Abstract
Endophytic bacteria promote plant growth, reduce stress caused by biotic and abiotic factors, and can trigger active defense reactions in plants. This study aimed to evaluate enzyme activity of in vitro jojoba (Simmondsia chinensis) plants inoculated with endophytic bacteria. In vitro shoots of female and male plants were inoculated with strains of Azospirillum brasilense (Cd), Methylobacterium aminovorans (JRR11), Rhodococcus pyridinivorans (JRR22) or co-inoculated with a mixture of JRR11 + JRR22. A total of 10 treatments were performed to evaluate shoot and root length; changes in key enzymes involved in plant defense (superoxide dismutase, catalase, peroxidase, ascorbate peroxidase and phenylalanine ammonia lyase) after post-inoculation (45 days). All endophytic bacteria strains used promoted plant growth and rhizogenesis. Differences were found in enzyme activity between female and male plants. The plants inoculated with JRR22 strain, showed the highest enzyme activity suggesting an induced systemic response and a potential increase in plant resistance to pathogen attack.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
New sources of renewable energy to help sustainable development, such as oils from non-edible vegetables and derivatives as biodiesel, are receiving increased attention because of their promising characteristics (Aly et al. 2008). Some of these characteristics include being biodegradable, non-toxic, carbon neutral, and clean-burning fuels with almost zero sulfur content. Jojoba oil has an ideal chemical structure for biodiesel as it contains long chain monoesters of fatty acids connected directly to fatty alcohols. Likewise, this oil releases considerable energy on combustion, and it is stable at high operating temperatures (Al-Hamamre and Al-Salaymeh 2014). Jojoba (Simmondsia chinensis) is a native shrub of southwestern United States of America and northwestern Mexico; it can grow in semi-arid regions and tolerate temperatures in the range from 5 to 54 °C; it also requires small amounts of water, fertilizers, and little maintenance (Gentry 1958).
In recent years, research on this plant has focused on increasing seed production; however, the limiting factor has been that the plant has a high genetic variability resulting in a high diversity in oil content and quality (Al-Hamamre and Al-Salaymeh 2014). The presence of pathogenic fungi that causes losses in plants, especially young plants, has also been reported (Baqir-Hussain et al. 2014). Some alternatives to solve these limitations are in vitro cultures and the use of beneficial bacteria (Daros-Salla et al. 2014). The first one allows the establishment of plantations with the desired proportions of female and male plants creating uniformity, high yields, early production, and cost reduction. The use of beneficial bacteria induces plant defense and lowers susceptibility to diseases caused by pathogens (El-Deeb et al. 2013).
Endophytic bacteria are among the beneficial bacteria that colonize plants without apparently causing any damage (Fedorov et al. 2013). These bacteria can benefit plant development through multiple mechanisms of action, directly by producing indole-3-acetic acid (IAA) and facilitating nutrient absorption in soil, or indirectly by antagonizing plant pathogens (Jain et al. 2013). For example, the genus Rhodococcus, endophytic bacteria associated with plants such as Panxi plateau, has an antagonistic activity against pathogens and also promotes growth (Zhao et al. 2011). Similarly, the genus Methylobacterium promotes plant growth, induces systemic resistance, and inhibits pathogen infection (Jain et al. 2013) besides synthesizing several antioxidant regulators and genes associated with stress (PhyR), especially when it is associated with Arabidopsis thaliana (Gururani et al. 2013).
The responses of plants to inoculation with growth-promoting bacteria may be physical or biochemical, which can include reinforcement of the plant cell wall, production of antimicrobial phytoalexins and pathogenesis-related proteins (PRs), increased ability to synthesize defense enzymes, such as peroxidase (PO), polyphenol oxidase (PPO), phenylalanine ammonia lyase (PAL), and synthesis of secondary metabolites (El-Deeb et al. 2013; Gururani et al. 2013). Therefore, the aim of this study was to evaluate the enzyme activity of in vitro jojoba (S. chinensis) plants inoculated with endophytic bacteria.
Materials and methods
Plant material and culture conditions
Assays were performed using shoots of approximately 3 cm long obtained from female and male plants for introduction in vitro. The basal culture medium for multiplication was prepared with mineral salts (Murashige and Skoog 1962); B5 vitamins (Gamborg et al. 1968), 100 mg L−1 myoinositol, 3% sucrose, 0.7% agar, N-6-benzyladenine (BA: 4.44 µM), pH 5.8. The root induction medium (RIM) consisted of a concentration of 50% of MS salts, 3% sucrose, indole-3-butyric acid (IBA: 49.6 µM), 0.6% agar; the shoots were cultured on RIM for 6 days. Subsequently, each shoot was transferred to medium rooting (RM), which consisted of a concentration of 50% of MS salts, 0.6% agar, 3% sucrose, pH 5.8 and hormone-free (Llorente and Apóstolo 2013). Inoculation was made at the time of transferring them to the RM by adding 0.1 mL of bacterial culture (106 cfu mL−1) at the base of each explant (Larraburu et al. 2010). Treatments without inoculation were controlled. Cultures were incubated in a growth chamber at 24 ± 2 °C, with light intensity of 100 µmol m−2 s−1 under a 16 h photoperiod.
Bacterial strains and culture conditions
The strain of Azospirillum brasilense Cd (American Type Culture Collection; ATCC 29710) was used as control; also isolates M. aminovorans JRR22 (KT964148), Rhodococcus pyridinivorans JRR11 (KT985910) (Perez-Rosales et al. 2017) were used for wild jojoba plant roots. Bacteria were grown in Erlenmeyer flaks (250 mL) with 150 mL of medium for A. brasilense Cd (Larraburu et al. 2010) and 150 mL of minimal media for M. aminovorans JRR22 and R. pyridinivorans (Kumar et al. 2012). Strains were incubated at 30 ± 1 °C at 120 rpm for 72 h. The experiments were performed in a completely randomized design, which consisted of a 2 × 5 factorial arrangement with eight repetitions per treatment where the factors were the two jojoba genotypes (male and female plants) and bacterial inoculation. The treatments are shown in Table 1.
Chlorophyll and Carotenoids
Eighteen plants were used per treatment; shoot and root were assessed after 45 days. To measure chlorophyll (Chl) and carotenoid (Car) content, 0.5 g of fresh leaf were mixed with 5 mL acetone. After centrifuging for 5 min at 4000 rpm, absorbance was measured at 649 and 665 nm for Chl and 480 and 510 nm for Car, estimated on spectrophotometer (Perveen et al. 2013).
Enzyme activity
Enzyme activity was determined in leaves and roots separately; 0.5 g of fresh tissue was homogenized in 5 mL of 0.1 M phosphate buffer (pH 7.0), 10 mM EDTA and 1% PVP (polyvinylpyrrolidone w/v). The extracts were centrifuged at 10, 000 g at 4 °C for 15 min (Kumar et al. 2012), and the supernatant was collected for determination of enzyme assays and protein content as described by Bradford method (Bradford 1976).
Superoxide dismutase activity
Superoxide dismutase (SOD; EC 1.15.1.1) activity was determined using the system xanthine/xanthine oxidase as O2·− generator and nitroblue tetrazolium (NBT) as detector (Suzuki 2000). The reaction mixture contained 25 µL of enzyme extract, 25 µL of xanthine oxidase (0.1 U mL−1 in ammonium sulfate 2 M) and 1450 µL of sodium–carbonate solution (50 mM, xanthine 0.1 mM, NBT 0.025 mM NBT, 0.1 mM EDTA). The mixture was quantified in a spectrophotometer at 560 nm for 300 s; SOD activity was expressed in units of SOD mg−1 protein.
Catalase activity
Catalase (CAT; EC1.11.1.6) activity was determined by monitoring H2O2 decomposition as described by Aebi (1984). The reaction mixture contained 50 mM KH2PO4 (pH 7.0), 10 mM H2O2, and 10 µL of enzyme extract. Decrease in absorbance of H2O2 was recorded at 240 nm for 3 min. The enzyme activity was expressed as units of CAT mg−1 protein.
Peroxidase activity
Peroxidase (POX; EC 1.11.1.7) activity was determined by mixing 500 µL of enzyme extract with 1350 µL distilled water, 125 µL 0.1 mM phosphate buffer (pH 6.5), 24.5 µL at 5% H2O2 and 500 µL of 1 mM pyrogallol. The reaction was monitored at 420 nm for 90 s (Zhou and Leul 1999). POX activity was expressed in units POX mg−1 protein.
Ascorbate peroxidase activity
Ascorbate peroxidase (APX; EC1.11.1.11) activity was measured according to Nakano and Asada (1981) method. Fresh leaf and root material (0.5 g) were ground in 5 mL of 50 mM phosphate buffer (pH 7.0), 1 mM EDTA, 1% PVP and centrifuged at 10,000 rpm at 4 °C for 10 min. APX activity was determined in supernatant by the decrease in ascorbate absorbance at 290 nm due to its enzymatic breakdown; 1 mL of reaction buffer containing 0.5 mM ascorbic acid, 0.1 mM H2O2 and 0.5 mL of enzyme extract. The reaction was run for 5 min and the activity was calculated using the extinction coefficient (2.8 mM−1 cm−1) for ascorbate, which was expressed as µM min−1 mg−1protein.
PAL activity
PAL (EC 4.3.1.5) activity was determined with 100 µL of enzyme extract, 900 µL of 6 mM l-phenylalanine solution in 500 mM Tris HCl (pH 8). The reaction mixture was incubated in a water bath at 37 °C for 70 min. The reaction stopped by adding HCl 5 N; absorbance was read at 290 nm. Trans-cinnamic acid (1 mg mL−1) was used as standard and expressed as µg trans-cinnamic acid min−1 mg−1 protein (Paynet et al. 1971).
Statistical analysis
The results are shown as mean ± standard error. Significant differences were observed between treatments and plant sex with two-way ANOVA; means were compared using Tukey’s (P ≤ 0.05). All statistical analyses were performed using the software STATISTICA 6.0 (Stat Soft, 1999).
Results and discussion
Inoculation effect on jojoba growth and rhizogenesis
Several studies have shown the use of endophytic bacteria for increasing plant growth (Andressen et al. 2009; El-Deeb et al. 2013; Larraburu and Llorente 2015). Certain bacteria appeared to have a beneficial effect on the explants in vitro, increasing multiplication and rooting, explant quality, recalcitrant organ and embryogenesis (Orlikowska et al. 2017). Biotization of jojoba explants with Azotobacter chroococum also increased the number of shoots generated per bud and multiplication rate significantly (Andressen et al. 2009). In this study, we found that inoculations with the strains M. aminovorans (JRR 22) and R. pyridinivorans (JRR11) increased shoot and root length in jojoba plants of both sexes compared to the control treatment (Table 1), likely related to the ability of these two strains to produce IAA (Perez-Rosales et al. 2017). Endophytic bacteria can release phytohormones that can be absorbed by plant roots, thus promoting plant growth (Benson et al. 2014). According to Abitha et al. (2014) inoculating with IAA-producing bacteria increases plant growth and rhizogenesis in vitro culture. Likewise, plant growth-promoting rhizobacterium (A. brasilense strain Cd) stimulated in vitro rooting of jojoba. The inoculated explant reached a rooting percentage of 86% and rooted 5 days earlier than the control. They also showed a significant increase in the mean number of roots per shoot, and exhibited less callus production than the control (Carletti et al. 1998; Larraburu et al. 2010). The endophytic bacteria isolated from jojoba roots might result in more vigorous plants, promoting productivity.
Chlorophyll and carotenoids
Carotenoids act as light-harvesting pigment and protect Chl from degradation; in our results, we found a higher concentration of pigments in inoculated plants when compared with the control in plants of both sexes (P ≤ 0.05). In male plants, we found increased concentration of chlorophyll α, β, total and carotenoids when the plants were inoculated with the strain JRR11 (R. pyridinivorans) (Fig. 1a–d). While in female plants, increased concentration of pigments was observed in the treatment where co-inoculation was performed. Benson et al. (2014) reported similar results in Naravelia zeylanica plant inoculated with Achromobacter xylosoxidan, showing a significant increase in chlorophyll content (46.3%). The combinations of plant growth promoting rhizobacterial strains significantly increased plant height, chlorophyll and protein content in Solanum nigrum when compared to the uninocultated control. Microbial inoculation increased 5.96 mg g−1 of chlorophyll (Megala and Paranthaman 2017).
SOD activity
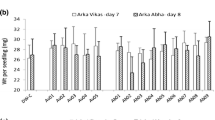
Scavenging free radicals is an important mechanism in which several antioxidant enzymes play a role as part of the plant defense response. The main reactive oxygen species (ROS) formed after electron transfer are superoxide radicals (O2·−), which are dismutated to H2O2 by SOD (Gururani et al. 2013). SOD enzyme activity in leaves of female and male plants was higher in the presence of the strain M. aminovorans (JRR22) when compared with other treatments (P < 0.05) (Fig. 2a). This result is consistent with Giri et al. (2013) who reported that the strains of the genus Methylobacterium increased SOD activity, resulting in the elimination of free radicals and increasing plant resistance to possible stress because M. aminovorans might have induced defense mechanisms in jojoba plants.
Effect of endophytic bacterial inoculation on enzymatic activity of jojoba S. chinensis. SOD enzyme activity in leaves (a) and roots (b); CAT enzyme activity in leaves (c) and roots (d); POX enzyme activity in leaves (e) and roots (f); APX enzyme activity in leaves (g) and roots (h); PAL enzyme activity in leaves (i) and roots (j). Data are means ± SE (n = 8). Different letters indicate significant differences according to Tukey’s test (P < 0.05)
Although it is known that SOD is the first line of defense in cells (Fedorov et al. 2013), in this study, no significant differences were found in the roots between treatments except for the control in female plants (Fig. 2b). Our results are consistent with Ibáñez et al. (2014) who reported that SOD activity was not expressed in Vicia sativa when inoculated with Bacillus sp.
CAT activity
In our study, significant differences were found in CAT activity in leaves (Fig. 2c) of female plants inoculated with A. brasilense (Cd) when compared to those with the other treatments (Fig. 2c). The association of plants inoculated with Azospirillum spp. has been reported to have higher activity of CAT, showing that this interaction is beneficial as the plant is alert to possible stress (El-Deeb et al. 2013). The female and male plants co-inoculated with JRR11 and JRR 22 strains had the lowest CAT activity (Fig. 2c), possibly because this treatment did not stimulate the enzyme activity, which was consistent with Baqir-Hussain et al. (2014).
CAT activity was lower in roots compared with leaves. In female plants, the activity of this enzyme was higher in plants inoculated with the strain M. aminovorans (JRR22) than in those not inoculated (Fig. 2d) while male plants inoculated with the same strain had the highest CAT activity (Fig. 2d). These results are similar to those observed in leaves, suggesting that bacterial inoculation plays an important role in reducing ROS and serves as a defense barrier to possible stress (Jha and Subramanian 2013).
POX activity
POX is a key enzyme plant defense response, participating in lignin biosynthesis by the oxidation of phenolic compounds, thus strengthening the cell wall (Jha and Subramanian 2013). No significant differences between treatments were found (P < 0.05) in POX activity in leaves of male and female plants; however, the treatments of the co-inoculated plants had the highest POX activity (Fig. 2e).
In roots of female plants co-inoculated with JRR11 and JRR22 strains had the highest POX activity (Fig. 2f), which was consistent with the results of Daros-Salla et al. (2014) in eucalyptus plants inoculated with Streptomyces sp. that had higher PPO and POX activities. Similarly, Indiragandhi et al. (2008) reported that inoculation with Methylobacterium strains increased POX activity conferring resistance to foliar pathogen-Pseudomonas syringae, which is important since pathogens are responsible for generating ROS restricting growth of pathogenic bacteria. Roots of male plants inoculated with A. brasilense (Cd) showed the highest POX activity; however, no significant differences were found compared to the control.
APX activity
APX has a role in protecting cells against deleterious effects caused by ROS production and accumulation, which increased notably under environmental stress (Gururani et al. 2013). APX activity increased in male plants compared with female plants, both in leaves and roots in all treatments (Fig. 2g, h). Leaves of female and male plants co-inoculated with JRR11 and JRR22 strains showed higher APX compared to other treatments (P < 0.05) (Fig. 2g, h). However, APX activity in roots of jojoba plants inoculated with strains of A. brasilense (Cd) was higher than in the other treatments (Fig. 2h). Jain et al. (2013) reported that the activity of this enzyme provided stability to the cell membrane with less water loss by perspiration and elimination of H2O2 in the chloroplast and cytosol of plant cells, so bacterial inoculation provided increased expression of this enzyme.
PAL activity
PAL plays an important role in biosynthesis of various defense chemicals in phenylpropanoid metabolism besides PAL activity that could be induced during plant–pathogen interactions (Nagendran et al. 2014). In our study the PAL activity in leaves of female plants was higher in the treatment inoculated with the strain M. aminovorans (JRR22) than with the other treatments (P < 0.05); however, other plants inoculated with the treatments showed lower PAL activity in comparison with the control (Fig. 2i). The leaves of inoculated male plants did not show differences compared with the control. The PAL activity is an indirect measure when the plant is under stress, as it shows a reflection of the immune system (Larraburu and Llorente 2015). Our results suggest that in the jojoba plants inoculated with the strain M. aminovorans (JRR22) PAL activity was higher, as mentioned by Jain and Kumar-Choudhary (2014) who suggested that the organism degraded phenylalanine to obtain NH3, carbon, or both.
Moreover, PAL activity was lower in roots compared with leaves. In male plants no significant differences were found (P < 0.05) between control and inoculated plants. While PAL activity in female plants was lower in the co-inoculation treatment (Fig. 2j). Our results were consistent with Ting et al. (2010) who found that the activities of POX and PAL and total phenol and lignin content increased in banana seedlings inoculated with endophytic bacteria Serratia marcescens, a strain capable of stimulating host defense as their main mode of defense mechanism.
In our study, the endophytic bacteria M. aminovorans (JRR11) and R. pyridinivorans (JRR22) promoted growth and increased rhizogenesis in in vitro cultures of jojoba. Likewise, the strain JRR22 increased SOD and PAL activities. The co-inoculated treatment with strains JRR22 and JRR11 increased POX and APX activities. The strain A. brasilense (Cd) increased CAT activity in leaves and roots. In general, activities of the enzymes CAT and PAL were lower in roots than in leaves. Inoculation with endophytic bacteria increased the activities of antioxidant enzymes providing protection from oxidative stress, avoiding cell membrane damage, and inhibiting photosynthesis. There were differences in enzyme activities between female and male plants. Further studies should be performed on the association of jojoba plants and endophytic bacteria to explain these results.
References
Abitha B, Manoharan-Melvin J, Balathandayutham K, Tongmin S, Chandrasekaran R (2014) Role of Achromobacter xylosoxidans AUM54 in micropropagation of endangered medicinal plant Naravelia zeylanica (L.) DC. J Plant Growth Regul 33:202–213
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Al-Hamamre Z, Al-Salaymeh A (2014) Physical properties of (jojoba oil + biodiesel), (jojoba oil + diesel) and (biodiesel + diesel) blends. Fuel 123:175–188
Aly MA, Amer EA, Al-Zayadneh WA, Negm-Eldin AE (2008) Growth regulators influence the fatty acid profiles of in vitro induced jojoba somatic embryos. Plant Cell Tissue Organ Cult 93:107–114
Andressen D, Manoochehri I, Carletti S, Llorente B, Tacoronte M, Vielma M (2009) Optimization of the in vitro proliferation of jojoba [Simmondsia chinensis (Link) Schn.] by using rotable central composite design and inoculation with rhizobacteria. Bioagro 21:41–48
Baqir-Hussain M, Ahmad-Zahir Z, Naeem-Asghar H, Asgher M (2014) Can catalase and exopolysaccharides producing rhizobia ameliorate drought stress in wheat? Int J Agric Biol 16:3–13
Benson A, Joe MM, Karthikeyan B, Sa T, Rajasekaran C (2014) Role of Achromo-bacter xylosoxidans AUM54 in micropropagation of endangered medicinal plant Naravelia zeylanica (L.) DC. J Plant Growth Regul 33:202–213
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carletti SM, Llorente BE, Cáceres EA, Tandecarz J (1998) Jojoba inoculation with Azospirillum brasilense stimulates in vitro root formation. Plant Tissue Cult Biotechnol 4:165–174
Daros-Salla T, Ramos da Silva T, Vieira-Astarita L, Romanato-Santarém E (2014) Streptomyces rhizobacteria modulate the secondary metabolism of Eucalyptus plants. Plant Physiol Biochem 85:14–20
El-Deeb B, Fayez K, Gherbawy Y (2013) Isolation and characterization of endophytic bacteria from Plectranthus tenuiflorus medicinal plant in Saudi Arabia desert and their antimicrobial activities. J Plant Interact 8:56–64
Fedorov DN, Ekimov GA, Doronina NV, Trotsenko YA (2013) 1-Aminocyclopropane-1-carboxylate (ACC) deaminases from Methylobacterium radiotolerans and Methylobacterium nodulans with higher specificity for ACC. FEMS Microbiol Lett 343:70–76
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gentry HS (1958) The natural history of Jojoba (Simmondsia chinensis) and its cultural aspects. Econ Bot 12(3):261–295
Giri DD, Kumar A, Shukla PN, Singh R, Singh PK, Deo-Pandey K (2013) Salt stress tolerance of methylotrophic bacteria Methylophilus sp. and Methylobacterium sp. isolated from coal mine spoils. Pol J Microbiol 62:273–280
Gururani MA, Upadhyaya CP, Baskar V, Venkatesh J, Nookaraju A, Won-Park S (2013) Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ROS-scavenging enzymes and improved photosynthetic performance. J Plant Growth Regul 32:245–258
Ibáñez SG, Merini LJ, Barros GG, Medina MI, Agostini E (2014) Vicia sativa-rhizospheric bacteria interactions to improve phenol remediation. Int J Environ Sci Technol 11:1679–1690
Indiragandhi P, Anandham R, Kim K, Yim W, Madhaiyan M, Sa T (2008) Induction of defense responses in tomato against Pseudomonas syringae pv. tomato by regulating the stress ethylene level with Methylobacterium oryzae CBMB20 containing 1-aminocyclopropane-1-carboxylate deaminase. World J Microbiol Biotechnol 24:1037–1045
Jain S, Kumar-Choudhary D (2014) Induced defense–related proteins in soybean (Glycine max L. Merrill) plants by Carnobacterium sp. SJ–5 upon challenge inoculation of Fusarium oxysporum. Planta 239:1027–1040
Jain A, Singh A, Singh S, Bahadur-Singh S (2013) Microbial consortium-induced changes in oxidative stress markers in pea plants challenged with Sclerotinia sclerotiorum. J Plant Growth Regul 32:388–398
Jha Y, Subramanian RB (2013) Paddy plants inoculated with PGPR show better growth physiology and nutrient content under saline conditions. Chil J Agric Res 73:213–219
Kumar S, Singh N, Mangal M, Dhawan AK (2012) Biotechnological advances in jojoba [Simmondsia chinensis (Link) Schneider]: recent developments and prospects for further research. Plant Biotechnol Rep 6:97–106
Larraburu E, Llorente B (2015) Azospirillum brasilense enhances in vitro rhizogenesis of Handroanthus impetiginosus (pink lapacho) in different culture media. Ann For Sci 72:219–229
Larraburu EE, Llorente BE, Apóstolo NM (2010) Anatomy and morphology of photinia (Photinia X 3 fraseri Dress) in vitro plants inoculated with rhizobacteria. Trees 24:635–642
Llorente BE, Apóstolo NM (2013) In vitro propagation of Jojoba. In: Lambardi M, Ozudogru EA, Jain SM (eds) Protocols for micropropagation of selected economically-important horticultural plants, methods in molecular biology. Springer, Berlin, pp 19–31 https://doi.org/10.1007/978-1-62703-074-8_2
Megala S, Paranthaman (2017) Effect on the plant growth promoting rhizobacteria (PGPR) increasing plant height, chlorophyll and protein content of Solanum nigrum. Int J Appl Res 3:147–150
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Nagendran K, Karthikeyan G, Mohammed-Faisal P, Kalaiselvi P, Raveendra M, Prabakar K, Raguchander T (2014) Exploiting endophytic bacteria for the management of sheath blight disease in rice. Biol Agric Hortic 30:8–23
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Orlikowska T, Nowak K, Reed B (2017) Bacteria in the plant tissue culture environment. Plant Cell Tissue Organ Cult 128:487–508
Paynet M, Martin C, Girand M (1971) Activité phenylalanine ammonia lyase et hypersensibilite au virus de la mosaique du tabac. Académie des Sciences, Paris, pp 537–539
Perez-Rosales E, Alcaraz-Meléndez L, Puente ME, Vázquez-Juárez R, Quiroz-Guzmán E, Zenteno-Savín T, Morales-Bojórquez E (2017) Isolation and characterization of endophytic bacteria associated with roots of jojoba [Simmondsia chinensis (Link) Schneid]. Curr Sci 112(2):1–6
Perveen S, Anis M, Aref IM (2013) Lipid peroxidation, H2O2 content, and antioxidants during acclimatization of Abrus precatorius to ex vitro conditions. Biol Plant 57(3):417–424
Suzuki K (2000) Measurement of Mn-SOD and Cu, Zn-SOD. In: Taniguchi N, Gutteridge J (eds) Experimental protocols for reactive oxygen and nitrogen species. Oxford University Press, New York, pp 91–95
Ting SY, Meon S, Kadir J, Radu S, Singh G (2010) Induction of host defense enzymes by the endophytic bacterium Serratia marcescens, in banana plantlets. Intern J Pest Manag 56:183–188
Zhao K, Penttinen P, Guan T, Xiao J, Chen Q, Xu J, Lindstrom K, Zhang L, Zhang X, Strobel GA (2011) The diversity and anti-microbial activity of endophytic actinomycetes isolated from medicinal plants in Panxi plateau, China. Curr Microbiol 62:182–190
Zhou WJ, Leul M (1999) Uniconazole-induced tolerance of rape plants to heat stress in relation to changes in hormonal levels, enzyme activities and lipid peroxidation. Plant Growth Regul 27:99–104
Acknowledgements
Centro de Investigaciones Biológicas del Noroeste S.C. (CIBNOR) staff Norma Ochoa-Alvarez of the Microbiological Diagnostic Laboratory; Margarito Rodriguez-Alvarez and Sergio Real-Cosio of the Plant Biotechnology Laboratory; Orlando Lugo–Lugo and Norma O. Olguín-Monroy of the Oxidative Stress Laboratory for technical support provided in this study; and Diana Fischer for English edition. The authors would like to acknowledge the Plant Tissue Culture Laboratory (CULTEV) at Universidad Nacional de Luján, Argentina for providing A. brasilense Cd strains.
Funding
The authors of this study thank Consejo Nacional de Ciencia y Tecnología (CONACYT) for scholarship No 331467. This work was supported by Sistema Nacional de Inspección y Certificación de Semillas y Sistema Nacional de Recursos Fitogenéticos (SNICS-SINAREFI) under the Jojoba Net project BEI-JOJ-13-4.SNICS-SINAREFI-BEI-JOJ-13-4.
Author information
Authors and Affiliations
Contributions
EPR developed the experiments and analysis. LAM director and advisor of the experiments and results. MEP microbiology advisor. RVJ genetic analysis advisor. TZS enzymatic analysis advisor. EMB statistical analysis advisor.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Sergey V. Dolgov.
Rights and permissions
About this article
Cite this article
Perez-Rosales, E., Alcaraz-Meléndez, L., Puente, M.E. et al. Endophytic bacteria isolated from wild jojoba [Simmondsia chinensis L. (Schneider)] roots improve in vitro propagation. Plant Cell Tiss Organ Cult 135, 515–522 (2018). https://doi.org/10.1007/s11240-018-1483-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-018-1483-9