Abstract
In this report we address the changes in the expression of the genes involved in ROS scavenging and ethylene biosynthesis induced by the inoculation of plant growth-promoting rhizobacteria (PGPR) isolated from potato rhizosphere. The two Bacillus isolates used in this investigation had earlier demonstrated a striking influence on potato tuberization. These isolates showed enhanced 1-aminocyclopropane-1-carboxylic acid deaminase activity, phosphate solubilization, and siderophore production. Potato plants inoculated with these PGPR isolates were subjected to salt, drought, and heavy-metal stresses. The enhanced mRNA expression levels of the various ROS-scavenging enzymes and higher proline content in tubers induced by PGPR-treated plants contributed to increased plant tolerance to these abiotic stresses. Furthermore, the photosynthetic performance indices of PGPR-inoculated plants clearly exhibited a positive influence of these bacterial strains on the PSII photochemistry of the plants. Overall, these results suggest that the PGPR isolates used in this study are able to confer abiotic stress tolerance in potato plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant growth-promoting rhizobacteria (PGPR) are plant-associated microorganisms that are known to induce plant defenses and confer beneficial effects such as increased plant growth and low susceptibility to diseases caused by pathogens (Kloepper and others 2004a; van Loon and Glick 2004; Dimkpa and others 2009b). PGPR have been proven to counteract the activities of other harmful soilborne microorganisms, thus promoting plant growth (Glick 1995). Some PGPR also elicit physical or chemical changes related to plant defense, a process called “induced systemic resistance” (ISR) (van Loon and Glick 2004). Although it is well known that ISR triggered by PGPR confers resistance against pathogen-induced plant diseases, a few published reports suggest the role of PGPR as elicitors of abiotic stresses in plants (Yang and others 2009). Strains with plant growth-promoting activity have been identified from various genera of which Pseudomonas and Bacillus are the most extensively studied (Kumar and others 2011). It has been proposed that these bacteria produce phytohormones, antibiotics, and siderophores in the rhizosphere, thus inducing systemic resistance to plant pathogens (Gutierrez-Manero and others 2001; Whipps 2001; Idris and others 2007; Richardson and others 2009). The underlying mechanisms of plant growth promotion by PGPR have been comprehensively described in several articles (van Loon 2007; Kloepper and others 2004b; Yang and others 2009). PGPR belonging to Bacillus spp. are frequently isolated from the rhizosphere, and most have shown favorable effects on plant growth, higher yield, and disease tolerance (Vessey 2003; Compant and others 2005; Wahyudi and others 2011). Bacillus mucilaginosus has been observed particularly for potassium- (Wu and others 2005) and phosphate-solubilizing abilities (Idriss and others 2002). Inoculation of Azotobacter and Bacillus increased the yield of wheat by 30 and 43 %, respectively (Kloepper and others 1991). Similarly, the inoculation of Bacillus with Bradyrhizobium japonicum was found to enhance the growth of soybean plants to increasing nodulation levels (Bai and others 2003). In another report, Woitke and others (2004) demonstrated the ability of B. subtilis to induce stress tolerance to salinity in hydroponically grown tomato plants.
Potato is the third most common food crop in the world, with a high yield per hectare; however, the potato has been relatively sensitive to yield losses resulting from soil salinity, drought, and low nutrient availability (van der Linden and others 2011). Although Bacillus strains have been previously isolated from the potato rhizosphere (Sessitsch and others 2003; Berg and others 2005; Calvo and others 2010), there have been no reports suggesting their role in abiotic stress tolerance in potato plants. Earlier, we had identified 13 PGPR isolates from the potato rhizosphere and demonstrated their influence on in vitro potato tuberization (Nookaraju and others 2011). Next, we selected two Bacillus isolates from among the 13 exerting a significant positive influence on in vitro potato tuberization to investigate their influence on abiotic stress tolerance in potato plants. The putative changes in the antioxidant pathway gene expression and photosynthetic efficiency, conferred by inoculation with PGPR, were also studied.
Materials and Methods
Plant Material and Growth Conditions
Potato cultivar ‘Taedong Valley’ was propagated in culture tubes (25 × 150 mm) containing semisolid basal MS medium (Murashige and Skoog 1962) supplemented with 30 g l−1 sucrose. The pH of the medium was set at 5.7 and gelled with 8 g l−1 agar (Duchefa, Germany). The cultures were maintained in a growth chamber at 22 ± 2 °C and a 16 h light period with a light intensity of 100 μmol m−2 s−1 using white fluorescent lamps. Four-week-old in vitro rooted shoots were planted in pots (10 cm wide and 10 cm deep) containing sterilized biopeat (Seminis Asia Ltd., Korea) and transferred to the greenhouse for hardening.
Screening of PGPR Isolates for Stress Tolerance
All the PGPR isolates (described by Nookaraju and others 2011) were screened for abiotic stress tolerance as described by Upadhyay and others (2011). The intrinsic resistance of these PGPR isolates was determined by monitoring their growth on nutrient agar medium supplemented with various concentrations of NaCl (50–400 mM). A control plate containing 0.05 % NaCl (w/v) was also maintained and incubated for 48 h at 28 °C. A sensitivity test against heavy-metal toxicity was performed using different concentrations of ZnCl2 (10–50 mM). The effect of ZnCl2 on bacterial growth was determined by spectrophotometry (560 nm) after 24 h of incubation at 28 °C in a nutrient broth. To determine the sensitivity of the PGPR isolates against polyethylene glycol (PEG6000), a tryptic soy broth (TSB) of varying PEG concentrations (0–50 %) was prepared and inoculated with 10 μl of overnight-grown bacterial cultures in TSB. The cultures were incubated at 28 °C for 24 h in a shaker at 180 rpm and growth was estimated by measuring the absorbance at 600 nm (Sandhya and others 2009).
Estimation of ACC Deaminase Activity and IAA Production by Bacterial Isolates
PGPR strains were assayed for 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity by monitoring their growth on the DF medium (Dworkin and Foster 1958) supplemented with 3 mmol l−1 ACC as the only source of nitrogen (Penrose and Glick 2003). Plates containing DF medium without any nitrogen source were also kept, serving as the negative control to exclude the possibility of free nitrogen fixers. Plates were then inoculated with 10 μl of overnight-grown bacterial culture and incubated at 25 °C, and colonies were monitored daily for up to 3 days (Smyth and others 2011).
Bacterial auxin indole-3-acetic acid (IAA) production was tested using LB broth and the Salkowski colorimetric assay (Gutierrez and others 2009). PGPR isolates were grown in the LB broth supplemented with l-tryptophan (500 μg ml−1) at 25 °C in darkness at 160 rpm for 5 days, and the supernatant was harvested by centrifugation on the sixth day. Then 1 ml supernatant was added to 1 ml Salkowski’s reagent (12 g l−1 FeCl3 in 429 ml l−1 H2SO4) (Glickman and Dessaux 1995). The mixture was kept for 30 min at room temperature and absorbance was measured at 535 nm. Auxin concentrations were estimated using the standard curve of IAA, prepared from serial dilutions of IAA (Sigma-Aldrich, St. Louis, MO, USA) stock solutions, with IAA concentration ranging from 0 to 2 mg l−1 and R 2 coefficient of 0.996.
Mineral Solubilization Activity of the Bacterial Strains
To determine the phosphate-solubilizing capabilities, the PGPR strains were analyzed, as described earlier (Miller and others 2010), on Pikovskaya’s agar (Sundara Rao and others 1963), whereas the phytate screening medium (PSM) agar (Kerovou and others 1998) was used to estimate the phytate-mineralizing capabilities. CAS (Chrome Azurol S) agar plates were prepared for the assessment of siderophore production capabilities, as described earlier (Schwyn and Neilands 1987).
Inoculation of Plants with PGPR Isolates and Stress Treatments
PGPR strains 4 (Bacillus pumilus str. DH-11) and 6 (Bacillus firmus str. 40) (Nookaraju and others 2011) were maintained at −80 °C in TSB amended with 20 % glycerol. The inocula were prepared by streaking the strains from −80 °C onto plate count agar (BD Biosciences, San Jose, CA, USA) plates, incubating the plates at 28 °C for 24 h, and scraping the bacterial cells off the plates in a sterile 10 mM MgSO4 buffer. The sterile substrate was bacterized to achieve 108 CFU/g and PGPR were applied to the roots of potted plants growing under glasshouse conditions. A second inoculum dose was applied by a soil drench (108 CFU g−1 substrate) 1 week after the first inoculation. Control plants of all treatments were mock-inoculated with 10 mM MgSO4 buffer and kept in parallel, as described by Barriuso and others (2008).
Two weeks after inoculation with PGPR strains, the plants were subjected to different abiotic stress treatments. Three plants were used for each category of stress treatment. For salt stress, the plants were watered with 200 mM NaCl solution; for drought stress, the plants were watered with 10 % PEG; and for heavy-metal challenge stress, the plants were watered with 20 mM ZnCl2 solution for a week. Potato plants not inoculated with any of the PGPR isolates served as controls. Each treatment entailed a minimum of three replicates and the experiment was repeated three times. To examine the colonization of PGPR strains, plants were carefully uprooted and the soil adhering to the roots and tubers was collected for reisolation of PGPR strains (4 and 6) as described earlier (Nookaraju and others 2011). CFUs were determined on soil agar plates after an appropriate dilution in sterile distilled water and were counted after a 48 h incubation at 28 °C.
Determination of Hydrogen Peroxide Levels and Enzyme Activity
The ferrous ammonium sulfate/xylenol orange method, as described by Cheeseman (2006), was used to determine the H2O2 content of the leaf tissues from control and PGPR-inoculated potato plants. H2O2 concentrations were estimated using the standard curve prepared from serial dilutions of H2O2, with concentrations ranging from 0 to 6 μM and R 2 coefficient of 0.940. Data were normalized and expressed as micromolar H2O2/g fresh weight (FW) of leaves. For protein extraction, leaf samples were homogenized in the presence of liquid nitrogen. Protein was extracted with an extraction buffer containing 0.1 M potassium phosphate buffer (pH 7.5), 50 % (v/v) glycerol, 16 mM MgSO4, 0.2 mM PMSF, and 0.2 % PVPP.
For the estimation of ascorbate peroxidase (APX) activity, the leaves were homogenized in 100 mM sodium phosphate buffer (pH 7.0) containing 5 mM ascorbate and 1 mM EDTA. The homogenate was filtered and centrifuged at 16,000×g for 20 min at 4 °C and the resulting supernatant was tested to determine the antioxidant enzyme activities. The protein content was determined based on the method of Lowry and others (1951) using bovine serum albumin (BSA) as the standard.
Catalase (CAT) activity was determined by monitoring the decomposition of H2O2 at 240 nm, as described by Aebi (1974). The reaction was initiated by adding 10 mM H2O2 to the reaction mixture containing 50 mM potassium phosphate buffer (pH 7.0) and plant extract, 3 ml in volume. One unit of CAT is defined as the amount of enzyme that liberates half the peroxide oxygen from 10 mM H2O2 solution in 100 s at 25 °C.
To determine the superoxide dismutase (SOD) activity, a method described by Beyer and Fridovich (1987) was followed. The reaction mixture (30.25 ml) contained 50 mM potassium phosphate buffer (pH 7.8), 9.9 mM methionine, 57 mM nitroblue tetrazolium (NBT), and an appropriate volume of plant extract. The reaction was initiated by light illumination. One unit of SOD is defined as the amount of enzyme needed to exhibit 50 % dismutation of the superoxide radical. APX activity was determined spectrophotometrically at 290 nm, as described by Chen and Asada (1989). The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM ascorbate, 0.2 mM H2O2, and a suitable volume of enzyme extract.
Glutathione reductase (GR) activity was determined by measuring the oxidation of NADPH at 340 nm (extinction coefficient of 6.2 mM cm−1), as described by Rao and others (1989). The reaction mixture was composed of 100 mM potassium phosphate buffer (pH 7.8), 2 mM EDTA, 0.2 mM NADPH, 0.5 mM glutathione (oxidized form, GSSG) and the enzyme extract in 1 ml volume. The reaction was initiated by the addition of NADPH at 25 °C.
Dehydroascorbate reductase (DHAR) activity was determined as described by Nakano and Asada (1981). The reaction mixture contained 50 mM phosphate buffer (pH 7.0), 20 mM reduced glutathione, 2 mM dehydroascorbate, and 100 μl crude enzyme. DHAR activity was assayed at 25 °C by adhering to the absorbance of GSH-dependent production of AsA at 265 nm (extinction coefficient of 6.2 mM−1 cm−1).
Expression Analysis of Stress-responsive Genes
Total RNA was isolated from the potato plant leaves using Tri reagent (Sigma) and treated with DNase I for reverse transcription reaction, and first-strand cDNA was synthesized using a random hexamer as the primer and SuperScript-II RT (Invitrogen, Carlsbad, CA, USA). The real-time PCR amplification of the ROS pathway genes (SOD, CAT, APX, DHAR, and GR) was carried out in the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) using gene-specific primers (Hemavathi and others 2011), as listed in Table 1. The PCR was performed using the SYBR® Green PCR kit (Bio-Rad) with actin as an internal control. The PCR conditions included 5-min initial denaturation at 95 °C followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s, and an extension at 72 °C for 2 min. Comparative threshold (Ct) values were normalized to actin control and compared to obtain relative expression levels.
Estimation of Proline Content
Proline was estimated (Bates and others 1973) from the PGPR-inoculated and noninoculated control potato plants subjected to various abiotic stress treatments. Approximately 500 mg of tuber tissue was crushed to a fine powder in the presence of liquid nitrogen and homogenized in 10 ml of 3 % aqueous sulfosalicylic acid. The homogenate was then filtered and 2 ml of the filtrate was mixed with 2 ml acid-ninhydrin and 2 ml of glacial acetic acid in a test tube for 1 h at 100 °C. The reaction was terminated by placing the test tubes on ice followed by proline extraction with 4 ml toluene. The chromophore-containing phase was collected and kept at room temperature for 15 min before measuring the absorbance at 520 nm. The proline concentration was determined from a standard curve and calculated on a fresh weight basis (μmol proline g−1 FW).
Estimation of Photosynthetic Performance
Chlorophyll fluorescence transients of dark-adapted intact leaves of the potato plants were measured using a Pocket PEA (Plant Efficiency Analyzer, Hansatech Ltd., Norfolk, UK). The 2 h dark adaptation was done with specially provided clips that fit onto the leaves. Data were recorded up to 1 s, at a rate of 10 μs for the first 2 ms and at the rate of 1 ms thereafter (Strasser and others 2000). The transients were induced by a red (650 nm) diode with 3,000 μE s−1 m−2. The maximum fluorescence (F m) and the minimum fluorescence (F 0) of dark-adapted tissues were used to calculate the F v/F m ratio. The parameter F v/F m [(F m−F 0)/F m] reflects the maximum quantum yield of photosystem II (PSII) or the potential quantum efficiency if all the PSII centers were open (Maxwell and Johnson 2000). Thus, the measurement of F v/F m provides a measure of the intactness of the PSII/LHC (light harvesting complex) unit and indicates the probability of a trapped photon within the reaction center to cause a photochemical event. Any change in the state of PSII causes a decrease in the value of F v/F m (Maxwell and Johnson 2000). The optimal value of F v/F m varies between 0.79 and 0.83 for most plant species (Björkman and Demmig 1987; Johnson and others 1993), and lower values indicate that the plant is either under stress or lacking an optimal health state. The measured values of leaf samples were also used to calculate the performance index (PItotal or PI) through a series of JIP equations (Strasser and others 1999, 2000; Yusuf and others 2010; Gururani and others 2012) in the Biolyzer software program. The PI is the sum of overall expressions that represent a kind of internal force of the sample to resist constraints from outside thereby indicating the vitality of the samples used.
Statistical Analysis
All treatments contained at least three replicates and the experiments were repeated three times. The means were analyzed using the Statistical Analysis Software package program 9.1 (SAS Institute, Cary, NC, USA). Statistical differences were determined using one-way analysis of variance, and standard error was calculated by using the n values of each experiment.
Results
Physiological Characterization of the Bacterial Strains
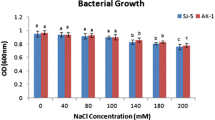
All the PGPR strains used in this study were isolated in our laboratory from the potato rhizosphere and were found to positively influence in vitro potato tuberization, suggesting the significant role of the bacteria inhabiting the potato rhizosphere in tuberization (Nookaraju and others 2011). Among the isolates, two Bacillus isolates (Bacillus pumilus str. DH-11 and Bacillus firmus str. 40) exerted a significant positive influence on potato tuberization under both in vitro and ex vitro conditions. All the isolated bacterial strains were tested in vitro for a range of activities that are attributed to the positive effects of plant growth-promoting bacteria. Five bacterial strains were able to mineralize phytates, whereas four isolates exhibited ACC deaminase activity (Table 2). Bacillus strains 4 and 6 produced the highest auxin level in vitro (Table 2). However, no correlation was found between auxin production and in vitro tuberization. All these bacterial strains were also screened for the abiotic stress tolerance as mentioned in the Materials and Methods section. Four isolated PGPR strains (isolates 4, 6, 7, and 12) showed stress tolerance in terms of better growth on the stress-inducing medium (data not shown). However, two Bacillus strains (4 and 6, showing maximum effect on tuberization) exhibited higher tolerance on different stress-inducing media. The growth of PGPR strain 4 was better on the medium with NaCl (400 mM), whereas strain 6 showed better growth on the medium containing ZnCl2 (40 mM). Both these strains were able to grow on the medium supplemented with 20 % PEG. Higher concentrations of NaCl, ZnCl2, and PEG were found to be lethal for all bacterial strains. It was also observed that the inoculation of these two strains of PGPR on potato facilitated better plant growth compared with the noninoculated plants. The inoculated plants showed better growth in terms of plant height, number of leaves, and number of tubers under stress conditions (Table 3).
Plants Inoculated with PGPR Showed Enhanced Tolerance to Different Abiotic Stresses
Two bacterial strains showing maximum tolerance to different abiotic stresses and exhibiting other superior characteristics, as indicated in Table 2, were used to inoculate the potato plants before applying the abiotic stress on the plants. The first inoculation was done at the time of potato hardening. After 1 week, the second inoculation of PGPR was done. After another 2 weeks, different abiotic stresses were applied (PEG 10 %, 200 mM NaCl, and 20 mM ZnCl2). These stresses were observed to be toxic to the potato plant without PGPR inoculation. However, the PGPR-inoculated plants growing under stress conditions showed better growth and tolerated these stresses significantly better than the noninoculated plants. Interestingly, plant survival was better after inoculation with both strains (Fig. 1). However, overall plant growth, leaf number, tuber size, and tuber yield were better in potato plants inoculated with PGPR strain 4 (Table 3).
Effect of PGPR (isolates 4 and 6) inoculation on 4 week-old potato plants growing under different abiotic stress conditions induced by a 200 mM NaCl, b 10 % PEG, and c 20 mM ZnCl2. C potato plants without any PGPR inoculation; 4 potato plants inoculated with PGPR isolate 4; 6 potato plants inoculated with PGPR isolate 6
PGPR Inoculation Induces the Higher Activity of ROS-Scavenging Enzymes in the Plant
The analysis of the soil adhered to a plant’s roots and tubers after harvesting indicated that PGPR (isolates 4 and 6) had successfully colonized the root system in all inoculated plants (104–107 CFU/g tissue on a fresh weight basis) growing under different stress conditions. To appreciate the effect of abiotic stress treatments (drought, salt, and heavy metal) on the antioxidant capacity of PGPR-inoculated plants (isolates 4 and 6), the hydrogen peroxide levels as well as the enzymatic activities of the major ROS scavengers were analyzed. Hydrogen peroxide levels were significantly higher in control plants under all the applied stress treatments than in the PGPR (isolates 4 and 6)-inoculated plants (Fig. 2). Plants inoculated with PGPR isolate 6 recorded the least amount of H2O2 accumulation under PEG (10 %) and NaCl (200 mM) stress conditions. The specific activity of APX increased 1.2 times in strain 4, including inoculated plants under drought stress conditions, 1.4 times under salt stress, and 1.3 times under heavy-metal stress (Fig. 3a). The specific activity of SOD significantly increased in the PGPR-inoculated plants growing under these stresses compared with that in noninoculated plants under the same stress conditions. The SOD activity in the inoculated plants was approximately 1.7 times higher under PEG stress, 2.4 times higher under salt stress, and 1.7 times higher under heavy-metal stress (Fig. 3b). The specific activity of GR also showed a similar trend (1.6–2.1 times increase), with maximum expression in the inoculated plants under heavy-metal stress compared with that in noninoculated control plants (Fig. 3c). A significant increase in the specific activity of CAT (up to 1.8 times under drought stress, 1.8 times under salt stress, and up to 1.7 times higher under heavy-metal stress) was also observed in the PGPR-inoculated plants (both strains) growing under stress conditions compared with that in noninoculated control conditions (Fig. 3d). Similarly, the specific activity of DHAR also increased significantly (ca. 1.3–1.7 times) in the inoculated plants growing under stress conditions compared with that in the noninoculated plants (Fig. 3e). The specific activities of these antioxidant enzymes were positively correlated with their mRNA expression levels as measured by quantitative real-time PCR analyses (Fig. 4a–e).
Estimation of H2O2 in leaves of control potato plants (C), PGPR isolate 4-inoculated plants (4), and PGPR isolate 6-inoculated plants (6) sampled in the glasshouse. Values represent the mean ± SE from three independent assays with three replicates for each treatment. Different letters in each column indicate significant differences (p ≤ 0.05) in H2O2 content between treatments after Tukey’s test (n = 5)
Specific enzyme activities of APX (a), SOD (b), GR (c), CAT (d), and DHAR (e) in S. tuberosum stressed with PEG (10 %), NaCl (200 mM), and ZnCl2 (20 mM). Values represent the mean ± SE from three independent assays with three replicates for each treatment. Different letters in each column indicate significant differences (p ≤ 0.05) in enzyme activities between treatments after Tukey’s test (n = 5). C potato plants without any PGPR inoculation; 4 potato plants inoculated with PGPR isolate 4; 6 potato plants inoculated with PGPR isolate 6
Real-time mRNA expression of genes coding ROS-scavenging enzymes APX (a), CAT (b), GR (c), SOD (d), and DHAR (e) from PGPR-inoculated and noninoculated control (C) potato plants growing under various abiotic stress conditions. Fold expression values are normalized to those of actin control. Different letters in each column indicate significant differences (p ≤ 0.05) in relative gene expression between treatments after Tukey’s test (n = 5). C potato plants without any PGPR inoculation; 4 potato plants inoculated with PGPR isolate 4; 6 potato plants inoculated with PGPR isolate 6
Accumulation of Higher Proline in PGPR-Inoculated Plant Tubers
Proline is a common osmolyte in higher plants and accumulates in response to stress. The change in the proline content was measured in the tubers from PGPR-inoculated and noninoculated plants growing under stress conditions. A significant increase in the proline content was recorded in PGPR-inoculated plant tubers (ca. 1.5–2.6 times) grown under stress conditions compared with that in noninoculated plant tubers (Table 4). The increase in proline content was greater in the inoculated plant tubers grown under salt stress than under PEG and heavy-metal stresses.
PGPR-Inoculated Potato Plants Exhibited an Increase in the Photosynthetic Efficiency
The two PGPR bacterial isolates were tested in S. tuberosum for their influence on the photosynthetic efficiency of plants under different abiotic stress conditions. The maximal photochemical efficiency (F v/F m) is the most common parameter used in fluorescence and is inversely proportional to damage in the PSII reaction centers (Farquhar and others 1989). The F v/F m ratio ranged between 0.4 and 0.8 and was observed to be most sensitive to drought treatment than to salt and heavy-metal treatments (Table 5). The F v/F m values of PGPR-inoculated plants were higher than those of the noninoculated control plants growing under the same stress conditions. This observation was further confirmed with the estimation of the performance index (PI), essentially an indicator of sample vitality. The PI value is considered an overall expression indicating a type of internal force of the sample to resist external constraints. The PI of PGPR-inoculated plants was significantly higher than that of the noninoculated control plants under abiotic stress conditions in all but one treatment (Fig. 5). The difference between PI values of plants inoculated with PGPR isolate 6 and control plants growing under salt stress was statistically nonsignificant. Interestingly, the PGPR isolate 4-inoculated potato plants had higher PI values under all the stress treatments than the PGPR isolate 6-inoculated and noninoculated control plants.
Performance index (PItotal) of control (C, no PGPR inoculation) and PGPR (strains 4 and 6)-inoculated intact leaves of potato plants growing under stress conditions induced by PEG (10 %), NaCl (200 mM), and ZnCl2 (20 mM). Values represent the mean value from five leaves per plant and three replicates for each treatment. C potato plants without any PGPR inoculation; 4 potato plants inoculated with PGPR isolate 4; 6 potato plants inoculated with PGPR isolate 6
Discussion
Inoculation of potato plants with some of the rhizobacteria strains isolated and identified in our laboratory induced plant tolerance to different abiotic stresses. These bacteria revealed characteristics of plant growth-promoting bacteria such as ACC deaminase activity, IAA production, and mineral solubilization activity. Potato plants inoculated with two bacterial strains belonging to Bacillus showed significant tolerance to salinity, heavy-metal, and drought stresses induced by NaCl, ZnCl2, and PEG, respectively. Inoculation with these bacteria not only induced significant changes in the gene expression of different ROS-scavenging enzymes but also enhanced the photosynthetic efficiency of inoculated plants, which proved to be a reason for the tolerance of abiotic stress. Earlier, these strains showed a significant influence on potato tuberization under in vitro and ex vitro conditions by inducing lipoxygenase (LOX) activity and other tuberization hormones (Nookaraju and others 2011; Upadhyaya and others 2011).
Abiotic stresses, including water deficiency, lead to ROS formation by misdirecting the electrons in the photosystems. Plants respond to water-deficit conditions by increasing their osmolyte production, which in turn increases the osmotic potential within the cell (Farooq and others 2009). Additionally, the compounds secreted by the rhizosphere bacteria belong to osmolytes. A positive correlation between glycine betaine production by PGPR and enhanced drought tolerance was reported in plants (Yuwono and others 2005). As mentioned earlier, these rhizobacteria revealed ACC deaminase activity, IAA production, and mineral solubilization activity. Earlier, it had been demonstrated that ACC deaminase activity of Achromobacter piechaudii confers tolerance to water deficit in tomato and pepper plants (Mayak and others 2004). Similarly, PGPR isolates belonging to Pseudomonas and Bacillus genera were reported to enhance resistance to water stress in green gram plants (Sarvanakumar and others 2011).
Ethylene is a plant hormone that is involved in the regulation of many physiological responses (Arshad and Frankenberger 2002; Owino and others 2006). Ethylene was originally regarded as a stress hormone because of its synthesis at accelerated rates in response to a variety of stress signals such as mechanical wounding, flooding, chemicals and metals, drought, extreme temperatures, and pathogen infection (Kende 1993; Johnson and Ecker 1998). ACC is the immediate precursor of ethylene in higher plants (Yang and Hoffman 1984) and its regulation has been described as the principal mechanism by which bacteria exert beneficial effects on plants under abiotic stress (Saleem and others 2007). Certain microorganisms have been reported to contain the enzyme ACC deaminase that hydrolyzes ACC into ammonia and α-ketobutyrate (Glick and others 1994, 1998; Mayak and others 1999; Shaharoona and others 2006) instead of converting it into ethylene. The cleavage of ACC by ACC deaminase-containing rhizobacteria reduces the ACC and ethylene levels in the rhizoplane, thereby providing a sink for the ACC. These reduced ACC levels in turn decrease the levels of endogenous ethylene, thus eliminating the potentially inhibitory effects of higher ethylene concentrations (Glick and others 1998). In our study, the two Bacillus strains used to inoculate the stressed potato plants showed significant ACC deaminase activity, which provides direct evidence of the beneficial effect of these bacteria on the plants.
The two PGPR isolates, strains 4 and 6, used in this study were identified as Bacillus pumilus strain DH-11 and Bacillus firmus strain 40, respectively (Nookaraju and others 2011). Besides other beneficial properties, Bacillus sp., as with most other bacteria, are known to reveal SOD activities and mitigate the oxidative stress in living systems. The bacterial SODs play an important role in their survival in the rhizosphere by facilitating the removal of free radicals (Wang and others 2007). In general, the survival of the bacterial strains is attributed to their contribution to the alleviation of abiotic stress and plant growth promotion (Dimkpa and others 2009b). Abiotic stress conditions cause an increase in ROS formation at the cellular level. ROS such as superoxide radical (O2 −), hydrogen peroxide, and hydroxyl radicals (OH) cause lipid peroxidation of membranes (Sgherri and others 2000; Hemavathi and others 2010). The antioxidant enzymes of the cell have the ability to remove the free radicals produced during abiotic stress conditions. These enzymes also protect the membranes and DNA from damage. To monitor the putative changes in the expression of genes encoding the ROS-scavenging enzymes, the PGPR-inoculated and noninoculated potato plants were subjected to different stresses induced by PEG, NaCl, and ZnCl2. The mRNA expression of SOD and APX in bacteria-inoculated plants growing under stress conditions increased considerably when compared with that in the noninoculated stressed plants. Similarly, the mRNA expression levels of genes encoding for other antioxidative enzymes such as CAT, DHAR, and GR also increased in the bacteria-treated plants. These results were consistent with the semiquantitative RT-PCR results (data not shown) where the expression of the antioxidative pathway genes was more pronounced in the PGPR-inoculated plants than in the noninoculated plants.
Plant growth-promoting bacteria are known to release metal-chelating substances such as iron-chelating siderophores into the rhizosphere. It has been suggested that siderophore-producing bacteria influence the uptake by plants of various metals, including Fe, Zn, and Cu (Carrillo-Castaneda and others 2003, 2005; Egamberdiyeva 2007; Dimkpa and others 2008, 2009a). Similar to these findings, the bacterial isolates 4 and 6 showed enhanced siderophore production. These reports clearly indicate that the rhizobacteria can also influence the bioavailability of metal ions required by the plants. As soil conditions affect the metal valences, rhizobacteria and other microorganisms also impact the availability of the metals by acidifying the surrounding rhizosphere, as reported by Dimkpa and others (2009b).
Bacterial inoculation has also been reported to positively influence root growth, total aerial biomass, leaf area index, and proline accumulation in the leaves and roots. Higher proline accumulation in potato plants inoculated with bacteria indicated higher plant tolerance to water stress. Creus and others (2004) also reported reduced grain yield losses in the grains of Azospirillum-inoculated wheat exposed to drought conditions. Besides observing the increase in relative water content, water potential, and apoplastic water fraction, they also measured the volumetric cell wall moduli of elasticity and hypothesized the “elastic adjustment” as a crucial factor for increased drought tolerance (Creus and others 2004).
Photosynthesis is the most important process on earth, directly or indirectly influencing the growth and survival of every organism. Therefore, it cannot be examined simply as an isolated phenomenon but must be studied within the context of whole-plant regulation. To assess the changes in the photosynthetic machinery of potato plants subjected to various abiotic stresses, we measured the photosynthetic efficiency of these plants, represented by the expression F v/F m, which indicates the maximum quantum yield of PSII. It has been suggested that abiotic stresses such as drought and salinity decrease the quantum efficiency of PSII photochemistry (Briantais and others 1996). Earlier, several reports were published where these parameters were employed to examine the photosynthetic efficiency of different crop plants subjected to various stresses like drought, salinity, and heavy metal (de Ronde and others 2004; Yusuf and others 2010; Mathur and others 2011). The F v/F m values were in the range of 0.45–0.71 in all the stressed samples of untreated control plants, whereas it ranged from 0.79 to 0.83 in samples treated with either of the two PGPR strains (Table 5). Additionally, the performance index (PI) of the plants, which indicates the sample vitality, was also recorded. The overall PI represents a single multiparametric expression that combines all three independent functional steps of photosynthesis: the density of the reaction centers in the chlorophyll pool, trapped excitation energy, and its conversion to the electron transport (Strasser and others 1999; Tsimilli-Michael and others 2000). The PI values shown in Fig. 5 clearly indicate that the photosynthetic performance of PGPR-treated plants under all stress conditions significantly increased, indicating the positive influence of the PGPR isolates on the photosynthetic machinery of the plants. Potato plants treated with PGPR isolate 4 (Bacillus pumilus strain DH-11) exhibited a higher overall PI in all the stress treatment situations, whereas in all stress situations the overall PI of untreated control plants was significantly lower than that of the plants treated with either of the PGPR strains.
In conclusion, the results shown here demonstrate that the inoculations with rhizobacteria strains 4 and 6 protected S. tuberosum against abiotic stress factors like water deficit, salinity, and heavy-metal toxicity. Plant tolerance to these stresses correlated with the increased expression levels of ROS-scavenging enzymes APX, SOD, CAT, DHAR, and GR, suggesting that inoculation with these strains triggered abiotic stress-related defense pathways under stressed conditions. These observed effects were consistent with the relative mRNA expression levels as determined by RT-PCR and real-time PCR. This study also proved that the use of photosynthetic parameters as a tool to investigate the changes in the photosynthetic machinery of the plants under stress conditions is a promising approach that can be applied to other crops as well. Furthermore, this study would facilitate follow-up studies on metabolites produced by these PGPR isolates that could be the potential candidate elicitors responsible for enhanced plant tolerance to various abiotic stresses.
References
Aebi H (1974) Catalases. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 2. Academic Press, New York, pp 673–684
Arshad M, Frankenberger WT (2002) Ethylene: agricultural sources and applications. Kluwer Academic/Plenum Publishers, New York
Bai Y, Zhou X, Mith DL (2003) Enhanced soybean plant growth resulting from coinoculation of Bacillus strain with Bradyrhizobium japonicum. Crop Sci 43:1774–1781
Barriuso J, Ramos SB, Gutierrez Manero FJ (2008) Protection against pathogen and salt stress by four plant growth-promoting rhizobacteria isolated from Pinus sp. on Arabidopsis thaliana. Phytopathology 98:666–672
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Berg G, Krechel A, Ditz M, Sikora RA, Ulrich A, Hallmann J (2005) Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi. FEMS Microbiol Ecol 51:215–229
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Björkman O, Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence at 77 K among vascular plants of diverse origins. Planta 170:489–504
Briantais JM, Dacosta JG, Ducruet JM, Moya I (1996) Heat stress induces in leaves an increase of the minimum level of chlorophyll fluorescence F0: a time-resolved analysis. Photosynth Res 48:189–196
Calvo P, Ormeño-Orrillo E, Martínez-Romero E, Zúñiga D (2010) Characterization of Bacillus isolates of potato rhizosphere from Andean soils of Peru and their potential PGPR characteristics. Braz J Microbiol 41:899–906
Carrillo-Castaneda G, Munoz JJ, Peralta-Videa JR, Gomez E, Gardea-Torresdey JL (2003) Plant growth-promoting bacteria promote copper and iron translocation from root to shoot in alfalfa seedlings. J Plant Nutr 26:1801–1814
Carrillo-Castaneda G, Munoz JJ, Peralta-Videa JR, Gomez E, Gardea-Torresdey JL (2005) Modulation of uptake and translocation of iron and copper from root to shoot in common bean by siderophore-producing microorganisms. J Plant Nutr 28:1853–1865
Cheeseman JM (2006) Hydrogen peroxide concentrations in leaves under natural conditions. J Exp Bot 57:2435–2444
Chen GX, Asada K (1989) Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30:987–998
Compant S, Duffy B, Nowak J, Clement C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Creus CM, Sueldo RJ, Barassi CA (2004) Water relations and yield in Azospirillum-inoculated wheat exposed to drought in the field. Can J Bot 82:273–281
de Ronde JA, Cress WA, Kruger GHJ, Strasser RJ, van Staden J (2004) Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene, during heat and drought stress. J Plant Physiol 161:1211–1224
Dimkpa C, Svatoš A, Merten D, Büchel G, Kothe E (2008) Hydroxamate siderophores produced by Streptomyces acidiscabies E13 bind nickel and promote growth in cowpea (Vigna unguiculata L.) under nickel stress. Can J Microbiol 54:163–172
Dimkpa C, Merten D, Svatoš A, Büchel G, Kothe E (2009a) Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J Appl Microbiol 107:1687–1696
Dimkpa C, Weinand T, Asch F (2009b) Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ 32:1682–1694
Dworkin M, Foster J (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:592–601
Egamberdiyeva D (2007) The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl Soil Ecol 36:184–189
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–212
Farquhar GD, Wong SC, Evans JR, Hubick KT (1989) Photosynthesis and gas exchange. In: Jones HG, Flowers TJ, Jones MB (eds) Plants under stress. Cambridge University Press, Cambridge, pp 47–69
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41:109–117
Glick BR, Jacobson CB, Schwarze MMK, Pasternak JJ (1994) 1-aminocyclopropane-1-carboxylic acid deaminase mutants of the plant growth promoting rhizobacteria Pseudomonas putida GR12-2 do not stimulate canola root elongation. Can J Microbiol 40:911–915
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol 190:63–68
Glickman E, Dessaux Y (1995) A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61:793–796
Gururani MA, Upadhyaya CP, Strasser RJ, Woong YJ, Park SW (2012) Physiological and biochemical responses of transgenic potato plants with altered expression of PSII manganese stabilizing protein. Plant Physiol Biochem 58:182–194
Gutierrez CK, Matsui GY, Lincoln DE, Lovell CR (2009) Production of the phytohormone indole-3-acetic acid by the estuarine species of the genus Vibrio. Appl Environ Microbiol 75:2253–2258
Gutierrez-Manero FJ, Ramos B, Probanza A, Mehouachi J, Talon M (2001) The plant growth promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberelins. Physiol Plant 111:206–211
Hemavathi, Upadhyaya CP, Nookaraju A, Young KE, Chun SC, Kim DH, Park SW (2010) Enhanced ascorbic acid accumulation in transgenic potato confers tolerance to various abiotic stresses. Biotechnol Lett 32:321–330
Hemavathi, Upadhyaya CP, Akula N, Kim HS, Jeon JH, Ho OM, Chun SC, Kim DH, Park SW (2011) Biochemical analysis of enhanced tolerance in transgenic potato plants overexpressing d-galacturonic acid reductase gene in response to various abiotic stresses. Mol Breed 28:105–115
Idriss EE, Makarewicz O, Farouk A, Rosner K, Greiner R, Bochow H, Ritchter T, Boriss R (2002) Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth-promoting effect. Microbiology 148:2097–2109
Idriss EE, Iglesias DJ, Talon M, Borriss R (2007) Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol Plant Microbe Interact 20:619–626
Johnson PR, Ecker JR (1998) The ethylene gas signal transduction pathway: a molecular perspective. Annu Rev Genet 32:227–254
Johnson GN, Young AJ, Scholes JD, Horton P (1993) The dissipation of excess excitation energy in British plant species. Plant Cell Environ 16:673–679
Kende H (1993) Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 44:283–307
Kerovou J, Lauraeus M, Nurminen P, Kalkkinen N, Apajalahti J (1998) Isolation, characterization, molecular gene cloning, and sequencing of a novel phytase from Bacillus subtilis. Appl Environ Microbiol 64:2079–2085
Kloepper JW, Zablotowicz RM, Tipping EM, Lifshitz R (1991) Plant growth promotion mediated by bacterial rhizosphere colonizers. In: Keister DL, Cregan PB (eds) The rhizosphere and plant growth. Kluwer Academic Publishing, Dordrecht, pp 315–326
Kloepper JW, Reddy MS, Rodríguez-Kabana R, Kenney DS, Kokalis-Burelle N, Martinez-Ochoa N, Vavrina CS (2004a) Application of rhizobacteria in transplant production and yield enhancement. Acta Hortic 631:217–229
Kloepper JW, Ryu CM, Zhang SA (2004b) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266
Kumar A, Prakash A, Johri BN (2011) Bacteria in agrobiology: crop ecosystems. In: Maheshwari DK (ed) Bacillus as PGPR in crop ecosystems. Springer, Berlin, pp 37–59
Lowry OH, Rogebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mathur S, Jajoo A, Mehta P, Bharti S (2011) Analysis of elevated temperature-induced inhibition of photosystem II using chlorophyll a fluorescence induction kinetics in wheat leaves (Triticum aestivum). Plant Biol 13:1–6
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Mayak S, Tirosh T, Glick BR (1999) Effect of wild type and mutant plant growth-promoting rhizobacteria on the rooting of mung bean cuttings. J Plant Growth Regul 18:49–53
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci 166:525–530
Miller SH, Browne P, Prigent-Combaret C, Combes-Meynet E, Morrissey JP, O’Gara F (2010) Biochemical and genomic comparison of inorganic phosphate solubilization in Pseudomonas species. Environ Microbiol Rep 2:403–411
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plantarum 15:473–497
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nookaraju A, Kappachery S, Yu JW, Park SW (2011) Rhizobacteria influence potato tuberization through enhancing lipoxygenase activity. Am J Potato Res 88:441–449
Owino WO, Manabe Y, Mathooko FM, Kubo Y, Inaba A (2006) Regulatory mechanisms of ethylene biosynthesis in response to various stimuli during maturation and ripening in fig fruit (Ficus carica L.). Plant Physiol Biochem 44:335–342
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118:10–15
Rao IM, Arulanantham AR, Terry N (1989) Leaf phosphate status, photosynthesis, and carbon partitioning in sugar beet. II. Diurnal changes in sugar phosphates, adenylates and nicotinamide nucleotides. Plant Physiol 90:820–826
Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339
Saleem M, Arshad M, Hussain S, Bhatti AS (2007) Perspectives of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J Ind Microbiol Biotechnol 34:635–648
Sandhya V, Ali SKZ, Minakshi G, Reddy G, Venkateswarlu B (2009) Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol Fertil Soils 46:17–26
Saravanakumar D, Kavino M, Raguchander T, Subbian P, Samiyappan R (2011) Plant growth promoting bacteria enhance water stress resistance in green gram plants. Acta Physiol Plant 33:203–209
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Sessitsch A, Kan FY, Pfeifer U (2003) Diversity and community structure of culturable Bacillus spp. populations in the rhizospheres of transgenic potatoes expressing the lytic peptide cecropin B. Appl Soil Ecol 22:149–158
Sgherri CLM, Maffei M, Navari-Izzo F (2000) Antioxidative enzymes in wheat subjected to increasing water deficit and rewatering. J Plant Physiol 157:273–279
Shaharoona B, Arshad M, Zahir ZA (2006) Effect of plant growth promoting rhizobacteria containing ACC deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vigna radiate L). Lett Appl Microbiol 42:155–159
Smyth EM, McCarthy J, Nevin R, Khan MR, Dow JM, O’Gara F, Doohan FM (2011) In vitro analyses are not reliable predictors of the plant growth promotion capability of bacteria: a Pseudomonas fluorescens strain that promotes the growth and yield of wheat. J Appl Microbiol 111:683–692
Strasser RJ, Srivastava A, Tsimilli-Michael M (1999) Screening the vitality and photosynthetic activity of plants by fluorescence transient. In: Behl RK, Punia MS, Lather BP (eds) Crop improvement for food security. SSARM, Hisar, pp 79–126
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanisms, regulation and adaptation. Taylor & Francis, London, pp 445–483
Sundara Rao WVB, Bajpai PD, Sharma JP, Subbaiah BV (1963) Solubilization of phosphorus solubilizing organisms using P as tracer and the influence of seed bacterization on the uptake by the crop. J Indian Soc Soil Sci 11:209–219
Tsimilli-Michael M, Eggenberg P, Biro B, Koves-Péchy K, Voros I, Strasser RJ (2000) Synergistic and antagonistic effects of arbuscular mycorrhizal fungi and Azospirillum and Rhizobium nitrogen-fixers on the photosynthetic activity of alfalfa, probed by polyphasic chlorophyll a fluorescence transient O-J-I-P. Appl Soil Ecol 15:169–182
Upadhyay SK, Singh JS, Singh DP (2011) Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 21:214–222
Upadhyaya CP, Venkatesh J, Gururani MA, Asnin L, Sharma K, Ajappala H, Park SW (2011) Transgenic potato overproducing L-ascorbic acid resisted an increase in methylglyoxal under salinity stress via maintaining higher reduced glutathione level and glyoxalase enzyme activity. Biotechnol Lett 33:2297–2307
van der Linden CG, Anithakumari AM, van Culemborg M, Visser RGF (2011) Dissecting the genetics of abiotic stress tolerance in potato. In: Plant & animal genomes XIXth conference, San Diego, 15–19 January 2011
van Loon LC (2007) Plant responses to plant growth-promoting rhizobacteria. Eur J Plant Pathol 119:243–254
van Loon LC, Glick BR (2004) Increased plant fitness by rhizobacteria. In: Sandermann H (ed) Molecular ecotoxicology of plants. Springer, Berlin, pp 177–205
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Wahyudi AT, Astuti RP, Widyawati A, Meryandini A, Nawangsih AA (2011) Characterization of Bacillus sp. strains isolated from rhizosphere of soybean plants for their use as potential plant growth promoting rhizobacteria. J Microbiol Antimicrob 3:34–40
Wang YJ, Wang HM, Yang CH, Wang Q, Mei RH (2007) Two distinct manganese-containing superoxide dismutase genes in Bacillus cereus: their physiological characterizations and roles in surviving in wheat rhizosphere. FEMS Microbiol Lett 272:206–213
Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52:487–512
Woitke M, Junge H, Schnitzler WH (2004) Bacillus subtilis as growth promotor in hydroponically grown tomatoes under saline conditions. Acta Hortic 659:363–369
Wu SC, Cao ZH, Li ZG, Cheung KC, Wonga MH (2005) Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: a greenhouse trial. Ganoderma 125:155–166
Yang SF, Hoffmann NE (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35:155–189
Yang J, Kloepper J, Ryu C (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4
Yusuf MA, Kumar D, Rajwanshi R, Strasser RJ, Tsimilli-Michael M, Govindjee Sarin NB (2010) Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: physiological and chlorophyll a fluorescence measurements. Biochim Biophys Acta 1797:1428–1438
Yuwono T, Handayani D, Soedarsono J (2005) The role of osmotolerant rhizobacteria in rice growth under different drought conditions. Aust J Agric Res 56:715–721
Acknowledgments
This research project was supported by the Konkuk University research support program. The authors are thankful to Prof. Reto J. Strasser, Bioenergetics Laboratory, University of Geneva, Switzerland, for providing the Plant Efficiency Analyzer used in this study.
Conflict of interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gururani, M.A., Upadhyaya, C.P., Baskar, V. et al. Plant Growth-Promoting Rhizobacteria Enhance Abiotic Stress Tolerance in Solanum tuberosum Through Inducing Changes in the Expression of ROS-Scavenging Enzymes and Improved Photosynthetic Performance. J Plant Growth Regul 32, 245–258 (2013). https://doi.org/10.1007/s00344-012-9292-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-012-9292-6