Abstract
The aim of this study was to evaluate whether the interaction of Vicia sativa with a bacterial strain capable of using phenol as sole carbon and energy sources can moderate adverse effects of this pollutant in plant tissues. A bacterial strain identified as Bacillus sp., isolated from a heavily polluted environment, was inoculated at different stages of growth. In root elongation assay, inoculated seeds showed higher values of relative root elongation and germination index than uninoculated ones in the presence of high phenol concentrations. Thus, common vetch–Bacillus sp. association could be important at the first stages of development allowing this plant to grow in highly polluted environments. Besides, phenol removal was largely accelerated in phenol-spiked soils, after 48 h of treatment with uninoculated/inoculated plants rather than by adsorption or biodegradation of the bacterial strain. Peroxidase and ascorbate peroxidase activities increased significantly in uninoculated plants, while superoxide dismutase activity, chlorophyll, malondialdehyde, and H2O2 levels of aerial parts remained unaltered in uninoculated/inoculated plants treated with the pollutant, demonstrating that the efficient response to oxidative damage did not depend on the inoculation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increase in the quality-of-life standards and the awareness of environmental issues has become the remediation of polluted sites in a priority for society (Segura et al. 2009). Phenol is a highly toxic pollutant produced by various industries. Thus, its utilization presents severe risks to human health and the environment, where its accumulation has been frequently reported (Yang et al. 2011). Because of the high cost of physicochemical strategies for the remediation of this compound, biological tools are a very attractive option for cleaning up contaminated sites. In this sense, biological remediation of contaminated environment includes, among others, the use of plants that can extract, contain, degrade, and/or immobilize contaminants (Wang et al. 2011). The realization that plants serve as mankind by cleanup of toxic contaminants is quite old, but the problems of contaminated land sites, water bodies and groundwater, and spoiled air worldwide have increased many folds due to anthropogenic activities during second half of the twentieth century and hence deserve special attention (Singh et al. 2007). Thus, in the last years, scientists have begun to explore the possibility of using soil bacteria, including biodegradative bacteria, plant growth-promoting bacteria, and bacteria that facilitate phytoremediation by other means as part of an effort to make this technology more efficient (Glick 2010). Consequently, a convergence of phytoremediation and microbial bioremediation strategies led to a more successful approach to remediate contaminants, particularly organic compounds (Gerhardt et al. 2009; Vangronsveld et al. 2009). Furthermore, microbe-assisted phytoremediation has a great potential to become cost-efficient and cost-effective ways of removing organic compounds from the environment (Gerhardt et al. 2009).
As it is well known, plants growing on land have to cope with a multitude of environmental stress, such as drought, salinity, radiation, extremes of temperature, and pollutants. (Ashraf 2009). This resulted in a rapid increase in reactive oxygen species (ROS) levels, which in plants paradoxically serve as both signaling molecules and as undesirable cytotoxic by-products. Under normal photosynthetic conditions, ROS are produced in the electron transport chains of chloroplasts and mitochondria, and are the by-products of metabolic pathways in the peroxisome (Liu et al. 2009). Whereas under normal growth conditions the production of ROS in cells is low (240 μM s−1 O2 ·− and a steady-state level of 0.5 μM H2O2 in chloroplasts), they can reach high concentrations during stress through pathways such as photorespiration, mitochondrial respiration, and from the photosynthetic apparatus (240–720 μM s−1 O2 ·− and a steady-state level of 5–15 μM H2O2) (Ashraf 2009). Since the production and removal of ROS must be strictly controlled in order to maintain a redox balance, plants possess both enzymatic and non-enzymatic mechanisms to diminish cytotoxic damage such as lipid peroxidation, protein modification, and DNA damage caused by high ROS concentrations (Dinakar et al. 2012). The enzymatic mechanisms are designated to minimize the concentration of O2 ·− and H2O2 and consist of several enzymes like peroxidase (POD), superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), and glutathione peroxidase (GPx). Scavenging of O2 ·− by SOD results in the production of H2O2, which is removed by APX or CAT. However, both O2 ·− and H2O2 are not as toxic as the (OH·), which is formed by the combination of O2 ·− and H2O2 in the presence of trace amounts of Fe2+ and Fe3+ and which has no specific scavenging enzymes in biological systems (Gong et al. 2011). Regarding non-enzymatic antioxidants, these include pigments, glutathione, ascorbate α-tocopherol, and others (Dinakar et al. 2012).
Vicia sativa is a plant species cultivated as food for humans and livestock, for soil improvement, and it is also an alternate legume in many areas, especially for dryland farming (Muccifora and Bellani 2013). In a previous work, we have studied V. sativa capability to tolerate and remove phenol from aqueous solutions. As an extension of the work, we analyzed whether the association between plant and microbe can improve the phytoremediation process. Firstly, growth, removal of the pollutant in liquid cultures, phenol degradation pathway, and the presence of plant growth promotion properties were evaluated in the bacterial strain. Then, we studied the effect of the interaction between plant and bacteria in the presence of the organic pollutant at different stages of growth. Remediation in pots’ assays was performed: analyzing phenol residual concentration and different parameters of the antioxidant response (POD, SOD, and APX activities; H2O2 and MDA content).
Materials and methods
Plant materials
The experiments were carried out with seeds and plants of V. sativa sp. Nigra.
Bacterial strain
The bacterial strain, namely BAC S, which was kindly provided by Dr Eloísa Pajuelo (Faculty of Pharmacy, University of Seville, Spain), was isolated from an area contaminated by As and heavy metals after a toxic mine spill (Carrasco López 2007). This bacterial strain was subjected to morphological, physiological, and biochemical tests for its characterization and identification. The isolate was characterized by Gram staining kit (Britania) using an Axiolab (Zeiss) microscope. Different commercially available kits for bacterial biochemical analysis were used in this study (BioMerieux® SA). The protocols followed the manufacturer’s instructions. Some biochemical features like the hemolytic activity and penicillin resistance were also performed.
Analyses of 16S rDNA sequences
The nucleotide sequence of the nearly full-length 16S rDNA of the bacterial isolate was directly obtained by Macrogen Laboratories (Korea) and has been deposited in GenBank data bank under accession number JX294295.
Sequence analyses of 16S rDNA were performed by using the algorithm BLASTN (Altschul et al. 1997) to identify similarities and to perform alignments. Phylogenetic analyses were conducted using BioEdit (Hall 1999) and MEGA version 4 (Tamura et al. 2007) softwares.
Phenol tolerance test
Phenol tolerance was tested on agar plates containing Tryptone-Yeast extract (TY) medium (Vincent 1970), with increasing concentrations of the pollutant. To prepare phenol-enriched medium, a stock phenol solution (5,000 mg L−1) was filter-sterilized and individually added to different flasks containing autoclaved TY medium with 1.3 % (w/v) of agar, to provide final concentrations of 100, 300, and 500 mg L−1. Then, the medium was placed on Petri dishes and they were incubated for 7 d at 28 °C. TY without phenol was used as control. Phenol used in all experiments was purchased from Merck. Solutions were prepared with deionized water and all substances used were of analytical reagent grade.
Phenol degradation assay and determination of its biodegradation pathway
The ability of the bacterial strain to remediate phenol from a liquid medium was assayed in vitro in a modified MS medium (Murashige and Skoog 1962). A stock phenol solution (5,000 mg L−1) was filter-sterilized and individually added to the different flasks containing autoclaved modified MS medium, to provide final concentrations of 100, 200, and 500 mg L−1. The different flasks were then inoculated with a culture grown over night (107 cfu mL−1) on TY medium and incubated at 25 ± 2 °C on an orbital shaker at 150 rpm. Optical density (OD) at 600 nm was followed every 24 h as a measurement of growth. The residual amount of phenol extracted from modified MS medium was determined following a spectrophotometric assay (Wright and Nicell 1999). Results were expressed as percentage of residual phenol, which is defined as the ratio between the concentration of phenol removed and the initial concentration of phenol in the incubation medium (Coniglio et al. 2008).
The determination of phenol biodegradation pathway was carried out through the methodology described by Pradhan and Ingle (2007). Increase in absorbance at 260 nm and at 375 nm was recorded due to the formation of cis, cis muconic acid (ε = 16,000 M−1 cm−1), and 2-hydroxymuconate semialdehyde (ε = 36,500 M−1 cm−1), produced by the activity of cathecol 1,2 or cathecol 2,3 dioxygenase, respectively. One unit of enzyme activity was defined as the amount, which catalyzed the formation of 1 μmol of cis, cis muconic acid min−1, or 2-hydroxymuconate semialdehyde min−1. Results were expressed as specific activity, and protein was calculated by Bradford method (1976).
Plant growth-promoting characteristics
Phosphate solubilization ability
The ability of the strain to solubilize inorganic phosphate was assayed on National Botanical Research Institute′s phosphate growth medium (NBRIP medium) (Metha and Nautiyal 2001), containing tri-calcium phosphate [Ca3 (PO4)2]. An aliquot of fresh bacterial culture (107 cfu mL−1) was spotted onto the plate and incubated at 28 °C for 7 days. Formation of halo around the colonies indicated solubilizing ability.
Siderophore production
Siderophore production was done according to the method described by Schwyn and Neilands (1987). Chrome azurol S agar plates were spotted with bacterial cultures (approximately 107 cfu mL−1) and incubated at 28 °C for 2–7 days. The siderophore production was indicated by the development of yellow–orange halo around the colonies.
Utilization of ACC in batch culture
The presence of 1-aminocyclopropane-1-carboxylate (ACC) deaminase enzyme was assayed according to the methodology described by Belimov et al. (2005), with few modifications. The bacterial strain was incubated for 12 days at 25 ± 2 °C in flasks containing a minimum saline medium (Glick 1995). The medium was supplemented with different nitrogen sources such as ACC 3 mM or (NH4)2SO4 15 mM. Bacterial growth was monitored every 48 h by measuring the OD 620 nm. The strain Pseudomonas putida aw4 was used as positive control.
Tryptophan-dependent auxin biosynthesis
Indole acetic acid (IAA)-like molecules production by bacterial strain was determined in the presence and absence of l-tryptophan, according to Glickmann and Dessaux (1995). The strain was grown in Luria–Bertani broth medium (Sambrook et al. 1989) for 72 h at 150 rpm. Then, 1 mL of each culture was centrifuged and supernatant was mixed with 2 mL Salkowsky reagent and incubated in darkness for 30 min at 130 rpm. When the development of pink color was observed, IAA-like molecules production was measured spectrophotometrically at 595 nm using IAA as standard. Azospirillum brasilense Cd strain was used as positive control.
Plant–bacteria interaction assays
Root elongation assay on filter paper
The plant root elongation-promoting (PREP) activity of bacterial isolate was determined according to Belimov et al. (2005). About 6 mL of bacterial suspension (105 cfu mL−1) or sterile water (uninoculated control) was added to sterile Petri dishes with filter paper. Bacterial suspensions and water were supplemented with 25, 100, and 500 mg L−1 of phenol (final concentrations). V. sativa seeds were surface-sterilized according to Ibáñez et al. (2012) and placed in each Petri dish (ten seeds per dish). The plates were covered by lid and incubated in a dark chamber at 25 ± 2 °C. The number of germinated seeds was counted after 7 days of treatment. Germination index (GI) and speed of germination index (S) were recorded according to Ahmed and Wardle (1994). The percentages of relative root elongation (E) and GI were calculated following standard methods (Barrena et al. 2009). These parameters were calculated using the Eqs (1), (2), and (3), respectively:
where N1, N2, N3,…, Nn are the proportions of seeds that germinate on day 1,2,3…n following the setup of the experiment; S varies from 100 (if all seeds germinate on the first day following the setup) to 0 (if no seeds have germinated by the end of the experiment)
Rhizoremediation of phenol in soil samples
The soil remediation ability of plant and bacteria interaction was tested using soil that has not been previously exposed to phenol and collected from an experimental area at National University of Río Cuarto. Their physicochemical properties are listed in Table 1. Soil samples, from a depth between 0 and 20 cm, were air-dried, passed through a 1-mm sieve, and sterilized in oven at 200 °C for 3 consecutive days. Between each cycle, soil samples remained at 25 °C for 24 h. Each plastic pot contained 140 mg of soil.
There were six treatments in this trial: soil with plants and without bacteria (T1 and T3), soil with bacteria and without plants (T2 and T4), and soil with plants and bacteria (T5 and T6). Soil without plants and bacteria was used as a control. Triplicates were set for each treatment and pots were randomized in a growing chamber at 25 ± 2 °C with a 12-h light/12-h dark regime. In T1, T3, T5, and T6, three disinfected and previously germinated (72 h) seeds of V. sativa were transferred to each pot and after 24 h, T2, T4, T5, and T6 were inoculated with 3 mL of Bacillus sp. (107 cfu mL−1). After 15 days of treatment, an aqueous solution of the pollutant (5,000 mg L−1) was individually added to treatments T3, T4, T6, and to control soil in order to reach a final concentration of 250 mg kg−1 of soil. The same volume of water was added to T1, T2, and T5 treatments. Soil samples were collected in a destructive fashion at time 0 (T0) and after 48 h of phenol addition, and stored at 4 °C for further analysis. Besides, at the same time, V. sativa plants were collected and roots were separated from aerial parts (shoot and leaves). Growth parameters (length and fresh weight of aerial parts) were determined and then samples were immediately frozen in liquid nitrogen. Frozen tissues were ground in liquid nitrogen with mortar and pestle and stored at −80 °C until extraction for the determination of antioxidative enzymes (POD, SOD, and APX), chlorophyll, H2O2, and MDA content.
Phenol extraction from soil samples
Phenol was extracted according to Hancock and Dean (1997). Briefly, 10 mL of methanol/water (60/40 v/v) was added to each gram of soil sample. The homogenate was sonicated 15 min, incubated on an orbital shaker (100 rpm, 1 h), and then centrifuged at 2,000 rpm 5 min. The obtained supernatant was filtered through a 0.45-μm Millipore filter. Phenol content in the filtrated supernatant was quantified by high-performance liquid chromatography (HPLC) (Hewlett Packard model 1100 pump, Palo Alto, CA, USA; Rheodyne manual injector with a 50 μl loop, Rheodyne, Cotati, CA, USA). Chromatographic separations were performed on a stainless steel, C18 reversed-phase column (150 mm × 4.6 mm i.d., 5 μm particle size; Luna-Phenomenex, Torrance, CA, USA). Phenol was detected by UV (Hewlett Packard model 1100 programmable UV detector, Palo Alto, CA, USA) at 270 nm (Masuda et al. 2001) and quantified by a data module Hewlett Packard Kayak XA (HP ChemStation Rev. A.06.01, Palo Alto, CA, USA). The mobile phase was methanol/water (50/50, v/v) at a flow rate of 1.0 ml min−1.
Results were expressed as residual phenol, which is defined as the ratio between the concentration of phenol removed and the initial concentration of phenol in soil samples (Coniglio et al. 2008).
Enzyme analyses
Total POD (EC1.11.1.7) activity was determined spectrophotometrically using o-dianisidine and H2O2 as substrates, according to the methodology described by Sosa Alderete et al. (2009). One unit of enzyme (U) was defined as the amount of enzyme, which generated 1 μmol of product in 1 min in the conditions described.
Superoxide dismutase (EC 1.15.1.1) activity was assayed by using the photochemical NBT method (Beauchamp and Fridovich 1973). One unit of SOD was defined as the quantity of enzyme required to inhibit the reduction of NBT by 50 %.
Total APX (EC1.11.1.6) activity was measured spectrophotometrically by monitoring the decline in A 290 as ascorbate (ε = 2.8 mM−1 cm−1) was oxidized, using the method of Hossain and Asada (1984).
Chlorophyll content
Chlorophyll “a” and “b” contents were determined using a colorimetric method using acetone as extraction solvent (Dere et al. 1998). The amount of chlorophyll “a” and “b” was calculated on the basis of mg of chlorophyll per gram of fresh tissue, according to the following formula (Lichtenthaler and Buschmann 2001):
Hydrogen peroxide
The hydrogen peroxide content was determined according to the method described by Sergiev et al. (1997).
MDA content
The extent of lipid peroxidation in terms of malondialdehyde (MDA) formation was determined following the method described by Heath and Packer (1968). The lipid peroxides were expressed as μmol MDA, by using an extinction coefficient of 155 mM−1 cm−1.
Statistical analysis
All dependent variables were subjected to the analysis of variance (ANOVA) without transformation. Treatment means of the untransformed data have been presented and were separated by Duncan′s multiple range tests at a probability level of P < 0.05 using the software STATISTICA (version 6.0).
Results and discussion
Identification of BAC S strain and sequence analysis of 16S rDNA
The bacterial strain was initially identified based on its colonial morphology on plates, its cellular appearance as viewed by light microscopy (Gram positive bacilli, 4–6 μm length), and the production of spores. Analysis of biochemical properties was performed according to standard protocols. The strain was nitrate positive, able to hydrolyze gelatin, and to use glucose as carbon source but not mannose, which are the characteristic criteria for Bacillus.
The nucleotide sequence of nearly full-length (1,228 bp approximately) 16S rDNA from isolate BAC S was determined and compared to sequences available in data banks. Phylogenetic 16S rDNA sequence analysis revealed that it was 99 % identical to that of Bacillus sp. and phylogenetic analysis revealed its closed relationship with B. cereus group (Fig. 1), which comprises species like B. thuringiensis, B. cereus, B. mycoides, and B. anthracis, among others. In this sense, it is well established that the majority of B. anthracis strains can be easily distinguished from other characterized strains of Bacillus cereus group by phenotypic characteristics (Vilas-Bôas et al. 2007). In this study, some biochemical traits of the strain BAC S like hemolytic activity, resistance to penicillin, and its motility (data not shown) suggested that it did not belong to B. anthracis species.
Tolerance test, phenol removal, and determination of phenol biodegradation pathway
BAC S strain could grow even at the highest concentration of the pollutant assayed (500 mg L−1) on TY plates. Moreover, BAC S could grow with phenol as sole carbon and energy source and could also remove it completely from liquid medium, as it is shown in Fig. 2. Besides, the time required to achieve this process increased with increasing concentrations of the contaminant. It is important to note that the bacterial strain, which was isolated from an area with high concentrations of inorganic pollutants, could remove similar phenol concentrations as microorganisms isolated from an area polluted with phenolic compounds (Bastos et al. 2000). Moreover, BAC S showed the ability to tolerate high concentrations of inorganic compounds and also phenol, which could be considered as a feature of great importance for bioremediation methodologies since many soils are contaminated with one or more metals, other inorganic compounds, radioactive material, or various organic compounds (Glick 2010).
Regarding phenol degradation, it is known that the cleavage of phenol aromatic ring involves two critical steps. These are, firstly, the monohydroxylation of the ring by a phenol hydroxylase at the ortho position of the preexisting hydroxyl group and this is followed by aromatic ring cleavage either by catechol 1,2-dioxygenase (C120), the ortho pathway, or catechol 2,3-dioxygenase (C230), the meta pathway (Tuan et al. 2011). The isolated bacterial strain showed catechol 1,2-dioxygenase activity (0.164 μmol min−1 mg−1 protein), while catechol 2,3-dioxygenase activity was not detected indicating that the oxidation of catechol takes place through ortho pathway.
Screening for phosphate solubilization ability, siderophore production, tryptophan-dependent auxin biosynthesis, and ACC deaminase activity
Enhancement of plant growth by members of Bacillus genera is well documented. In this sense, several mechanisms have been suggested for the phytoestimulatory activities of this group of plant growth-promoting bacteria (PGPB), including the production of phytohormones (like IAA), secretion of antibiotics, induction of systemic resistance, biocontrol activity, phytase activity (that contributes to plant growth promotion under phosphate limitation), ACC deaminase activity, production of siderophores, and phosphate solubilization (Ali et al. 2009 and references therein; Pereira et al. 2011).
Regarding the capabilities assayed in this study, the absence of halo around colonies indicated the inability of the bacterial strain BAC S to solubilize phosphate neither to produce siderophore. Besides, the bacterial strain did not grow in liquid medium with ACC as nitrogen source. On the other hand, Bacillus sp. produced 2 μg mL−1 of IAA in the presence of tryptophan, considered as a precursor of this phytohormone. In this sense, root exudates of plants are the natural source of tryptophan for rhizosphere microorganisms, which may enhance auxin biosynthesis within the rhizosphere that can induce a physiological response in the host plant (Ali et al. 2009). The majority of rhizospheric microorganisms that display beneficial effects on plant growth have been shown to produce IAA. In this sense, bacterial IAA showed an advantage to the rapid establishment of young seedlings roots, as it increases their ability to anchor themselves to the soil and to obtain water and nutrients from their environment, thus enhancing their chances for survival (Ali et al. 2009). In addition, the improved mineral and nutrient uptake and root exudation in turn stimulates bacterial proliferation on the roots (Glick 2010). Hence, the inoculation of various plant species with bacteria synthesizing IAA has resulted in increased root growth and/or enhanced formation of lateral roots and root hairs (Dimpka et al. 2009). This is an important property related with higher efficiencies in the removal process.
Plant–bacteria interaction assays
Root elongation assay on filter paper
As it was already mentioned, one strategy that overcomes some of the limitations of both conventional bioremediation and phytoremediation includes utilizing biodegradative bacteria together with plants (Glick 2010). Regarding phenol, the association of bacteria like Pseudomonas and Rhizobium has been reported to promote plant growth and also to increase the removal of the pollutant (Wang et al. 2011; Yang et al. 2011).
Since the beneficial effects of inoculation with plant growth-promoting root zone bacteria have been reported to be most significant under unfavorable conditions such as flooding, drought, metal toxicity (Belimov et al. 2005), or nutrient deficiency (Dimpka et al. 2009), we performed the PREP assay using different concentrations of the pollutant. As it is shown in Table 2, the highest concentration of the contaminant decreased significantly the speed of GI, of both inoculated and uninoculated seeds. Similar results were obtained for the relative root elongation (E) and GI. However, seeds inoculated with the bacterial strain showed higher values of these two parameters than uninoculated ones. This result could be related with the amount of IAA produced by Bacillus sp., which could have a beneficial effect over root growth under stressful conditions, like the presence of high phenol concentrations.
Rhizoremediation of phenol in soil samples
Although different biological and physicochemical treatments were developed to remediate polluted soils, frequently the best option resulted from the combination of them (Scullion 2006). Regarding biological treatments, the use of plants alone in bioremediation was successful but its combination with biodegradative and/or plant growth-promoting bacteria is particularly promising for the decontamination of polluted soils (Afzal et al. 2011).
Figure 3 showed the percentage of residual phenol after 48 h of treatment. T4 treatment (inoculated with Bacillus sp.) showed a decrease in phenol content, but it was not significantly different with respect to control soil (uninoculated and without plants). On the other hand, T6 treatment showed less than 40 % of the initial concentration of the pollutant, whereas T3 treatment had the minor amount of residual phenol (<20 %). According to the current knowledge, the fate of organic contaminants in the rhizosphere root system largely depends on their physicochemical properties. In this way, plants readily take up organics with a log K ow between 0.5 and 3.5. These compounds seem to enter the xylem faster than soil, and rhizosphere microflora can degrade them, even if the latter is enriched in degradative bacteria (Viehweger and Geipel 2010). Since phenol log K ow is 1.46, this value could explain the less amount of phenol determined in soils treated with V. sativa uninoculated plants. Besides, it is important to note the short time needed by this plant species to achieve phenol removal (48 h), considering that time is one of the challenges in a phytoremediation process. On the other hand, the presence of phenol (250 mg kg−1 of soil for 48 h) did not produce serious phytotoxic effects on common vetch plants, as it was observed by macroscopic analysis and by the growth parameters determined (Table 3).
Different enzymes belonging to the antioxidative defense system such as POD, SOD, and APX have important roles in protecting cells against deleterious effects caused by ROS, whose production and accumulation increased notably under environmental stress (Miller et al. 2010), like the presence of organic pollutants. In this work, an increase in total POD activity was observed in common vetch plants inoculated with Bacillus sp. (T5 and T6 treatments) compared to uninoculated plants (T1 and T3) (Fig. 4a). However, this difference was not observed after 48 h of treatment, neither in the presence of the contaminant nor in the presence of the bacterial strain. On the other hand, it is noteworthy the increase in POD activity of T3 after pollutant exposure, which was one of the treatment with an important decrease in the initial concentration of the pollutant. Furthermore, since POD is frequently cited as one of the main enzymes involved in phenol removal process, the increase in the activity of V. sativa uninoculated plants could be related with the decrease in the xenobiotic concentration. Regarding APX activity, common vetch uninoculated plants and treated with the contaminant (T3) showed a significant increase in this antioxidant enzyme after 48 h, with respect to T3 at the beginning of the treatment. Although it is well known that within cells, SODs constitute the first line of defense against ROS (Dinakar et al. 2012); in our study, we did not observe differences among the various treatments assayed or due to the presence of phenol (Fig. 4b). Similar results were previously obtained with V. sativa in vitro developed plantlets treated with 100 mg L−1 of phenol. These hydroponic cultures showed an increase in POD and APX activities, while SOD activity remained unaltered after 48 h of treatment (Ibáñez et al. 2012).
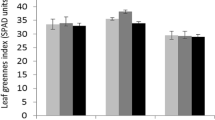
The metabolism associated with photosynthesis and respiration is particularly sensitive to free radical production under stressful conditions (Dinakar et al. 2012). Moreover, chlorophyll is the base of photosynthesis and a reduction in its content severely affect the photosynthetic capacity of the whole plant. Regarding this study, chlorophyll levels of plants treated with phenol (T3 and T6) were similar with those of control plants (T1 and T5) (Table 4). In this way, the results showed the absence of chlorosis in common vetch plants treated with phenol (250 mg kg−1 of soil for 48 h), which is related with a decrease in chlorophyll “a” concentrations due to iron deficiency or to a peroxidative breakdown of pigments (Viehweger and Geipel 2010).
Hydrogen peroxide is a versatile molecule involved in different cellular processes. Although H2O2 could act as a signaling molecule, high levels of it and other ROS (O2·−, OH−, etc.) may lead to unspecific oxidation of proteins and membrane lipids, resulting in an increased concentration of MDA. Thus, to estimate oxidative stress, MDA tissue content had been widely used as an indicator of lipid peroxidation and, thereby, of oxidative damage (Mediouni et al. 2009). Our results showed a decrease in H2O2 concentrations of roots in all the treatments while no changes were observed in the levels of this ROS in aerial parts. On the other hand, MDA content did not increase after 48 h of phenol treatment neither in roots nor in aerial parts of uninoculated/inoculated common vetch plants (Fig. 5). This result could be related with an efficient response of V. sativa antioxidant system, which could avoid deleterious effects caused by 48 h of exposure to phenol (250 mg kg−1 of soil). The results obtained in this study are in agreement with those previously obtained with hydroponic cultures of common vetch plants (Ibáñez et al. 2012). Moreover, this efficient response of the antioxidant system seems to be not dependent on the inoculation with a bacterial strain capable of using phenol as a sole carbon and energy source.
Conclusion
The complexity and heterogeneity of sites often polluted with multiple metals, metalloids, and organic compounds requires the design of integrated phytoremediation systems that combine different processes and approaches. In this sense, the results obtained in the present study could suggest a beneficial effect of the interaction between V. sativa and Bacillus sp. during germination stage and the first days of growth in the presence of high phenol concentrations. This interaction could be of great importance for common vetch plants development in environments highly polluted with this organic compound. Then, in latter stages of growth, common vetch could remove phenol efficiently with an adequate response of the antioxidative defense system.
References
Afzal M, Yousaf S, Reichenauer TG, Kuffner M, Sessitsch A (2011) Soil type affects plant colonization, activity and catabolic gene expression of inoculated bacterial strains during phytoremediation of diesel. J Hazard Mater 186:1568–1575
Ahmed M, Wardle D (1994) Allelopathic potential of vegetative and flowering ragwort (Senecio jacobea L.) plants against associated pasture species. Plant Soil 64:61–68
Ali B, Sabri AN, Ljung K, Hasnain S (2009) Quantification of indole-3-acetic acid from plant associated Bacillus spp. and their phytostimulatory effect on Vigna radiate (L.). World J Microbiol Biotechnol 25:519–526
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Gapped DJ (1997) BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv 27:84–93
Barrena R, Casals E, Colón J, Font X, Sánchez A, Puntes V (2009) Evaluation of the ecotoxicity of nanoparticles. Chemosphere 75:850–857
Bastos A, Moon D, Rossi A, Trevors J, Tsai S (2000) Salt-tolerant phenol-degrading microorganism isolated from Amazonian soil samples. Arch Microbiol 174:346–352
Beauchamp CO, Fridovich I (1973) Isozymes of SOD from wheat germ. Biochem Biophys Acta 317:50–54
Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR (2005) Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol Biochem 37:241–250
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carrasco López JA (2007) Rhizoremediation of soils polluted by heavy metals beyond Rhizobium-leguminosa symbiosis: analyses of copper contamination. PhD Thesis, University of Seville
Coniglio M, Busto V, González PS, Medina MI, Milrad S, Agostini E (2008) Application of Brassica napus hairy root cultures for phenol removal from aqueous solutions. Chemosphere 72:1035–1042
Dere S, Günes T, Sivaci R (1998) Spectrophotometric determination of chlorophyll A, B and total carotenoid contents of some algae species using different solvents. Turk J Bot 22:13–18
Dimpka C, Weinand T, Asch F (2009) Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ 32:1682–1694
Dinakar C, Djilianov D, Bartels D (2012) Photosynthesis in desiccation tolerant plant: energy metabolism and antioxidative stress defense. Plant Sci 182:29–41
Gerhardt K, Huang X, Glick B, Greenberg B (2009) Phytoremediation and rhizoremediation of organic soil contaminants: potential and challenges. Plant Sci 176:20–30
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41:109–117
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 3:367–374
Glickmann E, Dessaux Y (1995) A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61:793–796
Gong X, Chao L, Zhou M, Hong M, Luo L, Wang L, Ying W, Cai J, Songjie G, Hong F (2011) Oxidative damages of maize seedlings caused by exposure to a combination of potassium deficiency and salt stress. Plant Soil 340:443–452
Hall T (1999) Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hancock P, Dean J (1997) Extraction and fate of phenols in soils. Anal Commun 34:377–379
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hossain MA, Asada K (1984) Inactivation of ascorbate peroxidase in spinach chloroplasts on dark addition of hydrogen peroxide: its protection by ascorbate. Plant Cell Physiol 25:1285–1295
Ibáñez SG, Sosa Alderete LG, Medina MI, Agostini E (2012) Phytoremediation of phenol using V. sativa L. plants and its antioxidative response. Environ Sci Pollut Res 19:1555–1562
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. In: Wrolstad RE, Acree TE, An HJ et al (eds) Current protocols in food analytical chemistry. John Wiley & Sons, Inc, New York, pp F4.3.1–F4.3.8
Liu H, Weisman D, Ye Y, Cui B, Huang Y (2009) An oxidative stress response to polycyclic aromatic hydrocarbon exposure is rapid and complex in Arabidopsis thaliana. Plant Sci 176:375–382
Masuda M, Sakurai A, Sakakibara M (2001) Effect of reaction conditions on phenol removal by polymerization and precipitation using Coprinus cinereus peroxidase. Enzyme Microb Technol 28:295–300
Mediouni C, Ben Ammar W, Houlné G, Chabouté ME, Jemal F (2009) Cadmium and copper induction of oxidative stress and antioxidative response in tomato (Solanum lycopersicon) leaves. Plant Growth Regul 57:89–99
Metha S, Nautiyal C (2001) An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr Microbiol 43:51–56
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Muccifora S, Bellani LM (2013) Effects of copper on germination and reserve mobilization in Vicia sativa L. seeds. Environ Pollut 179:68–74
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15:473–479
Pereira P, Ibañez S, Agostini E, Etcheverry M (2011) Effects of maize inoculation with Fusarium verticillioides and with two bacterial biocontrol agents on seedlings growth and antioxidative enzymatic activities. Appl Soil Ecol 51:52–59
Pradhan N, Ingle AO (2007) Mineralization of phenol by a Serratia plymuthica strain G isolated from sludge simple. Int Biodeterior Biodegrad 60:103–108
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor, New York
Schwyn B, Neilands J (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Scullion J (2006) Remediating polluted soils. Naturwissenschaften 93:51–65
Segura A, Rodríguez-Conde S, Ramos C, Ramos JL (2009) Bacterial responses and interactions with plants during rhizoremediation. Microb Biotechnol 2:452–464
Sergiev I, Alexieva V, Karanov E (1997) Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Comput Rend Acad Bulg Sci 51:121–124
Singh RP, Dhania G, Sharma A, Jaiwal PK (2007) Biotechnological approaches to improve phytoremediation efficiency for environment contaminants. In: Singh SN, Tripathi RD (eds) Environmental bioremediation technologies. Springer, Berlín, pp 223–258
Sosa Alderete L, Talano M, Ibáñez S, Purro S, Agostini E, Milrad S, Medina MI (2009) Establishment of transgenic tobacco hairy roots expressing basic peroxidases and its application for phenol removal. J Biotechnol 139:273–279
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tuan NN, Hsieh H, Lin Y, Huang S (2011) Analysis of bacterial degradation pathways for long-chain alkylphenols involving phenol hydroxylase, alkylphenol monooxygenase and catechol dioxygenase genes. Bioresour Technol 102:4232–4240
Vangronsveld J, Herzig R, Weyens N, Boulet J, Adriaensen K, Ruttens A, Thewys T, Vassilev A, Meers E, Nehnevajova E, van der Lelie D, Mench M (2009) Phytoremediation of contaminated soils and groundwater: lessons from the field. Environ Sci Pollut Res Int 16:765–794
Viehweger K, Geipel G (2010) Uranium accumulation and tolerance in Arabidopsis halleri under native versus hypronic conditions. Environ Exp Bot 69:39–46
Vilas-Bôas GT, Peruca APS, Arantes OMN (2007) Biology and taxonomy of Bacillus cereus, Bacillus anthracis, and Bacillus thuringiensis. Can J Microbiol 53:673–687
Vincent JM (1970) A manual for the practical study of the root-nodule bacteria. Blackwell, Oxford
Wang Z, Xu Y, Zhao J, Fengmin L, Gao D, Xing B (2011) Remediation of petroleum contaminated soils through composting and rhizosphere degradation. J Hazard Mater 190:677–685
Wright H, Nicell JA (1999) Characterization of soybean peroxidase for the treatment of aqueous phenols. Bioresour Technol 70:69–79
Yang L, Wang Y, Song J, Zhao W, He X, Chen J, Xiao M (2011) Promotion of plant growth and in situ degradation of phenol by an engineered Pseudomonas fluorescens strain in different contaminated environments. Soil Biol Biochem 43:915–922
Acknowledgments
This work was supported by the grants from SECyT-UNRC, CONICET, Ministerio de Ciencia y Tecnología de la Provincia de Córdoba and PICTO FONCyT-SECyT-UNRC. SGI have a fellow from CONICET. LJM, GGB, and EA are members of the Research Career from CONICET (Argentina). We thank Mariela Woelke for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ibáñez, S.G., Merini, L.J., Barros, G.G. et al. Vicia sativa–rhizospheric bacteria interactions to improve phenol remediation. Int. J. Environ. Sci. Technol. 11, 1679–1690 (2014). https://doi.org/10.1007/s13762-013-0357-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-013-0357-2