Abstract
Background and aim
Phosphorus (P) addition is a common practice to alleviate P limitation in agricultural ecosystems. However, information regarding microbial communities’ response to P addition in grasslands remains limited. The present study aimed to investigate the response of bacterial communities from rhizosphere and root endosphere of Achnatherum inebrians to P addition, and assess the potential roles played by mutualistic endophyte Epichloë gansuensis in these processes.
Methods
The response of bacterial communities to P addition was investigated based on 16S rRNA sequencing. Soil properties were determined and Mantel test was employed to evaluate the main factors contributing to bacterial community alteration. Additionally, the root exudates were assessed by GC-MS.
Results
P addition influenced the bacterial community composition in both the rhizosphere and root endosphere of A. inebrians with (E+) or without (E−) E. gansuensis, while not affecting community diversity. Moreover, P addition increased the soil available P, total P, and pH levels, which exhibited significant correlation with the bacterial communities in both rhizosphere and root endosphere of A. inebrians. Additionally, P addition increased the exudation of xylose, glycine, alanine, mandelic acid, and lactic acid from E+ plant roots.
Conclusions
P addition shapes the bacterial communities in rhizosphere and root endosphere of A. inebrians by altering soil total P, available P, pH levels. Meanwhile, P addition modulates the root exudate profiles that mediated by E. gansuensis. This study provides new insights into the response of plant-soil-microbe ecosystem to P addition, which is helpful for the fertilization management during grassland sustainability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is an essential macronutrient and plays a pivotal role in almost all major physiological processes in plants, including photosynthesis, respiration, signal transduction, as well as the biosynthesis of ATP, nucleic acids, proteins, and phospholipids (Plaxton and Tran 2011; Tiziani et al. 2020). Inorganic orthophosphate (Pi), the primary form of P assimilated by plants, is one of the limiting factors that greatly affect plant growth and crop yields due to its poor mobility and low concentration in soil (Aslam et al. 2022). Meanwhile, limitation on biomass production caused by soil P deficiency would be aggravated because of the continuous increase in atmospheric nitrogen (N) deposition and N fertilizer utilization in the future (Li et al. 2016; Penuelas et al. 2013). Addition of Pi-containing fertilizers is a common practice to alleviate P limitation in farmland and grassland ecosystems (Bennett and Adams 2001; Cheng et al. 2022; Sarathchandra et al. 2001). Furthermore, improving P availability may be an effective management option for the restoration of degraded grasslands (Armitage et al. 2012; Reed et al. 2007). However, a large proportion of Pi that used as fertilizer is rapidly fixed by soil particle adsorption and chemical precipitation, leading to a decrease in Pi assimilation by plants and increasing the risk of environmental pollution (Richardson et al. 2001).
P addition also has a crucial impact on the soil microbial communities which play vital roles in regulating nutrient cycling by accelerating the decomposition of soil organic matter in terrestrial ecosystems (Ling et al. 2017). However, previous studies have shown that the responses of soil microbial communities to P addition are inconsistent, which may be related to multiple factors including soil conditions, plant species, P fertilizer types, and fertilizer application period (Huang et al. 2016; Lagos et al. 2016; Shi et al. 2020). It has been reported that P addition exerts positive, neutral, and even negative influence on the soil microbes in the temperate forests (Groffman and Fisk 2011; Thirukkumaran and Parkinson 2000, 2002). Similar results have also been observed in soil microbial communities of grasslands when exposed to P fertilizer application. Chen et al. (2014) reported that P input led to a shift from fungal-dominated to bacterial-dominated decomposition pathways in the P-limited and grazed grassland. While, Yan et al. (2021) found that P addition significantly altered the composition of soil fungal and bacterial communities in the temperate meadow, with decreasing the bacterial operational taxonomic unit (OTU) richness and increasing the fungal OTU richness. Additionally, other studies suggested that P fertilization did not significantly affect the soil microbial biomass or community structure in grasslands (Massey et al. 2016; Sarathchandra et al. 2001; Shi et al. 2020). These inconsistent results suggest that more trials should be carried out to determine the effects of P addition on soil microbial communities in different grassland types.
Plant-associated microbial communities play vital roles in plant growth and fitness via several mechanisms, such as stimulating growth, facilitating nutrient uptake, and conferring resistance to abiotic and biotic stress (Trivedi et al. 2020). In particular, some rhizosphere microorganisms are involved in enhancing the availability of essential nutrients (e.g., phosphorus, nitrogen, and sulfur) for plants. In turn, rhizosphere microorganisms acquire their primary carbon source from the exudates and deposits of plant roots (Brisson et al. 2022; Mimmo et al. 2018). The root exudates could drive the recruitment of beneficial microorganisms to the rhizosphere, which provides a crucial basis for the maintenance of distinct microbial community patterns required by host plants under specific environmental conditions (Trivedi et al. 2020; Zhalnina et al. 2018). Plants growing in the nutrient-deficient soils employ root exudates in multiple ways, for instance, serving as symbiotic signals and growth promoters of some soil microorganisms that involved in nutrient acquisition (Dakora and Phillips 2002). Chen et al. (2019a) reported that some bacterial genera, such as Arthrobacter, Bacillus, and Devosia, in the rhizosphere of wheat varied in response to the increased N input, which was significantly correlated with the organic acid levels secreted by roots. However, the effect of elevated P concentrations on the root exudate profiles of plants is still obscure.
Achnatherum inebrians is a perennial, cool-season grass species that can be found throughout the semi-arid grasslands in Northwest China (Li et al. 2004; Wang et al. 2020). In natural conditions, nearly all of A. inebrians are infected with the mutualistic seed-borne Epichloë endophytes which colonize all plant tissues with exception of the roots (Hou et al. 2021). Extensive research has suggested that the infection of Epichloë sp. enhances the resistance of host A. inebrians to various abiotic and biotic stresses, thereby facilitating the adaptation of this fungal symbiont to degraded grasslands (Chen et al. 2015; Hou et al. 2020b; Wang et al. 2020, 2022). In our previous study, it was demonstrated that Epichloë gansuensis improved A. inebrians growth under low-P condition by modulating the organic acid contents and increasing the phosphorus utilization efficiency (Liu et al. 2021). Additionally, the presence of E. gansuensis also could affect the rhizosphere microbial communities of A. inebrians with increasing the Shannon diversity of bacteria and the spore diversity of arbuscular mycorrhizal fungi (Hou et al. 2020b; Ju et al. 2020). Recently, Jin et al. (2022) suggested that E. gansuensis increased the secretion of organic acids and amino acids from host roots when exposed to Cd stress, and these exudates might recruit distinct rhizosphere bacterial communities to improve the resistance of hosts against Cd toxicity.
In the present study, we investigated the influence of P addition on soil properties, root exudates, and rhizosphere and root endosphere bacterial communities in E. gansuensis-infected (E+) and E. gansuensis-free (E−) A. inebrians. The objectives of this study were to address the following questions: (i) How does P addition influence the bacterial communities in rhizosphere and root endosphere of E+ and E− A. inebrians? (ii) How does P addition affect soil properties and root exudates of A. inebrians, and among these soil property indices, which would be the major factors that are strongly correlated with the bacterial communities of rhizosphere and root endosphere? and (iii) what potential roles might E. gansuensis play in these processes described above. We hypothesize that P addition could shape the bacterial communities in both rhizosphere and root endosphere of A. inebrians by affecting soil properties. Additionally, P addition could modulate the exudate profiles of A. inebrians roots, which may potentially be associated with E. gansuensis-mediated regulation. This study attempts to provide novel insights into the E. gansuensis-mediated adaptation of A. inebrians to P addition at the microbial level. These new findings may contribute to providing scientific management and guidance for the sustainable development of grasslands.
Materials and methods
Plant growth and P addition treatment
Seeds of Achnatherum inebrians with (E+) or without Epichloë gansuensis (E−) were obtained as described by Hou et al. (2020b). First, E+ and E− seeds were cleansed with 75% ethanol (5 min) followed by 1% NaClO (10 min) for surface sterilization, then washed with sterile distilled water for 6 times. After disinfection, E+ and E− A. inebrians seeds were planted in 12 pots with 6 seeds per pot, respectively. The size of the pot is 10 cm in the bottom diameter, 18.5 cm in the upper diameter, and 19.5 cm in height. Each pot was filled with sifted soil (2400 g, sampled from 10 to 30 cm of the profile and sieved through a 2 mm mesh) that collected from a fallow field located in Yuzhong campus, Lanzhou university (Yuzhong county, Gansu province, China). A week after germination, only three well-grown A. inebrians seedlings with same size were kept in each pot to avoid competition for space during growth, and plants were irrigated with 200 mL of distilled water once a week before P addition treatment. After one month of growth, six pots with E+ seedlings were treated with 200 mL of KH2PO4 solution (30 mM), and another six E+ pots were treated with 200 mL of distilled water as the control groups. The same procedure was repeated for E− pots. All samples were treated once a week for an 8-week period. After phosphate input, all A. inebrians plants continued to grow in the greenhouse for ten months, and during this period, each pot was watered with 200 mL distilled water once a week. Next, rhizosphere soil samples and roots of E+ and E− A. inebrians were collected for bacterial community analysis.
Rhizosphere soil collection and root sample preparation
First, the roots of three individual A. inebrians from one pot were mixed into a composite, and the bulk soils from roots were shaken off as much as possible. Then methods described by Tang et al. (2022) and McPherson et al. (2018) with a minor modification were used to collect rhizosphere soil samples and to sterilize the root surfaces for bacterial DNA extraction from the root endosphere. Briefly, roots without bulk soils were trimmed into 7–8 cm in length with a sterile scissor, and then transferred to a 50 mL sterile centrifuge tube containing 35 mL of autoclaved phosphate-buffered saline (PBS; NaCl 137 mM, KCl 2.7 mM, Na2HPO4 4.3 mM, KH2PO4 1.4 mM, pH 7.4). The rhizosphere soil samples were released by shaking the PBS solution for 2 min. Then the roots were transferred to a new centrifuge tube and placed on ice. The rhizosphere soil samples were resuspended by shaking, followed by centrifugation with 3000 g for 5 min at room temperature. The supernatant was discarded, and 1.5 mL of PBS solution was added to the pellet then vortexed. Immediately, the resuspended solution was transferred into a new 2 mL sterile Eppendorf tube and centrifuged at 12,000 g for 2 min at room temperature, followed by moving out the supernatant as much as possible. The rhizosphere soil samples were stored at −80 °C before DNA extraction.
Before the extraction of bacterial genome DNA from the root endosphere, the root surface was sterilized. In brief, the roots were first submerged in 35 mL disinfectant solution containing 50% bleach (available ClO−: 10%) with 0.01% Tween 20 in a 50 mL tube, and the tubes were shaken for 60 s. Then the disinfectant solution was replaced by 35 mL of 75% ethanol, and the tubes were shaken for another one minute, followed by rinsing the roots for five times with autoclaved ddH2O. The sterilized root samples were cut into 5 mm pieces and stored at −80 °C before DNA extraction. In addition, the rinsed water of the last wash was used to test the availability of root surface disinfection via plate incubation experiment and PCR analysis, and no bacterial growth or PCR products were detected.
DNA extraction, 16S rRNA sequencing and data analysis
Genomic DNA was extracted from 0.35 g rhizosphere soil sample and 0.5 g root sample using the MP BIO FASTDNA SPIN KIT (MP Bio, CA, USA) according to the manufacturer’s instructions. The purified DNA was quantified using NanoDrop 2000 (ThermoFisher scientific, MA, USA). The V5V7 region of the bacterial 16S rRNA gene was amplified using the 799F/1193R primer pairs (Zgadzaj et al. 2016), then PCR products were assessed by agarose gel electrophoresis before library construction. After the construction of sequencing library using the TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, CA, USA), the libraries were sequenced with the Illumina NovaSeq 6000 SP PE250 platform at Genesky Biotechnologies Co, Ltd. (Shanghai, China). After Illumina sequencing, the adaptor and primer sequences were removed using cutadapt plug-in of QIIME2 software (Bolyen et al. 2019), then we obtained the clean reads. The dada2 plug-in of QIIME2 software was used to denoise, splice the double-ended sequences, and remove the chimeric sequence (Callahan et al. 2016), eventually obtaining the valid data (Non-chimeric Reads).
Analyses of soil properties
Soil pH in moist samples (1:2.5 ratio of soil to ddH2O) was measured using a Sartorius PB-10 pH meter. The soil organic carbon (SOC) and available phosphorus were analyzed using method of Da and Sommers (1983). The total phosphorus (TP), total nitrogen (TN), ammonium N (NH4+-N; AN), and nitrate N (NO3−-N; NN) in soils were estimated by the method of Zhao et al. (2014). The activities of soil invertase, soil urease, and alkaline phosphatase were determined using the method of Hou et al. (2020a). The soil catalase activity was measured with the method of Guwy et al. (1999).
Root exudate extraction and determination
The extraction and determination of root exudates were performed mainly according to the method described by Jin et al. (2022) with a minor modification. In brief, the surface-sterilized seeds of E+ and E− A. inebrians were sown in a tray containing sterilized vermiculite with enough distilled water. After seed germination, seedlings were watered once a week with 400 mL of 1/2 Hoagland solution and 400 mL distilled water. After 35 days of growth under controlled conditions in an artificial light incubator (16 h light/8 h dark; 60% humidity; 25 °C; 90 µE·m−2·s−1), the plants were carefully pulled out, and then the vermiculite was washed off from the roots using tap water. Next, A. inebrians plants were transferred to a 50 mL sterile centrifuge tube, and plant roots were immersed in 30 mL of 1/2 Hoagland solution for 24 h to enhance the adaptation of plants to the hydroponic condition. Then, E+ and E− plants were treated with autoclaved 1/2 Hoagland solution with or without 30 mM KH2PO4 for four days, and the nutrient solution was renewed every day. The roots of E+ and E− plants were washed with sterile water, and transferred into the opaque conical bottles containing 20 mL of sterile water for the collection of root exudates. The roots were allowed to grow for another three days to release the root exudates into the water, and bottles were kept on shaking at 50 rpm for 2 h every day to increase dissolved oxygen levels in the water. Finally, root exudate solution was collected and freeze-dried, followed by analysis with GC-MS at BioTree Biomedical Technology Co, Ltd. (Shanghai, China). And the detailed procedure for GC-MS analysis was described by Bao et al. (2021).
Statistical analysis
The one-way analysis of variance (ANOVA) was employed to evaluate the effect of P addition and endophytic fungi on the soil nutrients and soil enzyme activities, and the level of statistically significant difference is P < 0.05. R (4.1.3 version) was used to determine the Shannon index, Principal coordinate analysis (PCoA), relative abundance of bacterial taxa at phylum and genus levels, co-network of soil bacterial communities at phylum level, redundancy analysis, heatmap, Spearman’s rank correlation coefficients, and Mantel’s test. Linear discriminant analysis (LDA) effect size (LEfSe) is an efficient algorithm for high-dimensional data comparisons and often used to detect the feature taxa with significantly different abundances as well as to evaluate the effect sizes by the LDA scores (Yang et al. 2019). LEfSe analysis was performed using the OmicStudio tools (http://www.omicstudio.cn/tool). Additionally, SIMCA-P 14.0 software was used to perform the principal component analysis (PCA) of root exudates. The criterion for significantly differential root exudates is VIP > 1 and P < 0.05. And metabolic pathways enriched by differential exudates were performed by MetaboAnalyst5.0 (https://www.metaboanalyst.ca/).
Results
The effect of E. gansuensis and P addition on the bacterial community diversity in rhizosphere and root endosphere
In this study, the 16S rRNA gene amplicon was firstly characterized with Illumina sequencing and then used to analyze the diversity and composition of bacterial communities in both roots and rhizosphere of A. inebrians after P addition. As shown in Fig. 1A, the infection of E. gansuensis significantly increased the Shannon index of the bacterial communities in the rhizosphere soil without P addition in A. inebrians. However, P addition eliminated this marked difference that identified in rhizosphere between E+ and E− plants, indicating that bacterial composition was influenced by the fertilization of phosphorus (Fig. 1A). In contrast, neither P addition nor E. gansuensis exhibited a significant effect on the Shannon index of bacterial communities in the root endosphere of A. inebrians (Fig. 1B). Principal coordinate analysis (PCoA) based on Bray-Curtis dissimilarity metrics showed a clear distance in the rhizosphere bacterial communities between E+ and E− A. inebrians under different P-input conditions (Fig. 1C). In contrast, no distinct separation was found in the bacterial communities of roots (Fig. 1D), and these results were further confirmed by the Adonis test as shown in Table 1. These data suggest that, instead of the root endophyte, the diversity of rhizosphere bacteria in A. inebrians was mainly influenced by P addition.
Response of bacterial communities in rhizosphere and roots of A. inebrians to P addition. Shannon index in rhizosphere (A) and root (B) bacterial communities; PCoA analysis of bacterial communities in rhizosphere (C) and roots (D). Different letters represent significant differences (ANOVA; P < 0.05). Values derive from five biological replicates
Bacterial community composition revealed by the 16S rRNA sequencing
All of the identified amplicon sequence variants (ASVs) were classified into 18 phyla and 45 genera within the rhizosphere and endophyte bacterial communities. Among these groups, the majority of ASVs belonged to phyla Proteobacteria, Bacteroidetes, and Actinobacteria (Fig. 2A), which accounted for more than 79.1% of the total bacterial sequences in rhizosphere as well as 97.1% in root endophytes. We noticed that P addition increased the relative abundance of Firmicutes in rhizosphere of both E+ (from 2.85 to 5.16%) and E− (from 3.38 to 9.04%) A. inebrians (Fig. 2A) and decreased the relative abundance of Acidobacteria in rhizosphere of E+ plants (from 3.27 to 2.27%; Fig. 2A). In the root endosphere of A. inebrians, the Actinobacteria relative abundance was increased by P addition, but it was decreased by the infection of E. gansuensis under both 0 (from 17.96 to 9.21%) and 30 mM (from 28.98 to 15.09%) P-input conditions (Fig. 2A). Additionally, comparative analysis suggested that rhizosphere bacteria showed a higher abundance of the genera Arthrobacter, Gemmatimonas, and Sphingomonas (Fig. 2B). In contrast, Niastella, Ohtaekwangia, and Ralstonia were the dominant genera that found in endophytic bacteria (Fig. 2B).
The composition of bacterial communities and LEfSe analysis. The relative abundance of bacterial communities at phylum level (A) and genus level (B) in rhizosphere and root endosphere of E+ and E− A. inebrians with different P additions. LEfSe analysis exhibiting the significant difference in rhizosphere bacteria of E+ plants under different P-input conditions (C), in rhizosphere bacteria under P addition in response to E. gansuensis (D), in root endophyte bacteria of E+ plants under different P addition (E), and in root endophyte bacteria with P addition in response to E. gansuensis (F). The threshold for taxa with significant difference is logarithmic discriminant analysis (LDA) score > 3 and P < 0.05
Next, the LEfSe analysis was used to exhibit the phyla and genera with significantly different abundance caused by P addition and infection of E. gansuensis. As shown in Fig. 2C, P addition significantly increased the abundance of Bacteroidetes and Firmicutes phyla, and Chryseobacterium, Bacillus genera, but significantly decreased the Chloroflexi and Acidobacteria phyla, along with Phenylobacterium and Sphingomonas genera in the rhizosphere of E+ A. inebrians. Under the P-input condition, the infection of E. gansuensis significantly increased the abundance of genera Pseudomonas, Arenimonas, and depressed phylum Firmicutes as well as genera Streptomyces, Ensifer in the rhizosphere (Fig. 2D). Additionally, P input increased the abundance of the genera Streptomyces and Flavitalea but decreased the Neorhizobium genus in E+ root endosphere (Fig. 2E). Meanwhile, the infection of E. gansuensis markedly induced the abundance of genera Pseudomonas and Fluviicola, but decreased the phylum Actinobacteria as well as the genera Lentzea and Rhodomicrobium in the root endosphere that treated with 30 mM P addition (Fig. 2F). Furthermore, the interspecific associations within bacterial communities were investigated by the co-occurrence network analysis. P addition decreased the number of total nodes, total edges, average degree, and density in rhizosphere networks of both E+ and E− A. inebrians plants, indicating the increased P fertilizer might low the complexity of rhizosphere bacterial community structure (Fig. S1 and Table S1). For the endophyte network, the infection of E. gansuensis increased the number of total edges, average degree, and density and centralization under different P-input conditions, suggesting E. gansuensis enhanced the connectedness of the bacterial community structure in root endosphere (Fig. S2 and Table S1).
Response of soil properties to P addition
Compared to the control, P addition significantly increased total P (TP), available P (AP), and pH levels (Fig. 3B, E, and G), but depressed the activity of catalase (Fig. 3K) in soils with both E+ and E− A. inebrians. In contrast, soil total N (TN), and activities of invertase, urease, and phosphatase were not significantly altered by P addition (Fig. 3A, H, I, and J). Intriguingly, E. gansuensis played opposite effects on the content of NO3−-N (NN) and NH4+-N (AN) after P fertilizer application, with significantly increasing NN while decreasing AN (Fig. 3C and D). Additionally, P input significantly induced the SOC in soil with E+ A. inebrians, and meanwhile, the infection of E. gansuensis markedly promoted the increase of SOC under P-input condition (Fig. 3F).
Soil properties regulated by P addition and E. gansuensis. The content of total N (TN, A), total P (TP, B), NO3−-N (NN, C), NH4+-N (AN, D), available P (AP, E), Soil Organic Carbon (SOC, F), pH (G), invertase (H), urease (I), phosphatase (J), and catalase (K) in soil with E+ and E− plants at 0 and 30 mM P additions
Relationship between bacterial community composition and soil properties
Pearson’s test suggested that AP was positively correlated with TP (P < 0.001) and pH (P < 0.001) (Fig. 4). Meanwhile, pH was positively correlated with TP (P < 0.001). It should be noted that catalase was negatively correlated with TP (P < 0.001), AP (P < 0.001), and pH (P < 0.001). The Mantel test indicated that TP, AP, and pH were strongly related to bacterial community composition in both rhizosphere and root endosphere, thus confirming our hypothesis. Additionally, AN was specifically related to the composition of rhizosphere bacteria with P < 0.01 (Fig. 4).
Furthermore, Spearman’s Rank correlation coefficient indicated that Acidobacteria and Chloroflexi were negatively correlated to SOC; Acidobacteria and Proteobacteria were positively correlated to AN and catalase in rhizosphere soil, respectively (Fig. 5A). The AP and pH exhibited similar correlation to the main bacterial phyla, showing significantly negative correlation with the Acidobacteria and Proteobacteria, and positive correlation with Actinobacteria, Firmicutes, and Nitrospirae in rhizosphere soil (Fig. 5A). Additionally, at the level of endophytic bacteria, Acidobacteria was negatively correlated to TN; Chlamydiae was positively correlated to the pH; Candidatus_Saccharibacteria and Planctomycetes were positively correlated to invertase (Fig. 5B). Meanwhile, Actinobacteria within endophytes was negatively correlated to NN, urease, and catalase (Fig. 5B).
Redundancy analysis (RDA) was used to further assess the correlation between soil properties and bacterial community composition. The results revealed that AP, TP, and pH exhibited positive correlations with phyla Verrucomicrobia, Actinobacteria, Firmicutes, and Nitrospirae in rhizosphere, and with phyla Candidatus_Saccharibacteria, Actinobacteria, Chlamydiae, Gemmatimonadetes, and Spirochaetes in root endosphere, respectively (Fig. S3A and S3C). Particularly, AP content (P = 0.011) and pH (P = 0.007) were the two major factors that influenced the bacterial community composition, explaining 24.22% and 25.26% of the variation, respectively (Table S2). The NN and AN were mainly correlated with phyla Planctomycetes, Proteobacteria, Acidobacteria, and Gemmatimonadetes in rhizosphere (Fig. S3A, Table S2). Moreover, catalase (P = 0.047) was identified as another factor that significantly correlated with the differences in the composition of rhizosphere bacterial communities, accounting for 42.04% of the variation (Fig. S3B, Table S2). The activity of urease was positively correlated with phyla Proteobacteria and Acidobacteria in root endosphere, explaining 35.91% of the variation with P = 0.026 (Fig. S3D, Table S2).
Response of root exudate profiles to the infection of E. gansuensis under P input condition
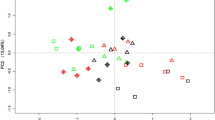
Root exudates of A. inebrians in response to the infection of E. gansuensis and P addition were investigated. Principal component analysis (PCA) suggested that P addition separated the root exudates of A. inebrians along the PC1 axis (Fig. 6A). Furthermore, root exudates of E+ and E− A. inebrians showed a distinct separation under P addition, indicating that E. gansuensis played important roles in regulating host root exudation (Fig. 6A). 30 mM P significantly altered 8 exudate metabolites in E+ roots with increasing xylose, lactic acid, glycine, 24,25-dihydrolanosterol, mandelic acid, and alanine but decreasing 3-hydroxynorvaline and sucrose (Fig. 6B). Meanwhile, pathway enrichment analysis indicated that these differentially expressed metabolites were mainly involved in glycine, serine and threonine metabolism, glyoxylate and dicarboxylate metabolism, as well as starch and sucrose metabolism (Fig. 6E). Additionally, after the infection of E. gansuensis, 8 differentially expressed exudates involved in vitamin B6 metabolism, sulfur metabolism, and TCA cycle were identified in roots under P-input condition (Fig. 6C and 6F). Intriguingly, we found that lactic acid, one of the up-regulated metabolites, was overlapped among these significantly altered exudates identified in both “30RE+ vs. 0RE+” and “30RE+ vs. 30RE−” comparisons, suggesting that E. gansuensis might play a pivotal role in promoting the P-induced secretion of lactic acid in roots of A. inebrians (Fig. 6D).
The effects of E. gansuensis and P addition on root exudates of A. inebrians. Principal component analysis (PCA) of root exudate profiles between E+ and E− A. inebrians at 0 and 30 mM P additions (A). The differential root exudates were identified between 30RE+ and 0RE+ (B) and between 30RE+ and 30RE− (C), respectively. Venn diagram analysis showing the differential root exudates between 30RE+ vs. 0RE+ and 30RE+ vs. 30RE− (D). KEGG pathway enrichment analysis of differential root exudates between 30RE+ and 0RE+ (E), and between 30RE+ and 30RE− (F). 30RE+ and 30RE− represent root exudates of E. gansuensis-infected and E. gansuensis-free A. inebrians at 30 mM P addition, respectively. 0RE+ represents root exudates of E. gansuensis-infected A. inebrians at 0 mM P addition
Discussion
In this study, new insights were presented to evaluate the effects of short-term P addition on rhizosphere and endophyte bacterial communities, soil properties, and root exudates of A. inebrians with or without E. gansuensis. P deficiency promotes the establishment of a symbiotic relationship between plant roots and mycorrhizal fungi (Alzate Zuluaga et al. 2021). It has been suggested that Neotyphodium coenophialum AR542E+ and N. coenophialum AR584E+ exhibited positive effects on general fungi in the tall fescue rhizosphere under conditions of lesser P availability (Ding et al. 2021). In contrast, it was found that P addition had a limited effect on the rhizosphere bacterial diversity (Fig. 1A), which was in agreement with the previous investigation showing that bacterial community α-diversity (Simpson and Shannon index) and richness (ACE and Chao1 index) in rhizosphere of the P-efficient soybean cultivars were not altered by the P fertilization (Tian et al. 2020). Furthermore, Shi et al. (2020) revealed that short-term P addition did not significantly affect the structure of soil microbial communities in timothy swards. On the other hand, some studies suggested the P availability is the key limiting factor for the soil bacterial communities in the grassland ecosystems, and these varied responses to P input might be attributed to soil types, fertilization frequencies, and land-use categories (Dong et al. 2020; Rooney and Clipson 2009; Wu et al. 2022; Yan et al. 2021). In the present study, the presence of E. gansuensis increased the Shannon index of rhizosphere bacteria in soil without P addition, which was consistent with the previous findings described by Hou et al. (2020b). The increased diversity of rhizosphere microbial communities could enhance plant nutrient acquisition, growth, and root health, thus improving the competitiveness of Epichloë fungus-host grass symbiont in natural habitats (Wang et al. 2020; Zhao et al. 2021).
The dominance of Actinobacteria, Proteobacteria, Bacteroidetes, and Firmicutes phyla is consistent with the distribution patterns of major bacterial taxa that found across a wide range of bulk and rhizosphere soils (Lauber et al. 2009; Widdig et al. 2019). As one of the most general phyla observed in the terrestrial ecosystems, Proteobacteria is a copiotrophic taxon and is mainly characterized by a rapid growth rate, response to labile C and P sources, and adapting to diverse rhizosphere conditions (Lagos et al. 2016). The negative correlation between Proteobacteria and pH has also been reported (Kim et al. 2016). Soil P status influences the incidence and diversity of soil bacteria that involved in P cycling (Ikoyi et al. 2018; Mander et al. 2012; Tan et al. 2012; Widdig et al. 2019). Our results showed that the relative abundance of Firmicutes was increased in response to the P addition in rhizosphere of A. inebrians with or without E. gansuensis, which was in accordance with the finding described in a long-term P input trial in the pasture soils (Tan et al. 2012). Firmicutes has a higher carbon mineralization rate and is regarded as the copiotrophic microbe with higher C availability in soil management (Fierer et al. 2007; Hegyi et al. 2021). The abundance of Acidobacteria in E+ rhizosphere was decreased due to P addition (Fig. 2A). This shift might be attributed to the higher soil pH in E+ A. inebrians since the abundance of Acidobacteria showed a strong negative correlation with pH value (Jones et al. 2009), and the similar conclusion was also observed in our RDA analysis (Fig. S3A) and Spearman’s rank correlation (Fig. 5A). It has been suggested that Acidobacteria is associated with the rhizosphere and helpful to increase the root nodulation and improve N mineralization in soil (Bulgarelli et al. 2013).
P addition increases the soil available P contents, which may decrease the number of phosphate solubilizing bacteria (PSB) in the soil (Tan et al. 2012; Yan et al. 2021). Bacillus and Pseudomonas are considered as the primary and efficient P-solubilizing bacteria in the terrestrial ecosystems (Chen et al. 2019b; Hu et al. 2018; Tian et al. 2020). However, P addition increased the relative abundance of Bacillus in E+ A. inebrians rhizosphere (Fig. 2C). A possible reason for the above divergence might be that Bacillus also plays important roles in the mobilization of other minerals (e.g., Fe and Ca), which drives the competition of this PSB in a high P-availability conditions (Ollinger et al. 2006; Tahir et al. 2019; Widdig et al. 2019). Interestingly, the soil with P addition showed a higher relative abundance of Pseudomonas in E+ rhizosphere, while a higher relative abundance of Bacillus was detected in E− A. inebrians rhizosphere (Fig. 2D), indicating that E. gansuensis might act as a factor in the selection of the rhizosphere PSB under P input condition.
Soil pH is widely considered as a major factor in shaping the structure of soil microbial communities (Aciego Pietri and Brookes 2009). In this study, P addition significantly increased the soil pH (Fig. 3G), and consistent results have been reported in other P addition experiments (Rooney and Clipson 2009; Shi et al. 2020). Additionally, our results also suggested that pH exhibited marked relationship not only with rhizosphere bacteria but also with endophytic bacteria in A. inebrians. The endophytic bacteria are considered to mainly originate from the rhizosphere, and soil pH is expected to play important roles in influencing the structure of endophytic bacterial communities by altering the rhizosphere bacterial communities as the intracellular pH is relatively stable in plants (Papik et al. 2020). In addition to the soil pH, N and P fertilization also altered the soil bacterial communities by influencing the nutrient availability (e.g., SOC, AP) (Ling et al. 2017; Ramirez et al. 2010). As expected, P addition increased the total P and available P content, which was in line with the previous findings (Huang et al. 2016; Ling et al. 2017). Increase in P availability could stimulate plant growth, shift the C allocation between aboveground and belowground tissues, and increase the litter inputs into soil, which consequently influence the microbial communities (Li et al. 2016; Wu et al. 2022). SOC supports a variety of soil functions, such as enhancing nutrient availability and retainment, promoting soil biological diversities and activities (Hoffland et al. 2020). It has been studied that the infection of N. coenophialum leads to a positive ecological consequence with increasing the total SOC due to the greater dry matter production of host tall fescue or the altered soil microbial dynamics that are responsible for the lower potential C mineralization (Buyer et al. 2010). Our results showed that SOC was specifically increased in E+ A. inebrians plots after P input (Fig. 3F), indicating the vital roles of E. gansuensis in improving soil properties. Furthermore, consistent with the previous studies, the oligotrophic phylum Chloroflexi exhibited a negative correlation with SOC (Fig. 5A), suggesting a potential balance of soil carbon dynamics mediated by this bacterial taxon (Goldfarb et al. 2011; Ren et al. 2018).
As much as 80% of organic P can be transformed to biological availability of P by the soil phosphatases (Sinsabaugh and Follstad Shah 2012). It was suggested that P addition could inhibit soil phosphatase activities in diverse terrestrial ecosystems, including forests, grasslands, and wetlands, based on the economic principle (Marklein and Houlton 2012). However, in this study, phosphatase showed non-response to P addition (Fig. 3J), probably because the presence of soil colloids might stabilize the activity of extracellular enzymes (Tian et al. 2016). As an indicator of aerobic microorganisms, catalase can protect the organisms from toxicity of peroxide that produced during degradation processes (Shen et al. 2018). The Pearson correlation coefficient suggested that catalase activity was negatively correlated with TP, AP, and pH (Fig. 4), which was in line with the report described by Wang et al. (2017).
Urease, a crucial enzyme involved in N-cycling, showed no response to P addition in our investigation, which was in accordance with the finding from two N-limited forests after 6 years of P addition (Chen et al. 2017). The potential reason for this phenomenon might be the sampling time after P addition, as the difference in urease activity between P-input soil and the control group gradually decreased over time (Chen et al. 2016). Invertase could promote the microbial enzymatic activities by degrading organic compounds into smaller size carbohydrates that are easy to be assimilated by microorganisms (Gao et al. 2019). In our study, P addition showed limited effects on the soil invertase activity, and consistent results were observed in an alpine meadow after P addition (Gao et al. 2016). Additionally, P addition did not significantly influence the contents of TN, AN and NN, indicating that P addition had a limited impact on soil N availability (Gao et al. 2016). The presence of E. gansuensis significantly altered the contents of AN and NN in soils with P addition, which might ascribe to alterations in the abundance of soil nitrogen cycling genes (e.g., nifH, AOB-amoA, nirK and nosZ) mediated by Epichloë endophytes in rhizosphere soil (Chen et al. 2022).
The root exudates serve as substrates as well as signaling molecules for microorganisms, providing a key bridge between plants and rhizosphere microbes. Numerous reports have indicated that plants can shape the composition and structure of microbial communities in the rhizosphere by regulating the secretion of root exudates (Huang et al. 2014; Ma et al. 2021). It has been suggested that the Epichloë endophyte is involved in regulating the root exudates of its host grass (Guo et al. 2015; Lee et al. 2021; Patchett and Newman 2021). Furthermore, Malinowski and Belesky (1999) revealed that the greater phosphorus assimilation in E+ tall fescue could lead to higher contents of phenolic metabolites in the root exudates of E+ plants than those of E− tall fescue. Additionally, in the pond-ditch circulation system, root exudates (e.g., lactic acid, amino acids, and proteins) are involved in the alteration of the relative abundance and diversity of rhizosphere microorganisms (Ma et al. 2021). In the present results, we observed a clear separation between E+ and E− samples in terms of exudate profiles under P-input condition (Fig. 6A), which supports our initial hypothesis. Additionally, we found that the contents of amino acids (glycine and alanine) and organic acids (lactic and succinic acid) were increased due to the presence of E. gansuensis under P-input condition (Fig. 6C). The foliar E. gansuensis may regulate root exudation in response to P addition by inducing transcriptional and metabolic changes in A. inebrians roots (Liu et al. 2021). Although the E. gansuensis-mediated exudate profiles in response to P addition have been investigated by hydroponic experiments, it is fascinating to further explore the potential correlation between root exudates and rhizosphere microbes in undisturbed soils to better understand the response of root-rhizosphere-microorganism ecosystems to P addition.
Conclusions
The present study investigated the response of bacterial communities in rhizosphere and root endosphere of E+ and E− A. inebrians to the short-term P addition and provided the potential regulatory factors from soil properties for these processes. We found that P addition showed limited effect on the bacterial community diversity, however, the composition and structure of bacterial communities were influenced in both rhizosphere and root endosphere of E+ and E− A. inebrians. P addition increased the soil pH and the contents of soil TP and AP and decreased the activity of catalase. But the activities of phosphatase, urease and invertase showed limited response to either P addition or E. gansuensis infection. Meanwhile, the higher contents of NO3−-N and SOC in soil were found due to the infection of E. gansuensis under P-input condition. Mantel test suggested that changes in soil AP, TP and pH mediated by P input were significantly associated with the bacterial communities of rhizosphere as well as root endosphere of A. inebrians. P addition also could reprogram the root exudates in E+ A. inebrians to enhance plant adaptation. Additionally, E. gansuensis increased the exudation of succinic acid and lactic acid from host roots compared to the E− roots under P-input condition. However, further investigations are necessary to elucidate the potential molecular mechanism for the E. gansuensis-regulated root secretion in response to P addition and to determine the functional alteration of microbial communities based on different gene markers under different P supply.
References
Aciego Pietri JC, Brookes PC (2009) Substrate inputs and pH as factors controlling microbial biomass, activity and community structure in an arable soil. Soil Biol Biochem 41:1396–1405. https://doi.org/10.1016/j.soilbio.2009.03.017
Alzate Zuluaga MY, Martinez de Oliveira AL, Valentinuzzi F, Tiziani R, Pii Y, Mimmo T, Cesco S (2021) Can inoculation with the bacterial biostimulant Enterobacter sp. strain 15S be an approach for the smarter P fertilization of maize and cucumber plants? Front Plant Sci 12:719873. https://doi.org/10.3389/fpls.2021.719873
Armitage HF, Britton AJ, Wal R, Woodin SJ (2012) Grazing exclusion and phosphorus addition as potential local management options for the restoration of alpine moss-sedge heath. Biol Conserv 153:17–24. https://doi.org/10.1016/j.biocon.2012.04.025
Aslam MM, Pueyo JJ, Pang J, Yang J, Chen W, Chen H, Waaseem M, Li Y, Zhang J, Xu W (2022) Root acid phosphatases and rhizobacteria synergistically enhance white lupin and rice phosphorus acquisition. Plant Physiol. https://doi.org/10.1093/plphys/kiac418
Bao Y, Yang N, Meng J, Wang D, Fu L, Wang J, Cang J (2021) Adaptability of winter wheat Dongnongdongmai 1 (Triticum aestivum L.) to overwintering in alpine regions. Plant Biol 23:445–455. https://doi.org/10.1111/plb.13200
Bennett LT, Adams MA (2001) Response of a perennial grassland to nitrogen and phosphorus additions in sub-tropical, semi-arid Australia. J Arid Environ 48:289–308. https://doi.org/10.1006/jare.2000.0759
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. https://doi.org/10.1038/s41587-019-0209-9
Brisson VL, Richardy J, Kosina SM, Northen TR, Vogel JP, Gaudin ACM (2022) Phosphate availability modulates root exudate composition and rhizosphere microbial community in a teosinte and a modern maize cultivar. Phytobiomes J 6:83–94. https://doi.org/10.1094/pbiomes-06-21-0041-r
Bulgarelli D, Schlaeppi K, Spaepen S, van Ver Loren E, Schulze-Lefert P (2013) Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838. https://doi.org/10.1146/annurev-arplant-050312-120106
Buyer JS, Zuberer DA, Nichols KA, Franzluebbers AJ (2010) Soil microbial community function, structure, and glomalin in response to tall fescue endophyte infection. Plant Soil 339:401–412. https://doi.org/10.1007/s11104-010-0592-y
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. https://doi.org/10.1038/nmeth.3869
Chen X, Daniell TJ, Neilson R, O’Flaherty V, Griffiths BS (2014) Microbial and microfaunal communities in phosphorus limited, grazed grassland change composition but maintain homeostatic nutrient stoichiometry. Soil Biol Biochem 75:94–101. https://doi.org/10.1016/j.soilbio.2014.03.024
Chen X, Jiang N, Condron LM, Dunfield KE, Chen Z, Wang J, Chen L (2019) Impact of long-term phosphorus fertilizer inputs on bacterial phoD gene community in a maize field, Northeast China. Sci Total Environ 669:1011–1018. https://doi.org/10.1016/j.scitotenv.2019.03.172
Chen L, Li X, Li C, Swoboda GA, Young CA, Sugawara K, Leuchtmann A, Schardl CL (2015) Two distinct Epichloë species symbiotic with Achnatherum inebrians, drunken horse grass. Mycologia 107:863–873. https://doi.org/10.3852/15-019
Chen Y, Sun T, Qian H, Fan J, He Y, Sun B (2016) Nitrogen mineralization as a result of phosphorus supplementation in long-term phosphate deficient soil. Appl Soil Ecol 106:24–32. https://doi.org/10.1016/j.apsoil.2016.04.019
Chen S, Waghmode TR, Sun R, Kuramae EE, Hu C, Liu B (2019) Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome 7:136. https://doi.org/10.1186/s40168-019-0750-2
Chen Z, White JF, Malik K, Chen H, Jin Y, Yao X, Wei X, Li C, Nan Z (2022) Soil nutrient dynamics relate to Epichloë endophyte mutualism and nitrogen turnover in a low nitrogen environment. Soil Biol Biochem 174:108832. https://doi.org/10.1016/j.soilbio.2022.108832
Chen H, Zhang W, Gurmesa GA, Zhu X, Li D, Mo J (2017) Phosphorus addition affects soil nitrogen dynamics in a nitrogen-saturated and two nitrogen-limited forests. Eur J Soil Sci 68:472–479. https://doi.org/10.1111/ejss.12428
Cheng H, Yuan M, Tang L, Shen Y, Yu Q, Li S (2022) Integrated microbiology and metabolomics analysis reveal responses of soil microorganisms and metabolic functions to phosphorus fertilizer on semiarid farm. Sci Total Environ 817:152878. https://doi.org/10.1016/j.scitotenv.2021.152878
Da N, Sommers LE (1983) Total carbon, organic carbon, and organic matter. Methods Soil Anal: Part 2 Chem Microbiol Prop 9:539–579
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245:35–47. https://doi.org/10.1007/978-94-017-1570-6_23
Ding N, Guo H, Kupper JV, McNear DH (2021) Phosphorus source and Epichloë coenophiala strain interact over time to modify tall fescue rhizosphere microbial community structure and function. Soil Biol Biochem 154:108125. https://doi.org/10.1016/j.soilbio.2020.108125
Dong J, Wang S, Niu H, Cui X, Li L, Pang Z, Zhou S, Wang K (2020) Responses of soil microbes and their interactions with plant community after nitrogen and phosphorus addition in a tibetan alpine steppe. J Soils Sediments 20:2236–2247. https://doi.org/10.1007/s11368-020-02586-3
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364. https://doi.org/10.1890/05-1839
Gao Y, Cooper DJ, Ma X (2016) Phosphorus additions have no impact on plant biomass or soil nitrogen in an alpine meadow on the Qinghai-Tibetan Plateau, China. Appl Soil Ecol 106:18–23. https://doi.org/10.1016/j.apsoil.2016.04.020
Gao Y, Huang H, Zhao H, Xia H, Sun M, Li Z, Li P, Zheng C, Dong H, Liu J (2019) Phosphorus affects enzymatic activity and chemical properties of cotton soil. Plant Soil Environ 65:361–368. https://doi.org/10.17221/296/2019-pse
Goldfarb KC, Karaoz U, Hanson CA, Santee CA, Bradford MA, Treseder KK, Wallenstein MD, Brodie EL (2011) Differential growth responses of soil bacterial taxa to carbon substrates of varying chemical recalcitrance. Front Microbiol 2:94–104. https://doi.org/10.3389/fmicb.2011.00094
Groffman PM, Fisk MC (2011) Phosphate additions have no effect on microbial biomass and activity in a northern hardwood forest. Soil Biol Biochem 43:2441–2449. https://doi.org/10.1016/j.soilbio.2011.08.011
Guo J, McCulley RL, McNear DH Jr (2015) Tall fescue cultivar and fungal endophyte combinations influence plant growth and root exudate composition. Front Plant Sci 6:183–196. https://doi.org/10.3389/fpls.2015.00183
Guwy A, Martin S, Hawkes F, Hawkes D (1999) Catalase activity measurements in suspended aerobic biomass and soil samples. Enzyme Microb Technol 25:669–676. https://doi.org/10.1016/S0141-0229(99)00115-5
Hegyi A, Nguyen TBK, Posta K (2021) Metagenomic analysis of bacterial communities in agricultural soils from vietnam with special attention to phosphate solubilizing bacteria. Microorganisms 9:1796–1813. https://doi.org/10.3390/microorganisms9091796
Hoffland E, Kuyper TW, Comans RNJ, Creamer RE (2020) Eco-functionality of organic matter in soils. Plant Soil 455:1–22. https://doi.org/10.1007/s11104-020-04651-9
Hou W, Wang J, Christensen MJ, Liu J, Zhang Y, Liu Y, Cheng C (2021) Metabolomics insights into the mechanism by which Epichloë gansuensis endophyte increased Achnatherum inebrians tolerance to low nitrogen stress. Plant Soil 463:487–508. https://doi.org/10.1007/s11104-021-04930-z
Hou W, Wang J, Nan Z, Christensen MJ, Xia C, Chen T, Zhang Z, Niu X (2020a) Epichloë gansuensis endophyte-infection alters soil enzymes activity and soil nutrients at different growth stages of Achnatherum inebrians. Plant Soil 455:227–240. https://doi.org/10.1007/s11104-020-04682-2
Hou W, Xia C, Christensen MJ, Wang J, Li X, Chen T, Nan Z (2020b) Effect of Epichloë gansuensis endophyte on rhizosphere bacterial communities and nutrient concentrations and ratios in the perennial grass species Achnatherum inebrians during three growth seasons. Crop Pasture Sci 71:1050–1066. https://doi.org/10.1071/cp20145
Hu Y, Xia Y, Sun Q, Liu K, Chen X, Ge T, Zhu B, Zhu Z, Zhang Z, Su Y (2018) Effects of long-term fertilization on phod-harboring bacterial community in Karst soils. Sci Total Environ 628–629:53–63. https://doi.org/10.1016/j.scitotenv.2018.01.314
Huang XF, Chaparro JM, Reardon KF, Zhang R, Shen Q, Vivanco JM (2014) Rhizosphere interactions: root exudates, microbes, and microbial communities. Botany 92:267–275. https://doi.org/10.1139/cjb-2013-0225
Huang J, Hu B, Qi K, Chen W, Pang X, Bao W, Tian G (2016) Effects of phosphorus addition on soil microbial biomass and community composition in a subalpine spruce plantation. Eur J Soil Biol 72:35–41. https://doi.org/10.1016/j.ejsobi.2015.12.007
Ikoyi I, Fowler A, Schmalenberger A (2018) One-time phosphate fertilizer application to grassland columns modifies the soil microbiota and limits its role in ecosystem services. Sci Total Environ 630:849–858. https://doi.org/10.1016/j.scitotenv.2018.02.263
Jin J, Huang R, Wang J, Wang C, Liu R, Zhang H, Deng M, Li S, Li X, Tang R, Li C (2022) Increase in Cd tolerance through seed-borne endophytic fungus Epichloë gansuensis affected root exudates and rhizosphere bacterial community of Achnatherum inebrians. Int J Mol Sci 23:13094–13112. https://doi.org/10.3390/ijms232113094
Jones RT, Robeson MS, Lauber CL, Hamady M, Knight R, Fierer N (2009) A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J 3:442–453. https://doi.org/10.1038/ismej.2008.127
Ju Y, Zhong R, Christensen MJ, Zhang X (2020) Effects of Epichloë gansuensis endophyte on the root and rhizosphere soil bacteria of Achnatherum inebrians under different moisture conditions. Front Microbiol 11:747. https://doi.org/10.3389/fmicb.2020.00747
Kim JM, Roh AS, Choi SC, Kim EJ, Choi MT, Ahn BK, Kim SK, Lee YH, Joa JH, Kang SS, Lee SA, Ahn JH, Song J, Weon HY (2016) Soil pH and electrical conductivity are key edaphic factors shaping bacterial communities of greenhouse soils in Korea. J Microbiol 54:838–845. https://doi.org/10.1007/s12275-016-6526-5
Lagos LM, Acuña JJ, Maruyama F, Ogram A, de la Luz Mora M, Jorquera MA (2016) Effect of phosphorus addition on total and alkaline phosphomonoesterase-harboring bacterial populations in ryegrass rhizosphere microsites. Biol Fertil Soils 52:1007–1019. https://doi.org/10.1007/s00374-016-1137-1
Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75:5111–5120. https://doi.org/10.1128/AEM.00335-09
Lee K, Missaoui A, Mahmud K, Presley H, Lonnee M (2021) Interaction between grasses and Epichloë endophytes and its significance to biotic and abiotic stress tolerance and the rhizosphere. Microorganisms 9:2186–2213. https://doi.org/10.3390/microorganisms9112186
Li C, Nan Z, Gao J, Tian P (2004) Detection and distribution of Neotyphodium-Achnatherum inebrians association in China. In: Proceedings of 5th international Neotyphodium/grass interactions symposium. United States Department of Agriculture, Arkansas, USA, pp 24–27
Li Y, Niu S, Yu G (2016) Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: a meta-analysis. Glob Change Biol 22:934–943. https://doi.org/10.1111/gcb.13125
Ling N, Chen D, Guo H, Wei J, Bai Y, Shen Q, Hu S (2017) Differential responses of soil bacterial communities to long-term N and P inputs in a semi-arid steppe. Geoderma 292:25–33. https://doi.org/10.1016/j.geoderma.2017.01.013
Liu Y, Hou W, Jin J, Christensen MJ, Gu L, Cheng C, Wang J (2021) Epichloë gansuensis increases the tolerance of Achnatherum inebrians to low-P stress by modulating amino acids metabolism and phosphorus utilization efficiency. J Fungi 7:390–413. https://doi.org/10.3390/jof7050390
Ma L, Yang L, Liu W, Zhang Y, Zhou Q, Wu Z, He F (2021) Effects of root exudates on rhizosphere bacteria and nutrient removal in pond-ditch circulation systems (PDCSs) for rural wastewater treatment. Sci Total Environ 785:147282. https://doi.org/10.1016/j.scitotenv.2021.147282
Malinowski DP, Belesky DP (1999) Neotyphodium coenophialum-endophyte infection affects the ability of tall fescue to use sparingly available phosphorus. J Plant Nutr 22:835–853. https://doi.org/10.1080/01904169909365675
Mander C, Wakelin S, Young S, Condron L, O’Callaghan M (2012) Incidence and diversity of phosphate-solubilising bacteria are linked to phosphorus status in grassland soils. Soil Biol Biochem 44:93–101. https://doi.org/10.1016/j.soilbio.2011.09.009
Marklein AR, Houlton BZ (2012) Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol 193:696–704. https://doi.org/10.1111/j.1469-8137.2011.03967.x
Massey PA, Creamer RE, Whelan MJ, Ritz K (2016) Insensitivity of soil biological communities to phosphorus fertilization in intensively managed grassland systems. Grass Forage Sci 71:139–152. https://doi.org/10.1111/gfs.12163
McPherson MR, Wang P, Marsh EL, Mitchell RB, Schachtman DP (2018) Isolation and analysis of microbial communities in soil, rhizosphere, and roots in perennial grass experiments. J Vis Exp 137:e57932. https://doi.org/10.3791/57932
Mimmo T, Pii Y, Valentinuzzi F, Astolfi S, Lehto N, Robinson B, Brunetto G, Terzano R, Cesco S (2018) Nutrient availability in the rhizosphere: a review. Acta Hortic 1217:13–28. https://doi.org/10.17660/ActaHortic.2018.1217.2
Ollinger J, Song KB, Antelmann H, Hecker M, Helmann JD (2006) Role of the Fur regulon in iron transport in Bacillus subtilis. J Bacteriol 188:3664–3673. https://doi.org/10.1128/JB.188.10.3664-3673.2006
Papik J, Folkmanova M, Polivkova-Majorova M, Suman J, Uhlik O (2020) The invisible life inside plants: deciphering the riddles of endophytic bacterial diversity. Biotechnol Adv 44:107614. https://doi.org/10.1016/j.biotechadv.2020.107614
Patchett A, Newman JA (2021) Comparison of plant metabolites in root exudates of Lolium perenne infected with different strains of the fungal endophyte Epichloë festucae var. lolii. J Fungi 7:148–177. https://doi.org/10.3390/jof7020148
Penuelas J, Poulter B, Sardans J, Ciais P, van der Velde M, Bopp L, Boucher O, Godderis Y, Hinsinger P, Llusia J, Nardin E, Vicca S, Obersteiner M, Janssens IA (2013) Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat Commun 4:2934–2944. https://doi.org/10.1038/ncomms3934
Plaxton WC, Tran HT (2011) Metabolic adaptations of phosphate-starved plants. Plant Physiol 156:1006–1015. https://doi.org/10.1104/pp.111.175281
Ramirez KS, Lauber CL, Knight R, Bradford MA, Fierer N (2010) Consistent effects of nitrogen fertilization on soil bacterial communities in contrasting systems. Ecology 91:3463–3470. https://doi.org/10.1890/10-0426.1
Reed SC, Seastedt TR, Mann CM, Suding KN, Townsend AR, Cherwin KL (2007) Phosphorus fertilization stimulates nitrogen fixation and increases inorganic nitrogen concentrations in a restored prairie. Appl Soil Ecol 36:238–242. https://doi.org/10.1016/j.apsoil.2007.02.002
Ren C, Wang T, Xu Y, Deng J, Zhao F, Yang G, Han X, Feng Y, Ren G (2018) Differential soil microbial community responses to the linkage of soil organic carbon fractions with respiration across land-use changes. For Ecol Manage 409:170–178. https://doi.org/10.1016/j.foreco.2017.11.011
Richardson A, Hadobas P, Hayes J, O’hara C, Simpson R (2001) Utilization of phosphorus by pasture plants supplied with myo-inositol hexaphosphate is enhanced by the presence of soil micro-organisms. Plant Soil 229:47–56. https://doi.org/10.1023/A:1004871704173
Rooney DC, Clipson NJ (2009) Phosphate addition and plant species alters microbial community structure in acidic upland grassland soil. Microb Ecol 57:4–13. https://doi.org/10.1007/s00248-008-9399-2
Sarathchandra S, Ghani A, Yeates G, Burch G, Cox N (2001) Effect of nitrogen and phosphate fertilisers on microbial and nematode diversity in pasture soils. Soil Biol Biochem 33:953–964. https://doi.org/10.1016/S0038-0717(00)00245-5
Shen F, Wu J, Fan H, Liu W, Guo X, Duan H, Hu L, Lei X, Wei X (2018) Soil N/P and C/P ratio regulate the responses of soil microbial community composition and enzyme activities in a long-term nitrogen loaded chinese fir forest. Plant Soil 436:91–107. https://doi.org/10.1007/s11104-018-03912-y
Shi Y, Ziadi N, Hamel C, Bélanger G, Abdi D, Lajeunesse J, Lafond J, Lalande R, Shang J (2020) Soil microbial biomass, activity and community structure as affected by mineral phosphorus fertilization in grasslands. Appl Soil Ecol 146:103391. https://doi.org/10.1016/j.apsoil.2019.103391
Sinsabaugh RL, Follstad Shah JJ (2012) Ecoenzymatic stoichiometry and ecological theory. Annu Rev Ecol Evol Syst 43:313–343. https://doi.org/10.1146/annurev-ecolsys-071112-124414
Tahir M, Ahmad I, Shahid M, Shah GM, Farooq ABU, Akram M, Tabassum SA, Naeem MA, Khalid U, Ahmad S, Zakir A (2019) Regulation of antioxidant production, ion uptake and productivity in potato (Solanum tuberosum L.) plant inoculated with growth promoting salt tolerant Bacillus strains. Ecotox Environ Safe 178:33–42. https://doi.org/10.1016/j.ecoenv.2019.04.027
Tan H, Barret M, Mooij MJ, Rice O, Morrissey JP, Dobson A, Griffiths B, O’Gara F (2012) Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol Fertil Soils 49:661–672. https://doi.org/10.1007/s00374-012-0755-5
Tang M, Liu J, Hou W, Stubbendieck RM, Xiong H, Jin J, Gong J, Cheng C, Tang X, Liu Y, Li Z, Wang J, Yi Y (2022) Structural variability in the bulk soil, rhizosphere, and root endophyte fungal communities of Themeda japonica plants under different grades of karst rocky desertification. Plant Soil 475:105–122. https://doi.org/10.1007/s11104-021-04969-y
Thirukkumaran CM, Parkinson D (2000) Microbial respiration, biomass, metabolic quotient and litter decomposition in a lodgepole pine forest floor amended with nitrogen and phosphorous fertilizers. Soil Biol Biochem 32:59–66. https://doi.org/10.1016/S0038-0717(99)00129-7
Thirukkumaran CM, Parkinson D (2002) Microbial activity, nutrient dynamics and litter decomposition in a Canadian rocky mountain pine forest as affected by N and P fertilizers. For Ecol Manage 159:187–201. https://doi.org/10.1016/S0378-1127(01)00432-7
Tian J, Lu X, Chen Q, Kuang X, Liang C, Deng L, Lin D, Cai K, Tian J (2020) Phosphorus fertilization affects soybean rhizosphere phosphorus dynamics and the bacterial community in karst soils. Plant Soil 475:137–152. https://doi.org/10.1007/s11104-020-04662-6
Tian J, Wei K, Condron LM, Chen Z, Xu Z, Chen L (2016) Impact of land use and nutrient addition on phosphatase activities and their relationships with organic phosphorus turnover in semi-arid grassland soils. Biol Fertil Soils 52:675–683. https://doi.org/10.1007/s00374-016-1110-z
Tiziani R, Pii Y, Celletti S, Cesco S, Mimmo T (2020) Phosphorus deficiency changes carbon isotope fractionation and triggers exudate reacquisition in tomato plants. Sci Rep 10:15970. https://doi.org/10.1038/s41598-020-72904-9
Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK (2020) Plant-microbiome interactions: from community assembly to plant health. Microbiology 18:607–621. https://doi.org/10.1038/s41579-020-0412-1
Wang J, Hou W, Christensen MJ, Li X, Xia C, Li C, Nan Z (2020) Role of Epichloë endophytes in improving host grass resistance ability and soil properties. J Agric Food Chem 68:6944–6955. https://doi.org/10.1021/acs.jafc.0c01396
Wang C, Huang R, Wang J, Jin J, Malik K, Niu X, Tang R, Hou W, Cheng C, Liu Y, Liu J (2022) Comprehensive analysis of transcriptome and metabolome elucidates the molecular regulatory mechanism of salt resistance in roots of Achnatherum inebrians mediated by Epichloë gansuensis. J Fungi 8:1092–1113. https://doi.org/10.3390/jof8101092
Wang R, Zhang H, Sun L, Qi G, Chen S, Zhao X (2017) Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci Rep 7:343–353. https://doi.org/10.1038/s41598-017-00472-6
Widdig M, Schleuss PM, Weig AR, Guhr A, Biederman LA, Borer ET, Crawley MJ, Kirkman KP, Seabloom EW, Wragg PD, Spohn M (2019) Nitrogen and phosphorus additions alter the abundance of phosphorus-solubilizing bacteria and phosphatase activity in grassland soils. Front Environ Sci 7:185–200. https://doi.org/10.3389/fenvs.2019.00185
Wu W, Wang F, Xia A, Zhang Z, Wang Z, Wang K, Dong J, Li T, Wu Y, Che R, Li L, Niu S, Hao Y, Wang Y, Cui X (2022) Meta-analysis of the impacts of phosphorus addition on soil microbes. Agric Ecosyst Environ 340:108180. https://doi.org/10.1016/j.agee.2022.108180
Yan Y, Sun X, Sun F, Zhao Y, Sun W, Guo J, Zhang T (2021) Sensitivity of soil fungal and bacterial community compositions to nitrogen and phosphorus additions in a temperate meadow. Plant Soil 471:477–490. https://doi.org/10.1007/s11104-021-05237-9
Yang WY, Lee Y, Lu H, Chou CH, Wang C (2019) Analysis of gut microbiota and the effect of lauric acid against necrotic enteritis in Clostridium perfringens and Eimeria side-by-side challenge model. PLoS ONE 14:e0205784. https://doi.org/10.1371/journal.pone.0205784
Zgadzaj R, Garrido-Oter R, Jensen DB, Koprivova A, Schulze-Lefert P, Radutoiu S (2016) Root nodule symbiosis in Lotus japonicus drives the establishment of distinctive rhizosphere, root, and nodule bacterial communities. Proc Natl Acad Sci USA 113:e7996–e8005. https://doi.org/10.1073/pnas.1616564113
Zhalnina K, Louie KB, Hao Z, Mansoori N, da Rocha UN, Shi S, Cho H, Karaoz U, Loque D, Bowen BP, Firestone MK, Northen TR, Brodie EL (2018) Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat Microbiol 3:470–480. https://doi.org/10.1038/s41564-018-0129-3
Zhao J, Zhang R, Xue C, Xun W, Sun L, Xu Y, Shen Q (2014) Pyrosequencing reveals contrasting soil bacterial diversity and community structure of two main winter wheat cropping systems in China. Microb Ecol 67:443–453. https://doi.org/10.1007/s00248-013-0322-0
Zhao M, Zhao J, Yuan J, Hale L, Wen T, Huang Q, Vivanco JM, Zhou J, Kowalchuk GA, Shen Q (2021) Root exudates drive soil-microbe-nutrient feedbacks in response to plant growth. Plant Cell Environ 44:613–628. https://doi.org/10.1111/pce.13928
Funding
This research was supported by the Natural Science Foundation of China (32001399), the Joint Fund of the Natural Science Foundation of China and the Karst Science Research Center of Guizhou Province (Grant No. U1812401), Natural Science Foundation of Gansu Province (22JR5RA451), Changjiang Scholars and innovative Research Team in University (IRT_17R50), the Fundamental Research Funds for the Central Universities (lzujbky-2021-ey01, lzujbky-2021-kb12) in Lanzhou University, the Open Project of State Key Laboratory of Plateau Ecology and Agriculture, Qinghai University (2021-KF-02), Technical Service Contract of Microbiology Mechanism in the Process of Themeda japonica Adapting to Different Grades of Rocky Desertification [21(0520)].
Author information
Authors and Affiliations
Contributions
Jianfeng Wang, Chao Wang, Jie Jin conceived the ideas and designed the experiment; Rong Tang, Rong Zheng, Rong Huang collected and analyzed the samples; Jianfeng Wang, Xueli Niu, Yang Yang, Chengzhou Zhao analyzed the data; Jianfeng Wang, Chao Wang prepared the Figures and wrote the manuscript; Yang Yang, Kamran Malik revised and edited the draft.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Luz E. Bashan.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 700 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Wang, J., Niu, X. et al. Phosphorus addition modifies the bacterial community structure in rhizosphere of Achnatherum inebrians by influencing the soil properties and modulates the Epichloë gansuensis-mediated root exudate profiles. Plant Soil 491, 543–560 (2023). https://doi.org/10.1007/s11104-023-06133-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06133-0