysis. Ind Crops Prod 113:64–74. https://doi.org/10.1016/j.indcrop.2018.01.014
Santos BS, Gomes AF, Franciscon EG, Oliveira JM, Baffi MA (2015) Thermotolerant and mesophylic fungi from sugarcane bagasse and their prospection for biomass-degrading enzyme production. Braz J Microbiol 46:903–910. https://doi.org/10.1590/S1517-838246320140393
Saroj P, Manasa P, Narasimhulu K (2022) Biochemical characterization of thermostable carboxymethyl cellulase and beta-glucosidase from Aspergillus fumigatus JCM 10253. Appl Biochem Biotechnol. 194:2503–2527. https://doi.org/10.1007/s12010-022-03839-2
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794. https://doi.org/10.1177/004051755902901003
Semwal S, Sivagurunathan P, Satlewal A, Kumar R, Gupta RP, Christopher J, Kumar R (2023) An efficient and cost- effective pretreatment of rice straw using steam explosion: a pilot scale experience. Waste Biomass Valori 1–12. https://doi.org/10.1007/s12649-023-02267-5
Shankar A, Saini S, Sharma KK (2022) Fungal-integrated second-generation lignocellulosic biorefinery: utilization of agricultural biomass for co-production of lignocellulolytic enzymes, mushroom, fungal polysaccharides, and bioethanol. Biomass Convers Biorefinery 1–15. https://doi.org/10.1007/s13399-022-02969-1
Sharma R, Rawat R, Bhogal RS, Oberoi HS (2015) Multi-component thermostable cellulolytic enzyme production by HN-1 using pea pod waste: appraisal of hydrolytic potential with lignocellulosic biomass. Process Biochem 50:696–704. https://doi.org/10.1016/j.procbio.2015.01.025
Sharma S, Kuila A, Sharma V (2017) Enzymatic hydrolysis of thermochemically pretreated biomass using a mixture of cellulolytic enzymes produced from different fungal sources. Clean Technol Environ Policy 19:1577–1584. https://doi.org/10.1007/s10098-017-1346-9
Sindhu R, Kuttiraja M, Preeti VE, Vani S, Sukumaran RK, Binod P (2013) A novel surfactant-assisted ultrasound pretreatment of sugarcane tops for improved enzymatic release of sugars. Bioresour Technol 135:67–72. https://doi.org/10.1016/j.biortech.2012.09.050
Sun F, Mukasekuru MR, Tan L, Ren J, Huang Z, Ren H, Zhang Z (2018) Optimization of on-site cellulase preparation for efficient hydrolysis of atmospheric glycerol organosolv pretreated wheat straw. J Chem Technol Biotechnol 93:2083–2092. https://doi.org/10.1002/jctb.5605
Tavares MP, Morgan T, Gomes RF, Mendes JPR, Castro-Borges W, Maitan-Alfenas GP, Guimaraes VM (2024) Comparative analysis of exoproteomes and their specificity for saccharification of sugarcane bagasse. Enzyme Microb Tech 173:110365. https://doi.org/10.1016/j.enzmictec.2023.110365
Velvizhi G, Goswami C, Shetti NP, Ahmad E, Pant KK, Aminabhavi TM (2022) Valorisation of lignocellulosic biomass to value-added products: paving the pathway towards low-carbon footprint. Fuel 313:122678. https://doi.org/10.1016/j.fuel.2021.122678
Velvizhi G, Jacqueline PJ, Shetti NP, Latha K, Mohanakrishna G, Aminabhavi TM (2023) Emerging trends and advances in valorization of lignocellulosic biomass to biofuels. J Environ Manage. 345:118527. https://doi.org/10.1016/j.jenvman.2023.118527
Vimala Rodhe A, Sateesh L, Sridevi J, Venkateswarlu B, Venkateswar Rao L (2011) Enzymatic hydrolysis of sorghum straw using native cellulase produced by T. reesei NCIM 992 under solid state fermentation using rice straw. 3 Biotech 1:207–215. https://doi.org/10.1007/s13205-011-0024-6
Wang Z, Hou X, Sun J, Li M, Chen Z, Gao Z (2018) Comparison of ultrasound-assisted ionic liquid and alkaline pretreatment of eucalyptus for enhancing enzymatic saccharification. Bioresour Technol 254:145–150. https://doi.org/10.1016/j.biortech.2018.01.021
Wyman CE, Balan V, Dale BE, Elander RT, Falls M, Hames B, Holtzapple MT (2011) Comparative data on effects of leading pretreatments and enzyme loadings and formulations on sugar yields from different switchgrass sources. Bioresource Technol 102:11052–11062. https://doi.org/10.1016/j.biortech.2011.06.069
Yang M, Zhang J, Kuittinen S, Vepsalainen J, Soininen P, Keinanen M, Pappinen A (2015) Enhanced sugar production from pretreated barley straw by additive xylanase and surfactants in enzymatic hydrolysis for acetone-butanol-ethanol fermentation. Bioresour Technol 189:131–137. https://doi.org/10.1016/j.biortech.2015.04.008
Zhang H, Wu J (2021) Statistical optimization of sodium hydroxide pretreatment and enzymatic hydrolysis of corn stover powder for enhancing sugar production using response surface methodology. Biomass Convers Biorefinery 13:7111–7125. https://doi.org/10.1007/s13399-021-01638-z
Zhang J, Ma X, Yu J, Zhang X, Tan T (2011) The effects of four different pretreatments on enzymatic hydrolysis of sweet sorghum bagasse. Bioresour Technol 102:4585–4589. https://doi.org/10.1016/j.biortech.2010.12.093
Zhang ZY, Harrison MD, Rackemann DW, Doherty WOS, Ohara IM (2016) Organosolv pretreatment of plant biomass for enhanced enzymatic saccharification. Green Chem. 18:360–381. https://doi.org/10.1039/c5gc02034d
Zhang H, Wei W, Zhang J, Huang S, Xie J (2018) Enhancing enzymatic saccharification of sugarcane bagasse by combinatorial pretreatment and Tween 80. Biotechnol Biofuels 11:309. https://doi.org/10.1186/s13068-018-1313-7
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Paramjeet Saroj performed material preparation, data collection and analysis. Paramjeet Saroj wrote the first draft of the manuscript. Korrapati Narasimhulu and Manasa P commented on previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saroj, P., P, M. & Narasimhulu, K. Enhanced reducing sugar production by blending hydrolytic enzymes from Aspergillus fumigatus to improve sugarcane bagasse hydrolysis. Environ Sci Pollut Res 31, 48085–48102 (2024). https://doi.org/10.1007/s11356-024-34246-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-34246-1