Abstract
This study aimed to examine the induction of defense responses in tomato elicited by Methylobacterium oryzae CBMB20 as a consequence of reduced stress ethylene level possibly through its ACC deaminase activity. Significantly increased activities of pathogenesis-related (PR) proteins and defense enzymes such as β-1,3-glucanase, phenylalanine ammonia-lyase, peroxidase and polyphenol oxidase were noted in M. oryzae CBMB20 pretreated and challenged with Pseudomonas syringae pv. tomato (Pst) compared to either control or M. oryzae-treated tomato plants in both growth chamber and greenhouse conditions. Increased PR proteins and defense enzyme activities were correlated with the reduction of stress ethylene level. M. oryzae CBMB20 reduced the stress ethylene level about 27% and 55% when challenged with Pst, in growth chamber and greenhouse on day 7 respectively and the effect was comparable to that of the chemical ethylene biosynthesis inhibitor AVG, L-α-(2-aminoethoxyvinyl)-glycine hydrochloride. As a consequence of reduced stress ethylene level and its effect on defense response in crop plants, the disease severity was reduced 26% in M. oryzae CBMB20-treated plants challenged with pathogen. Therefore, inoculation of M. oryzae CBMB20 would induce the defense enzymes and contribute to the enhanced resistance of tomato plants against the pathogen Pst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato (Lycopersicon esculentum Mill L.) is one of the most important vegetable crops of South Korea, cultivated over a 5,624 ha area under greenhouse conditions. Ninety nine percent of the tomatoes produced in Korea are exported to Japan (Ministry of Agriculture, Nature and Food quality, Republic of Korea 2006). Bacterial speck disease caused by Pseudomonas syringae pv. tomato, decreases production under greenhouse conditions despite its moderate economic importance to tomato production (Bashan and de-Bashan 2002). Earlier studies have demonstrated the efficacy of chemical sprays against bacterial speck of tomato caused by Pseudomonas syringae pv. tomato. However, use of agrochemicals to combat plant pathogens is environmentally and economically not benign, in addition to causing resistance development in pathogens and reduction in the beneficial organism population (Glick and Bashan 1997). Therefore, biological approaches as an alternative strategy for the use of synthetic chemicals to manage diseases of crop plants are receiving more attention.
Bio-control agents, mainly bacterial inoculants are believed to induce systemic defense responses in the plants besides other mechanisms including direct antagonism, antibiosis and siderophore production. Induction of defense responses by plant-growth-promoting bacteria (PGPB) is largely associated with the production of pathogenesis-related (PR) proteins like β-1,3-glucanase and the defense enzyme phenylalanine ammonia-lyase (PAL 4.3.1.5) and oxidative enzymes like peroxidase (PO) and polyphenol oxidase (PPO) (Compant et al. 2005). However, increased activities and accumulation of these enzymes depends mainly on the plant hormone ethylene (Boller 1991; Chen et al. 2003). Ethylene emission is an early response of plants to pathogen attack and it has been suggested that it acts as a signal for defense response during plant-microbe interactions (Fig. 1). On the other hand, timing of increased ethylene emission is paralleled with the development of disease symptoms in crop plants (Chen et al. 2003). The chemical ethylene biosynthesis inhibitor AVG is used to reduce the ethylene levels and eventually the disease symptoms. It is established that PGPB possessing 1-aminocyclopropane-1-carboxylate deaminase activity (EC 3.5.99.7) could reduce the stress ethylene level through the hydrolytic cleavage of the ethylene biosynthesis precursor 1-aminocyclopropane-1-carboxylate (Glick et al. 1998; Madhaiyan et al. 2006a) and yet be able to maintain the ethylene level sufficient for biological processes till the later stage of crop growth, thus reducing the disease symptoms in crop plants as proposed in Fig. 1 (Robison 2001; Wang et al. 2000).
A proposed model for the lowering of plant ethylene level by plant growth promoting Methylobacterium oryzae leading to eventual increase in defense enzymes activity as a consequence of induction systemic resistance against invading pathogen (based on Glick and Penrose 1998; Boller 1991; Huang et al. 1993). SAdomet is S-adenosylmethionine, SA is salicylic acid, ACS is 1-aminocyclopropane-1-carboxylate synthase, ACC is 1-aminocyclopropane-1-carboxylate, ACO is 1-aminocyclopropane-1-carboxylic acid oxidase, ACCD is 1-aminocyclopropane-1-carboxylate deaminase, α-KB is α-ketobutyrate. ⊥ Symbol shows the inhibition of ACS by salicylic acid (SA)
The genus Methylobacterium of class alphaproteobacteria, sometimes referred as pink pigmented facultative methylobacteria (PPFMs) are a physiologically interesting group of bacteria, able to grow on methanol, methylamine as well as on a variety of C2, C3 and C4 compounds (Lidstrom 2001) is receiving more attention as PGPB. Several beneficial aspects such as stimulation of seed germination, plant growth promotion, production of phytohormones and induction of defense responses in rice and peanut against Rhizoctonia solani and Aspergillus niger/Sclerotium rolfsi have been reported for Methylobacterium (Omer et al. 2004; Madhaiyan et al. 2004; 2006b). More recently, Ryu et al. (2006) and Anandham et al. (2007) documented the production of IAA, cytokinin and oxidation of sulfur by Methylobacterium oryzae CBMB20. Also, the lessening of plant stress ethylene level through the action of the bacterial enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase was reported for M. oryzae strains CBMB20 and CBMB110 (Glick et al. 1998; Madhaiyan et al. 2006a). This work aimed to test the induction of the defense response in tomato provided by M. oryzae CBMB20 against the foliar pathogen-Pseudomonas syringae pv. tomato as a consequence of reducing the stress ethylene level through its ACC deaminase activity.
Materials and methods
Bacterial strains, culture conditions and plant material
Methylobacterium oryzae strains CBMB20 (AY683045) and CBMB110 (AY683046) were grown in ammonium mineral salts (AMS) medium (Whittenbury et al. 1970) supplemented with 0.5% methanol (v/v) and cycloheximide (20 μg/ml). The bacterial pathogen P. syringae pv. tomato (Pst) maintained in King’s B (KB) liquid medium was obtained from the Department of Plant Medicine, Chungbuk National University, Republic of Korea. Tomato seeds (Lycopersicon esculentum cv. rokusanmaru) and seedlings susceptible to bacterial speck were obtained from commercial sources for growth chamber and greenhouse experiments respectively.
Siderophore production and antagonistic activity of M. oryzae
Siderophore production by M. oryzae strains CBMB20 and CBMB110 was tested on universal chrome azural S (CAS) agar plates (Schwyn and Neilands 1987). The antagonistic activity was tested by a dual culture method. Filter paper discs (6 mm) saturated with Methylobacterium suspension were placed over a bacterial lawn of Pst (108 c.f.u./ml) in nutrient agar medium. The plates were incubated for 3–5 days at 30°C and observed for clear inhibition zones around the discs.
In vitro salicylic acid (SA) production by M. oryzae
M. oryzae strains CBMB20 and CBMB110 were grown in 25 ml of liquid medium (Davis and Mingioli 1950) supplemented with 0.5% methanol at 30°C for 72 h in shaking incubator (150 rev/min). Bacterial cultures were harvested by centrifugation at 2,800g for 10 min at 4°C. The resulting supernatant was acidified with 1 M HCl to pH 1.5–2.0 and extracted with an equal volume of ethyl acetate. After evaporation of the organic phase, the dry residue was suspended in 3 ml of 97% methanol. SA concentration was determined by adding 5 μl of 2 mol FeCl3 and 3 ml of water to 1 ml of concentrated extract. The absorbance of the purple iron-SA complex, which developed in the aqueous phase, was measured at 527 nm (UV-spectrophotometer, UV-1601, Shimadzu, Japan) and compared with a standard curve of SA dissolved in ethyl acetate (Meyer and Abdallah 1992).
Antimicrobial effect of Salicylic acid (SA)
The antibacterial activity of SA on Pst was tested according to Cameron and Zaton (2004). Briefly, a single colony from a KB agar plate on which it had ben maintained for 24 h, was grown in KB liquid medium containing SA (0, 0.01, 0.01, 1.0 and 5 mM) at 120 rev/min for 28 h at 30°C. Bacterial culture growth was measured spectrophotometrically (O.D.600 nm) at 8, 20, 22, 24 and 28 h.
Growth chamber bioassay
Bacterial cells of 72-h-old cultures of M. oryzae strains CBMB20 and CBMB110 were harvested by centrifugation at 10,000g for 10 min at 4°C, washed twice and suspended in 0.03 M MgSO4. Homogenous bacterial suspensions were adjusted to O.D.600 nm = 0.15 (108 c.f.u./ml). The surface disinfected (70% ethanol-10 min; 1% sodium hypochlorite-5 min) tomato seeds were subjected to the following treatment for 4 h: (i) M. oryzae CBMB20, (ii) CBMB110, (iii) 1 × 10−4 M AVG (l-α-(2-aminoethoxyvinyl)-glycine hydrochloride) (Sigma-Aldrich Co. St Louis, Mo, USA) a chemical ethylene biosynthesis inhibitor (as positive control), and (iv) 0.03 M MgSO4 (as negative control).
Treated tomato seeds were axenically dried and placed in a Petri dish with moist sterile filter paper and incubated for 7 days at 24°C under dark. On day 7 germinated tomato seeds were transplanted into 150-ml plastic pots containing oven-sterilized commercial pot mixture (Sun Gro Horticulture Distribution, Inc., Seba Beach, Canada). Overnight-grown bacterial pathogen Pst cells were harvested by centrifugation at 10,000g for 10 min at 4°C and washed twice and suspended in saline (0.85% NaCl). Bacterial suspension was prepared by mixing 1 ml of saline-suspended bacterial cells with 20 ml of 0.025% Triton X-100 to a final concentration of 108 c.f.u./ml (O.D.600 nm = 0.4) and used as foliar spray on 4-week-old tomato plants until run-off by using a hand-held pneumatic sprayer. Pots were placed in growth chambers at 20 ± 1°C, photoperiod starting with 12 h dark followed by 12 h lights (18 μmol/m2/S) in a completely randomized design with three replications. Plants were watered regularly with sterile distilled water.
Greenhouse bioassay
Twenty one day old tomato seedlings were transplanted into 300-ml pots containing an oven-sterilized commercial pot mixture. After establishment the plants were subjected to the following treatments: (i) Control (0.03 M MgSO4), (ii) CBMB20 (O.D.600 nm = 0.15 = 108 c.f.u./ml), (iii) CBMB20 (108 c.f.u./ml) + Pst (O.D.600 nm = 0.4 = 1.5 × 107 c.f.u./ml) and (iv) Pst alone (1.5 × 107 c.f.u./ml). Methylobacterium and pathogen inocula were prepared as described for growth chamber bioassay and inoculation was performed with a hand-held pneumatic sprayer until run-off on 28- and 30-day-old tomato plants respectively. Plants were maintained in the greenhouse with a temperature regime of 28/22°C (day/night) and natural illumination. Twelve pots per treatment, each with a single plant were arranged in a completely randomized design with three replications.
Disease scoring was started after the initial development of the bacterial speck on 5 and 3 days after pathogen inoculation in growth chamber and greenhouse respectively. Disease severity was evaluated visually and scored using a disease index with a range of 0–3 (0-signifies a leaf showing no symptom or speck; 1-signifies 2–5 lesions/leaf; 2-signifies 6–10 lesions; and 3-signifies more than 10 lesions together or spread over each leaf) as described previously in Bashan and de-Bashan (2002). Leaf samples were collected on days 5, 10 and 1, 3, 5, 7, 10, 15 days respectively from growth chamber and greenhouse after challenge inoculation of the pathogen for ethylene, PR- protein and defense enzymes analysis.
Ethylene analysis
Tomato leaves (1 g) collected from growth chamber and greenhouse experiments were placed in 120 ml vials for 30 min to allow for the escape of wound ethylene and sealed with a rubber septum for 4 h. One ml sample of the headspace was injected into a Gas Chromatograph (dsCHROM 6200, Donam Instruments Inc., Republic of Korea) packed with Poropak-Q column at 70°C and equipped with a flame ionization detector. The amount of ethylene emission was expressed as nmol or pmol of ethylene/h/g fresh weight by comparing the standard curve generated with pure ethylene (Praxair, Praxair Korea Co., Ltd).
Enzyme extraction, PR-protein and defense enzyme assay
Leaf samples (1 g) collected from tomato plants were snap frozen and homogenized immediately with liquid nitrogen. The resulting powder was macerated for 30 s with 100 mM potassium phosphate buffer, pH 7.0 (1:1.25 w/v). Then the crude homogenates were centrifuged at 20,000g for 20 min at 4°C. The supernatants were kept in an ice bath and used for the determination of PR proteins and defense enzyme and dosing for Lowry protein estimation using bovine serum albumin (BSA) as a standard.
β-1,3-Glucanase activity was assayed using laminarin (from Laminaria digitata) (Sigma-Aldrich Co. St. Louis, Missouri, USA) as substrate (Liang et al. 1995). The assay mixture 0.1 ml consisted of 50 μl enzyme extract, 50 μl laminarin (10 mg/ml) in 50 mM sodium acetate buffer (pH 5.0) and was incubated at 37°C for 1 h and the amount of reducing sugar released from laminarin was determined by the termination of reaction with 1.5 ml dinitrosalicylic acid (DNS) reagent and boiling the mixture in a water bath for 5 min. Reducing sugar equivalents were measured at 530 nm. Enzyme activity was expressed as μg glucose/min/mg protein. Phenylalanine ammonia-lyase activity was measured according to the procedure described by El-Shora (2002). The assay mixture consisted of 1.9 ml of 100 mM Tris–HCl buffer (pH 8.5), 1 ml of 15 mM l-phenylalanine and 100 μl enzyme extract was incubated at 30°C for 15 min. The reaction was terminated by adding 200 μl of 6 M HCl and the absorbance of the solution was measured at 290 nm. One unit represents the conversion of 1 μmol l-phenylalanine to trans-cinnamic acid per min. The amount of trans-cinnamic acid synthesized was calculated using its molar absorptivity of 9630 M−1 cm−1 and expressed as nmol trans-cinnamic acid/min/mg protein.
Peroxidase activity was determined by mixing the 50 μl of enzyme extract with 2.85 ml of 100 mM phosphate buffer (pH 7.0) and 50 μl of 20 mM guaiacol (Fu and Huang 2001). The reaction was started by adding 20 μl of 40 mM H2O2 to the mixture and the initial rate of increase in the absorbance was measured at 470 nm over 1 min. One unit of activity was defined as a change in absorbance of 0.001/min. Polyphenol oxidase activity was determined according to Mayer et al. (1965). The reaction mixture consisted of 200 μl enzyme extract and 1.5 ml 0.1 M sodium phosphate buffer (pH 6.5). The reaction was started with the addition of 200 μl 0.01 M catechol and the activity was measured by observing the changes in absorbance at 420 nm. The activity of PO and PPO was expressed as changes in absorbance/min/mg protein.
For estimation of the total phenolics, fresh tomato leaves (1 g) were extracted with 10 ml of 80% methanol and agitated for 15 min at 70°C (Zieslin and Ben-Zaken 1993). One ml of the methanolic extract was added to 5 ml of distilled water and 250 μl of Folin-Ciocalteu reagent (1 M) and kept for incubation at 25°C for 30 min. The absorbance of the developed blue color was measured at 725 nm and expressed as μg of catechol/mg protein by using catechol as standard.
Statistical analysis
The data were statistically analysed by analysis of variance (ANOVA) using the general linear model version 9.1 developed by SAS institute Inc., Cary, NC, USA (SAS 2004). Means were compared using the least significant difference (LSD) P ≤ 0.05.
Results
Siderophore-negative M. oryzae strains CBMB20 and CBMB110 were found to produce 10.5 ± 0.40 and 8.0 ± 0.57 μg salicylic acid/ml of culture media. Though the strains were not able to show antagonism toward Pst in dual culture technique, SA produced by the M. oryzae CBMB20 inhibited the growth of Pst about 2-fold that of control over 20 h of incubation (Fig. 2).
In the growth chamber experiment, M. oryzae CBMB20 significantly (P ≤ 0.05) increased the PR protein and defense enzymes activity in tomato plants inoculated with pathogen. The level of ethylene recorded in plants pretreated with M. oryzae CBMB20 and challenge-inoculated with pathogen (3.52 ± 0.18) was on par with the ethylene level recorded in AVG and challenge-inoculated with pathogen (3.40 ± 0.16) and significantly less than that of the ethylene level in plants treated with pathogen alone (4.86 ± 0.20). The increased activity of β-1,3-glucanases, PAL, PO, PPO was noted in plants pretreated with M. oryzae CBMB20 compared with that of M. oryzae CBMB110 and comparable to that of AVG (Table 1). Based on the significant performance in the growth chamber experiment, M. oryzae CBMB20 was chosen for the greenhouse experiment.
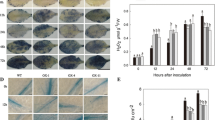
Under greenhouse conditions, ethylene production in uninoculated plants remained low compared to pathogen-inoculated plants and the latter showed a significant increase in ethylene level on a day after inoculation and reached maximum on day 7 (Fig. 3a). During this time period, pathogen-inoculated plants showed small, water-soaked lesions. Ethylene production then began to decrease and yellow haloes appeared around the water-soaked lesions on day 9 after pathogen inoculation (Fig. 4a and b). On day 12 the lesions coalesced and almost all of the leaf tissue was damaged, coinciding with a second peak in ethylene production (Figs. 3a and 4c). Plants pretreated with M. oryzae CBMB20 and challenge-inoculated with pathogen showed reduced ethylene level (Fig. 3a) and a concurrent increase in the activity of β-1,3-glucanases (204.2–288.8 ng glucose/min/mg protein) was also recorded (Fig. 3b).
Induction of defense response in tomato by Methylobacterium oryzae CBMB20 against Pseudomonas syringae pv. tomato under growth chamber condition resulting from reduced (a) ethylene level and increased activity of (b) β-1,3-glucanase, (c) Phenylalanine ammonia-lyase, (d) Peroxidase, (e) Polyphenol oxidase and (f) Phenolics. Each value represents the mean of three replications per treatment. Error bars indicate ± standard error (SE)
Efficacy of Methylobacterium oryzae on the severity of bacterial speck caused by P. syringae pv. tomato in tomato leaves on day (a) 3 after pathogen inoculation, (b) 9 after pathogen inoculation and (c) 12 after pathogen inoculation. (Top: P. syringae pv. tomato alone, Bottom: M. oryzae CBMB20 + Pst)
The activity of PAL started to increase from 30.25 to 44.79 nmol of trans-cinnamic acid/min/mg protein on day 1 through day 5 after pathogen inoculation in plants pretreated with M. oryzae CBMB20. The increase in PAL activity was stable till day 12 (Fig. 3c). PO and PPO activities were extremely high in treatment with M. oryzae CBMB20 and challenged with pathogen, compared to treatment with either pathogen or Methylobacterium alone (Fig 3d and e). Treatment with M. oryzae CBMB20 and challenge-inoculated with Pst increased the total phenols of the plants, ranging between 6.32 and 6.76 μg catechol/mg of protein and the increase was noticed throughout the experimental period. Plants treated with Pst alone showed a gradual decline in phenolic content (Fig. 3f).
In growth chamber experiments, the percent disease index (PDI) value observed in seedlings of CBMB20-treated seeds challenged with Pst was 10.5% and was comparable to that of plants treated with AVG. whereas, PDIs in the pathogen-treated plants was as high as 22% under growth chamber and 28% for greenhouse conditions (Fig. 5).
Percent disease index of tomato plants treated with Methylobacterium oryzae strains CBMB20 and CBMB110 and challenged with P. syringae pv. tomato. A hollow and solid bar indicates under growth chamber and greenhouse conditions respectively. Each value represents means of three replicates per treatment. Error bars indicate ± standard error (SE)
Discussion
It is established that the antagonistic activity of the PGPB was characterized by the production of siderophore (Compant et al. 2005). Therefore, the absence of antagonism against the Pst by the siderophore-negative M. oryzae strains CBMB20 and CBMB110 is not surprising. It was evident from the growth chamber experiment, where the spatial separation of Methylobacterium in rhizosphere and the bacterial pathogen in the phyllosphere excludes the possibility of direct antagonism leads to the conclusion that the defense response was induced. Induction of defense response through the application of PGPB often induces changes in plant chemistry that are correlated with an increase in disease resistance, which is more advantageous than antagonistic activity. Methylobacterium-elicited defense responses have been demonstrated in rice and peanut (Madhaiyan et al. 2004; 2006b). M. oryzae CBMB20 probably reduced the level of stress ethylene induced by pathogen attack and thus confers resistance to stress posed by phytopathogens, which is congruent with earlier findings (Robison et al. 2001; Wang et al. 2000; Glick et al. 1998).
The possible mechanism behind this protection might be due to the hydrolytic cleavage of the ethylene biosynthesis precursor ACC into α-ketobutyrate (α-KB) and ammonia by the bacterially produced ACC deaminase (Glick et al. 1998; Madhaiyan et al. 2006a), since the reduced level of ethylene is enough to induce PR proteins and defense enzymes; but not sufficient for the proliferation of the pathogen and eventually disease symptom development (Chen et al. 2003). It is evident from our study that treatment with M. oryzae CBMB20 showed reduced ethylene level (1.47 nmol/h/g fresh weight), which is within the level of 0.05 μl/l, enough to activate the biological processes in crop plants (Abeles 1992) and increased activity of PR proteins. The increased activity of defense enzymes and reduced level of ethylene in M. oryzae CBMB20 compared with M. oryzae CBMB110 could be explained by the magnitude of ACC deaminase activity of 94.48 and 24.74 nmol α-KB/h/mg protein respectively (Madhaiyan et al. 2006a). In a previous study, transgenic tomato expressing a bacterial ACC deaminase showed reduced symptoms of Verticillium wilt (Robison et al. 2001). Also, the inoculation of Methylobacterium to rice and peanut plants increased the activity of PR proteins (Madhaiyan et al. 2004; 2006b).
Ethylene production as a defense response immediately after pathogen attack might be originate from the portion of plant’s existing pool of ACC by ACC oxidase (Fig. 1). But the subsequent increase in ethylene level is due to the transcription of ACS by pathogen leading to disease symptom development (Hase et al. 2003). In this study, the reduced level of ethylene in plants treated with either CBMB20 alone or challenged with pathogen might be due to the action of ACC deaminase present in the Methylobacterium. Though we did not determine the SA level in plant after Methylobacterium treatment, it can be explained that the reduced stress ethylene level may also be due to the inhibition of ACS by SA (Huang et al. 1993) (Fig. 1) besides its antimicrobial activity (Cameron and Zaton 2004). It has been established that the plant growth-promoting bacteria able to produce SA are well known for their role in the induction of PR proteins and defense enzymes (Compant et al. 2005).
In tomato, seed treatment with Methylobacterium induced the plants to synthesize β-1,3-glucanase, PAL, PO and PPO. An additional increase in β-1,3-glucanase synthesis was observed in M. oryzae CBMB20-pretreated plants challenged with Pst in both growth chamber and greenhouse experiments. The increased activity of β-1,3-glucanase parallel with the level of ethylene reflects the role of ACC deaminase-containing Methylobacterium in induction of the defense response. Increased activity of β-1,3-glucanase was reported for rice and peanut when the seedlings raised from Methylobacterium-bacterized seeds and challenged with Rhizoctonia solani and Aspergillus niger/Sclerotium rolfsii respectively (Madhaiyan et al. 2004; 2006b).
Cinammic acid, the product of PAL, is directly linked to cell lignification processes and the highest levels of PAL activity usually occur about one day after initial infection of pathogen (Podile and Laxmi 1998). In this study also the highest PAL activity occurred about one day after initial infection of the pathogens and the activity was increased constantly in Methylobacterium-treated plants up to day 12, which is congruent with the results reported by earlier researchers (Podile and Laxmi 1998; Madhaiyan et al. 2006b). It is possible that the product of PAL, cinnamic acid directs the synthesis of the antimicrobial agent SA (Fig. 1). The PO and PPO activities are linked to lignification and generation of hydrogen peroxides at later stages of infection, which inhibit pathogens directly, or generation of other free radicals with antimicrobial activity, that restrict the development of pathogenic bacteria (Silva et al. 2004). Similar results were obtained in rice and peanut with a high accumulation of phenolic compounds when treated with Methylobacterium and R. solani and A. niger/S. rolfsi (Madhaiyan et al. 2004; 2006b).
The probable reason for the reduced bacterial speck disease symptoms in M. oryzae CBMB20-pretreated plants challenged with Pst might be due to the reduced ethylene levels resulting from the deaminase activity of M. oryzae CBMB20. The enhanced accumulation of PR proteins and defense enzymes as a result of reduced ethylene level substantiates the potential role of M. oryzae CBMB20 in disease resistant development in crop plants besides its potential for the direct plant growth-promoting activity through the production of phytohormone (IAA, cytokinin) and sulfur oxidation. The tested strain had been originally isolated from the surface-sterilized stem of rice plants, thus it may have the potential to grow endophytically within the plant system and could arrest the proliferation of the bacterial pathogens for instance, Pst causing systemic disease in crop plants. The findings of the present study support the use of M. oryzae CBMB20 as a potential biocontrol agent against bacterial speck. However, further studies under field conditions need to be conducted to test the efficacy of this biocontrol agent against bacterial speck and other foliar diseases of tomato.
References
Anandham R, Indiragandhi P, Kim K, Yim W, Madhaiyan M, Saravanan VS, Chung J, Sa TM (2007) Thiosulfate oxidation and mixotrophic growth of Methylobacterium oryzae. Can J Microbiol 53:869–876
Abeles FB, Morgan PW, Saltveit ME Jr. (1992) Ethylene in plant biology, 2nd edn. Academic Press, New York
Bashan Y, de-Bashan LE (2002) Protection of tomato seedlings against infection by Pseudomonas syringae pv. tomato by using the plant growth promoting bacterium Azospirillum brasilense. Appl Environ Microbiol 68:2637–2643
Boller T (1991) Ethylene in pathogenesis and disease resistance. In: Mattoo AK, Suttle JC (eds) The plant hormone ethylene. CRC Press, Boca Raton, FL, pp 293–314
Cameron RK, Zaton K (2004) Intercellular salicylic acid accumulation is important for age-related resistance in Arabidopsis to Pseudomonas syringae. Physiol Mol Plant Pathol 65:197–209
Chen N, Goodwin PH, Hsiang T (2003) The role of ethylene during the infection of Nicotiana tabacum by Colletotrichum destructivum. J Exp Bot 54:2449–2456
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Davis BD, Mingioli ES (1950) Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol 60:17–28
El-Shora HM (2002) Properties of phenylalanine ammonia-lyase from marrow cotyledons. Plant Sci 162:1–7
Fu JM, Huang BR (2001) Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ Exp Bot 45:105–114
Glick BR, Bashan Y (1997) Genetic manipulation of plant growth promoting bacteria to enhance biocontrol of phytopathogens. Biotechnol Adv 15:353–378
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol 190:63–68
Hase S, van Pelt JA, van Loon LC, Pieterse CMJ (2003) Colonization of Arabidopsis roots by Pseudomonas fluorescens primes the plant to produce higher levels of ethylene upon pathogen infection. Physiol Mol Plant Pathol 62:219–226
Huang YF, Chen CT, Kao CH (1993) Salicylic acid inhibits the biosynthesis of ethylene in detached rice leaves. Plant Growth Regul 12:79–82
Liang ZC, Hseu RS, Wang HH (1995) Partial purification and characterization of a 1,3-β-D-glucanase from Ganoderma tsugae. J Ind Microbiol 14:5–9
Lidstrom ME (2001) The aerobic methylotrophic bacteria. In: Dworkin M (ed) The prokaryotes. Berlin, Springer-Verlag, pp 223–244
Madhaiyan M, Poonguzhali S, Senthilkumar M, Seshadri S, Chung H, Yang J, Sundaram S, Sa TM (2004) Growth promotion and induction of systemic resistance in rice cultivar Co-47 (Oryza sativa L.) by Methylobacterium spp. Bot Bull Acad Sin 45:315–324
Madhaiyan M, Poonguzhali S, Ryu JH, Sa TM (2006a) Regulation of ethylene levels in canola (Brassica campestris) by 1-aminocyclopropane-1-carboxylate deaminase containing Methylobacterium fujisawaense. Planta 224:268–278
Madhaiyan M, Suresh Reddy BV, Anandham R, Senthilkumar M, Poonguzhali S, Sundaram SP, Sa TM (2006b) Plant growth-promoting Methylobacterium induces defense responses in groundnut (Arachis hypogaea L.) compared with rot Pathogens. Curr Microbiol 53:270–276
Mayer AM, Harel E, Shaul RB (1965) Assay of catechol oxidase a critical comparison of methods. Phytochemistry 5:783–789
Meyer JM, Abdallah MA (1992) The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physicochemical properties. J Gen Microbiol 107:319–328
Omer ZS, Tombolini R, Broberg A, Gerhardson B (2004) Indole-3-acetic acid production by pink-pigmented facultative methylotrophic bacteria. Plant Growth Regul 43:93–96
Podile AR, Laxmi VDV (1998) Seed bacterization with Bacillus subtilis AF1 increases phenylalanine ammonia-lyase and reduces the incidence of fusarial wilt in pigeon pea. J Phytopathol 146:255–259
Robison MM, Shah S, Tamot B, Pauls KP, Moffatt BA, Glick BR (2001) Reduced symptoms of Verticillium wilt in transgenic tomato expressing a bacterial ACC deaminase. Mol Plant Pathol 2:135–145
Ryu JH, Madhaiyan M, Poonguzhali S, Yim WJ, Indira Gandhi P, Kim KA, Anandham R, Young JC, Kim KH, Sa TM (2006) Plant growth substances produced by Methylobacterium spp. and their effect on tomato (Lycopersicon esculentum L.) and red pepper (Capsicum annuum L.) growth. J Microbiol Biotechnol 16:1622–1628
SAS Institute Inc. SAS 9.1® (2004) Companion for windows SAS. SAS Institute Inc., Cary, NC
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Silva HS, Aromeiro RS, Macagnan D, Halfeld-Vierira BA, Pereira MCB, Mounteer A (2004) Rhizobacterial induction of systemic resistance in tomato plants: non-specific protection and increase in enzyme activities. Biol Control 29:288–295
Wang C, Knill E, Glick BR, Défago G (2000) Effect of transferring 1-aminocyclopropane-carboxylic acid (ACC) deaminase genes into Pseudomonas fluorescens strain CHA0 and its gacA derivative CHA96 on their growth-promoting and disease-suppressive capacities. Can J Microbiol 46:898–907
Whittenbury R, Davies SL, Wilkinson JF (1970) Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol 61:205–218
Zieslin N, Ben-Zaken R (1993) Peroxidase activity and presence of phenolic substances in peduncles of rose flowers. Plant Physiol Biochem 31:333–339
Acknowledgements
The financial support provided by Brain Korea (BK21) through Ph.D. scholarship to P.I is greatly acknowledged. R.A and M.M. are supported by Korea Research Foundation. We acknowledge the partial financial support provided by Rural Development Administration. Critical reading of this paper by the learned referees is thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Indiragandhi, P., Anandham, R., Kim, K. et al. Induction of defense responses in tomato against Pseudomonas syringae pv. tomato by regulating the stress ethylene level with Methylobacterium oryzae CBMB20 containing 1-aminocyclopropane-1-carboxylate deaminase. World J Microbiol Biotechnol 24, 1037–1045 (2008). https://doi.org/10.1007/s11274-007-9572-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-007-9572-7