Abstract
Background and aims
Soil microorganisms play key roles in soil nutrient turnover and plant community composition; however, the soil microbial community composition and species diversity are often influenced by nutrient enrichment which may affect how soil microbes influence nutrient cycles and the plant community structure. The resistance of soil fungal and bacterial communities to nitrogen (N) and phosphorus (P) additions and whether the responses of the soil microbes and the plant community are simultaneous in a N-limited temperate meadow ecosystem are still unclear.
Methods
We carried out a 7-year experiment with N and P additions in a temperate meadow. The community structures of soil bacteria and fungi were examined based on high-throughput sequencing targeting the 16S rRNA and ITS genes, respectively.
Results
Nitrogen addition did not influence the community composition or species richness of bacteria, but it did alter the soil fungal community composition and increased fungal operational taxonomic unit (OTU) richness. Phosphorus addition significantly altered the soil fungal and bacterial community compositions, decreased the richness of bacterial OTUs, and increased the OTU richness of fungi. Proteobacteria (38.5%) and Acidobacteria (22.3%) were the most dominant bacteria. Ascomycota were the dominant fungi (42.6%) across all samples. The enrichment of available P in the soil due to P addition reduced the bacterial β-diversity, while the β-diversity of soil fungi was mainly influenced by the concentrations of soil N and P, as well as soil moisture.
Conclusions
The sensitivity of soil fungi and bacteria to P addition was stronger than that of N addition, and the response of the soil microbes to N and P additions was more sensitive than that of the plant community. Our results highlight the unequal sensitivity of the soil fungal and bacterial community composition and structure to N and P additions, thereby causing changes in above and belowground community composition and structures in the studied temperate meadow ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil microbes play an important role in determining ecosystem stability and multifunctionality (van der Heijden et al. 2008; Delgado-Baquerizo et al. 2016; Bennett et al. 2017). For instance, soil fungi can reduce greenhouse gas emissions, such as N2O and CH4 (Bender et al. 2014, 2015; Thompson et al. 2016; Storer et al. 2018), and alleviate warming potential (Cui et al. 2021). Soil microbial diversity plays an important role in regulating the soil carbon (C), nitrogen (N) and phosphorus (P) cycles because it accelerates the decomposition of litter (Kang et al. 2020) and mineralizes organic P (Jiang et al. 2021), thus affecting plant N and P uptake (Freedman et al. 2013; Mei et al. 2019). However, the community composition and structures of soil microbes are influenced by environmental changes, which should reduce the positive effect of soil microbes on nutrient cycles and alter the plant community structure. To date, how environmental changes influence soil microbial communities is still uncertain.
A series of global change factors such as climate warming, N deposition, and rising atmospheric carbon dioxide concentrations, significantly reduce biodiversity and decrease ecosystem functions (Vellend et al. 2017; Harrison 2020; Yang et al. 2021a, 2021b). For instance, N and P additions have been shown to alter the community composition and reduce the species diversity of soil microorganisms (Wang et al. 2018). The addition of N reduced the bacterial richness in soil by decreasing soil pH but it had no impact on fungal biomass in a tropical forest ecosystem in South China (Wang et al. 2018). Nitrogen addition was also found to alter the bacterial community composition because of changes in the soil pH and in the plant community composition in a temperate steppe (Zeng et al. 2016). Nitrogen deposition reduced the species richness of the active fungal community but had no influence on the bacterial community in a hardwood forest ecosystem, suggesting that the influence of N deposition on the soil bacterial community may be determined in a seasonally or temporally variable fashion (Freedman et al. 2015). However, N addition exerted little influence on soil bacterial diversity in a cedar creek ecosystem (Fierer et al. 2012), and a meta-analysis study also suggested that the effects of N application on soil microbial community compositions were inconsistent (Ramirez et al. 2012). These varying responses of the soil microbial community to N addition suggest that the influence of N enrichment on soil microbial diversity might be ecosystem-specific or be caused by local environmental differences in the soil nutrient status, community composition, and climate.
Increasing evidence has demonstrated that P deposition is becoming a significant P source across the globe (Ahn and James 2001; Vicars et al. 2010; Peñuelas et al. 2013; Zhu et al. 2016), and is a major determinant for the soil microbial community (Wan et al. 2015; Li et al. 2021). The change in soil available P concentration caused by P addition was a key parameter that shift the diversity and composition of the soil microbial community in previous studies (He et al. 2016; Ling et al. 2017). Previous results found that the addition of P increased the abundance of arbuscular mycorrhizal fungi and bacteria due to an increase in carbon availability and pH in subalpine meadows (Huang et al. 2016). Phosphorus addition alters the structure of soil fungal and bacterial communities by affecting phosphate and plant species in acidic grasslands (Rooney and Clipson 2009). Phosphorus addition changed the soil fungal community structure due to fertilization-mediated changes in soil pH in a P-deficient woodland (Nielsen et al. 2015). An increase in soil fungal and bacterial abundance might ameliorate the negative impact of N enrichment on the belowground community (Su et al. 2015) and then positively affect aboveground community composition. However, several studies also found that P addition had little influence on the community composition of soil microbes under N enrichment (He et al. 2016; Wang et al. 2018). These results suggest that the influence of P additions on the soil microbial community is inconsistent and might be determined by local environmental factors and the ecosystem type.
Although some studies have investigated the influence of N and P additions in tropical forests (He et al. 2016; Wang et al. 2018), species-rich meadows (Pan et al. 2014), semiarid steppes (Ling et al. 2017), the influences of N and P additions, and their interactive effects on soil bacterial and fungal community compositions in temperate meadow ecosystems are still not well understood. A previous study showed that N and P additions could alter the plant community structure (Zhao et al. 2019), but whether the response of soil microbes and plants to N and P additions are coordinated is still unclear. To understand the mechanisms by which N and P additions affect the soil microbial community composition and structure, we conducted a 7-year field experiment with N and P additions in the Songnen meadow, northeastern China. This study aims to clarify the influence of long-term N and P additions, and their interaction on the soil bacterial and fungal communities, to better understand the pathways by which N and P additions affect soil microbes, and to reveal new insights into possible changes in soil ecological functions under N and P additions in a temperate meadow. We hypothesized that: (1) the addition of N would decrease soil bacterial and fungal species diversity and alter the community structure because of a decline in soil pH caused by N addition, whereas P addition would have few impacts (Wang et al. 2018); (2) the soil fungi would be more sensitive than soil bacteria because the closer associations between plants and fungi (Li et al. 2020); and (3) the changes in the soil community structure induced by the addition of N and P would be determined by the changes in plant community composition caused by N and P addition (Cline and Zak 2015).
Material and methods
Experiment site
This experiment was performed in Songnen grassland (123°45′ E, 44° 45′ N), in western Jilin Province, northeastern China. The Songnen grassland is the largest temperate meadow in China, and has been seriously influenced by N deposition (Zhang et al. 2015, 2016; Wen et al. 2020). The altitude of the experimental area is 135–165 m. The annual rainfall at the experimental site is approximately 300–500 mm with a mean of 400 mm (Kang et al. 2020). The annual air temperature on average is 2. 4–2.7 °C. In this region, the soil type is Chernozem soil and it is characterized by a higher pH (7.5–9.0) and a low organic matter content (3–4%) (Zhang et al. 2016). Soil N is limited with a total soil N of 1.8 g kg−1 and a soil available P concentration of 2.5 mg kg−1 (Mei et al. 2019). The terrain in this area is flat, and the vegetation is relatively uniform. The vegetation is dominated by Leymus chinensis and some subordinate species, such as Carex duriuscula, Polygonum sibiricum, Thalictrum aquilegifolium, and Chloris virgata.
Experimental design
A completely randomized block factorial experimental design was used with two nutrient factors, and N and P additions were included in this experiment. There were four treatments: N addition (N), P addition (P), N + P addition (NP), and one control (C), with three replicates per treatment (Fig. 1). The experimental plot size was 2 × 2 m, with buffer zones (2 m in width) between the plots. Previous studies reported that the saturation rates of soil N and P additions for plant communities were 10.5 g N m−2 year−1 and 10 g P m−2 year−1 in grasslands in northern China (Bai et al. 2010; Zhao et al. 2019), respectively. For the additions of N and P, a NH4NO3 solution (10 g N m−2 year−1 in 10 L water) and Ca(H2PO4)2 solution (10 g P m−2 year−1 in 10 L water) was added to the plots before plant germination. In the NP addition treatment, NH4NO3 and Ca(H2PO4)2 solutions (10 g N and 10 g P m−2 year−1 in 10 L water) were simultaneously added to the experimental plot. To reduce the effect of water caused by the addition of N and P on the experimental results, the same amount of water (10 L water m−2 without N and P) as in N and P treatments was added to the control plots. The experiment began in May 2013.
Sampling and measurements
The soil was sampled in August 2019 and the following parameters were measured soil pH, the concentrations of soil total N, available P, soil moisture, and the community and composition structure of soil bacteria and fungi. In each plot, five soil cores (an inner diameter of 5 cm, 20 cm in-depth) were drilled randomly from three replicate blocks, and then homogenized thoroughly and sieved (2 mm) before chemical analysis. Soil total N and available P were determined according to Mei et al. (2019). Soil pH was measured using a glass electrode.
Plant community composition and productivity
To explore the relationship between the soil and plant community composition under N and P additions, the plant community composition was studied after seven years of nutrient addition. The plant species in each block were recorded on July 15, 2019 (peak growing season) according to the method of Zhao et al. (2019). Plant species were recorded using a modified point-frame method (1 × 1 m2), and the plant species number (species number m2) and density were recorded to calculate the Shannon–Wiener index H. Plants in two quadrants (50 × 50 cm2) in each plot were randomly cut in September 2019 to measure the aboveground biomass, which included the dead biomass from the previous year. After cutting the plants in the quadrants, the aboveground plants were weighed after drying for 48 h at 65 °C.
Soil DNA extraction and high-throughput sequencing
A Power Soil DNA Isolation Kit (MO BIO Laboratories) was used to extract soil DNA. The quantity and quality of DNA were evaluated, and the DNA was then stored at −80 °C before use. The 16S rRNA gene of bacteria (in the V3-V4 region) was amplified by the primers 338-F (5′- ACTCCTACGGGAGGCAGCA-3′) and 806-R (5′- GGACTACHVGGGTWTCTAAT-3′) (Mori et al. 2014). We amplified the rRNA gene of fungi (ITS1 region) using ITS1-F (5’-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2-R (5’-GCTGCGTTCTTCATCGATGC-3′) primers (White et al. 1990; Gardes and Bruns 1993).
We performed PCR amplification in a total volume of 50 μl including the buffer and DNA polymerase (Table S1). A description of the thermal cycling conditions of the first step of PCR can be found in Table S2. We purified the PCR products in the first step using DNA clean beads (VAHTSTM). The amplification PCR of the second round and conditions of thermal cycling can be found in Tables S3 and S4. The sequencing of bacteria and fungi was performed using the Illumina HiSeq2500 platform (2 × 250 paired ends).
Sequence analyses
The paired-end reads of the fungal ITS gene and bacterial 16S rRNA gene were processed, and the ITS region and 16S sequences were screened for quality control according to the methods of Guo et al. 2019. We clustered all the tags of >97% identity into operational taxonomic units (OTUs). The tags were classified into different taxonomies according to the Silva and UNITE databases for soil bacterial and fungal communities, respectively. There were 17,822 OTUs for soil bacteria and 4986 OTUs for soil fungi after removing those OTUs that did not belong to the soil bacterial or fungal community.
Data analyses
All analyses were performed using R version 3.6.0. (R Core Team 2019). To determine the influence of N and P additions, and their interactive effects on soil total N concentrations, available P concentrations, soil moisture, soil pH, plant species diversity (Shannon–Wiener index, H), aboveground biomass, soil bacterial and fungal α-diversity (the indices of ACE index, Chao1, Shannon and Simpson), and the dominant (> 1%) bacterial and fungal group abundances, two-way ANOVA was performed using R software. The N and P addition effects and their interaction on the community compositions of soil bacteria and fungi were tested by PERMANOVA using the vegan package with 104 permutations. We also visualized how taxonomic frequency and abundances responded to N and P additions using ternary plots. To explore the variations in soil fungal and bacterial species compositions in the fractions illustrated by plant diversity, productivity, soil N and P concentrations, moisture, and pH, we performed distance-based redundancy analysis (db-RDA) based on Bray–Curtis distance. Structural equation modeling (SEM) was performed to explore the pathways by which N and P additions affected soil bacterial and fungal richness according to the methods described by Yang et al. (2021a, 2021b).
Results
Plant and soil parameters
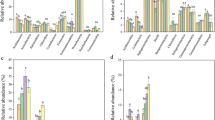
Nitrogen addition highly enhanced the soil total N concentration (F = 4.217, P = 0.025) but had no influence on the soil-available P concentration (F = 2.021, P = 0.27, Fig. 2a and b). In contrast, phosphorus addition improved the concentrations of both total N (F = 5.01, P = 0.022) and available P (F = 10.02, P < 0.01) in the soil (Fig. 2a and b). The soil total N (F = 4.327, P = 0.023) and available P (P < 0.05) concentrations in the NP addition treatment were higher than those in the control. For the soil total N concentration, there was no significant difference (F = 1.727, P = 0.17) between the P and NP addition treatments (Fig. 2a), and no obvious difference in the soil-available P concentration (F = 0.372, P = 0.65) between the P addition and NP addition treatments was detected (Fig. 2b). Significant interactive effects of N and P addition on soil total N concentration were observed (F = 7.371, P = 0.004). N and P additions significantly altered the plant community structure (Fig. S4). The addition of N decreased plant species diversity (F = 5.47, P = 0.018, Fig. 2c) but significantly increased aboveground biomass (F = 2.478, P = 0.033, Fig. 2d). Phosphorus addition did not affect plant species diversity (F = 1.051, P = 0.271, Fig. 2c) or aboveground biomass (F = 0.957, P = 0.375, Fig. 2d). The addition of NP had no impact on plant species diversity (F = 1. 570, P = 0.145, Fig. 2c) but led to an increase in aboveground biomass (F = 4.57, P = 0.012, Fig. 2d). No interactive effects of N and P additions on plant species richness (F = 0.78, P = 0.55, Fig. 2c) or aboveground biomass were detected (F = 1.077, P = 0.25, Fig. 2d).
Effects of the additions of nitrogen (N) and phosphorus (P) on the soil N (a) and P concentrations (b), plant species diversity (Shannon index, c) and aboveground biomass (d). C represents the control, N represents N addition, P represents P addition, and NP represents nitrogen plus phosphorus addition. * indicates P < 0.05; ** indicates P < 0.01; *** indicates P < 0.001; ns indicates no significance
OTU richness and α-diversity
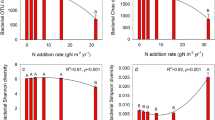
Rarefaction analysis was performed to compare the levels of bacterial and fungal diversities in terms of the total number of OTUs and the Chao 1 index, and the rarefaction results are presented in Supplementary Data Fig. S1. Nitrogen addition had no impact on the soil bacterial OTU richness (F = 1.097, P = 0.15), but it was significantly reduced in the P (F = 4.057, P = 0.021) and NP (F = 7.907, P = 0.002) addition treatments (Fig. 3a). The addition of N and P had no interactive effect on bacterial OTU richness (F = 2.017, P = 0.045). Both N (F = 10.101, P = 0.005) and P (F = 13.475, P - 0.003) additions increased the soil fungal OTU richness (Fig. 3b). The interactions between N and P additions significantly affected fungal OTU richness (F = 21.017, P = 0.001).
Effects of the additions of N and P on soil bacterial (a) and fungal (b) OTU numbers and the Chao1 index of soil bacterial (c) and fungal (d) communities. C represents the control, N represents nitrogen addition, P represents P addition, and NP represents nitrogen plus phosphorus addition. * indicates P < 0.05; ** indicates P < 0.01; *** indicates P < 0.001; ns indicates no significance
The addition of N (F = 5.201, P = 0.014) and P (F = 11.701, P = 0.002) remarkably decreased the Chao1 index of soil bacteria (Fig. 3c). The Chao1 index in the NP addition treatment was much lower than that in the N addition (F = 10.011, P = 0.002) and control (F = 34.08, P < 0.001, Fig. 3c) treatments. A significant primary effect of P addition on the Chao1 index of bacteria was noticed (F = 24.57, P < 0.001). The addition of N (F = 4.27, P = 0.024) and P (F = 5.18, P = 0.015) remarkably enhanced the Chao1 index of soil fungi, and no obvious difference was detected between NP addition and the addition of N and P (F = 1.08, P = 0.27, Fig. 3d).
Structural equation modeling analysis accounted for 17%, 73%, 29%, 31%, 30%, 80% and 76% of the variations in soil total N, available P, pH, aboveground net primary productivity (ANPP), plant species diversity (Shannon index), and the species richness of bacteria and fungi, respectively (Fig. 4). The addition of N and P caused an increase in soil fungal richness, most likely through their positive effect on soil total N and available P concentrations (Fig. 4). The positive changes in plant species diversity due to the decline in soil pH led to the shift in soil bacterial richness (Fig. 4).
Structural equation models of N addition and AM fungi as predictors of ecosystem functioning. Solid red arrows represent negative paths, solid green arrows represent positive paths, and dotted red and green arrows represent nonsignificant paths. * indicates P < 0.05, ** indicates P < 0.01, *** indicates P < 0.001, χ2 = 2.9, P = 0.40; root mean square error of approximation (RMSEA) = 0.40, P = 0.27; Akaike information criteria = 38.93
Soil bacterial and fungal community compositions
Proteobacteria (38.5%), Acidobacteria (22.4%), Actinobacteria (14.8%), and Gemmatimonadetes (8.3%) dominated the soil bacterial community across all the treatments (Fig. 5a). Phosphorus addition reduced the abundances of Proteobacteria (F = 3.18, P = 0.027) and Gemmatimonadetes (F = 4.31, P = 0.017) and increased the abundances of Acidobacteria (F = 7.11, P = 0.005) and Actinobacteria (F = 3.07, P = 0.031), while neither the addition of N (F = 1.07, P = 0.095) nor the addition of NP (F = 2.04, P = 0.068) influenced the dominant bacterial taxon abundances. For soil bacteria, Proteobacteria and Gemmatimonadetes were closely tied to P addition (Fig. S2a), while no phyla were closely tied to N addition or the control (Fig. S2a). Several families were affected by N and P additions.
Effect of N and P additions on the relative abundances of the soil bacterial (a) and fungal (b) groups. C represents the control, N represents N addition, P represents P addition, and NP represents nitrogen plus phosphorus addition. The dominant groups are shown (relative abundances >1%), while the rare groups (relative abundances <1%) are integrated into “other”
Ascomycota (42.7%), Basidiomycota (11.8%), Gemmatimonadetes (8.3%), and Chloroflexi (7.3%) dominated the soil fungal community across all samples (Fig. 5b). Nitrogen addition increased Ascomycota (F = 6.01, P = 0.012) and decreased Basidiomycota (F = 6.01, P = 0.012) abundances. The addition of P reduced the abundances of Basidiomycota (F = 3.47, P = 0.022) and Chytridiomycota (F = 26.03, P = 0.002). For the soil fungal composition, Ascomycota was closely tied to P addition (Fig. S2b), while no phyla were closely tied to N addition (Fig. S2b).
The db-RDA results showed significant shifts in the community compositions of bacteria (Fig. 6a) and fungi (Fig. 6b). The variation in the bacterial community were mainly influenced by the soil available P content (F = 10.14, P = 0.004) and soil moisture (F = 3.13, P = 0.021). Soil available P content (F = 8.75, P = 0.006), soil total N content (F = 4.22, P = 0.014), and soil moisture (F = 3.27, P = 0.031) were found to be the most important parameters influencing the community composition of soil fungi. The addition of P (F = 16.07, P = 0.003) and NP (F = 4.24, P = 0.022)) strongly altered the community composition of bacteria, while the addition of N had no effect (F = 1.03, P = 0.472), Fig. 7a). Significant main effects of N (F = 14.37, P = 0.003) and P additions (F = 11.13, P = 0.004) and interactive effect of P addition × N addition (F = 20.24, P = 0.001) on soil fungal community composition were observed (Fig. 7b).
Discussion
The influence of N and P additions on soil bacterial diversity
This study provides new insight into the impacts of N and P additions on bacterial and fungal communities in a temperate meadow. The addition of N was found to have no influence on soil bacterial OTU richness or α-diversity (Fig. 3), which is inconsistent with previous studies showing that N enrichment reduced soil bacterial richness in tropical forests, temperate steppes, and arctic tundra ecosystems (Campbell et al. 2010; Zeng et al. 2016; Wang et al. 2018; Yan et al. 2018). This might be partly explained by background nutrient availability or soil pH because many previous studies demonstrated that the diversity of soil bacteria is frequently affected by the decline in soil pH caused by N addition (Fierer and Jackson 2006; Wang et al. 2018). The soil pH at our experimental site was much higher than that in the above-studied ecosystem, and N addition did not influence soil pH (Fig. S3). This suggests that N addition has few effects on soil bacterial α-diversity because no significant changes in soil pH resulted from N addition in the highly alkalized N-limited temperate meadow (Fig. S4a). Moreover, soil microbial diversity is likely to increase with the enhancement of plant diversity (van der Heijden et al. 2008). Nitrogen addition highly decreased plant species diversity, but had no impact on soil bacterial richness, suggesting that the sensitivity of soil bacteria to N addition might lag compared to that of plant species. The results also suggest that the effects of N addition on the species diversities of plants and soil bacteria may not be the same (Fierer et al. 2012).
Phosphorus addition significantly reduced soil bacterial OTU richness and α-diversity which is not in agreement with earlier studies showing that P addition did not affect soil bacterial richness (Eo and Park 2016) and that soil bacterial diversity is enhanced with P addition in an agricultural ecosystem (Tan et al. 2013). First, one possible reason for this discrepancy is the competition between soil bacteria and fungi because P addition significantly increased soil fungal diversity, which might have reduced the competition and species diversity of soil bacteria. Second, a significant negative correlation between soil bacterial richness and soil P concentration was detected (Fig. S4b), suggesting that P addition might increase the competition for nutrients between soil bacteria and plants, and thereby reducing soil bacterial richness (Zhang et al. 2014). Moreover, P addition increased the soil available P concentration which might reduce phosphate solubilizing bacteria and indirectly decrease soil bacterial richness. However, the reduction in soil bacterial diversity may not have a negative influence on soil functionality because of the functional redundancies in the soil bacterial community (Pan et al. 2014).
Influences of N and P additions on soil fungal diversity
The addition of N increased soil fungal richness which is in agreement with previous studies that N fertilization highly enhances soil fungal richness in N-limited ecosystems (Weber et al. 2013; Mueller et al. 2014), this might be related to the increase in soil N availability caused by N addition (Fig. 4). Moreover, the increase in soil fungal richness might be due to the increase in plant aboveground biomass which should favor the growth of soil fungi by increasing the growth of plant belowground biomass and reducing N competition in the soil fungi. The results suggest that some fungal species are more adaptable and tolerant to N addition, and soil N and P concentrations had significant positive influences on soil fungal richness (Fig. S4c, d). The results also indicate that the influence of N addition on soil fungal diversity might depend on the type of grassland ecosystems and the dose of nutrient addition (Zhou et al. 2016).
Phosphorus addition significantly enhanced the soil fungal richness in our present study, which is inconsistent with a previous study that reported that P fertilization reduced the soil fungal species richness (He et al. 2016). One possible reason for this is that soil fungi can increase plant P uptake which might facilitate the associations between soil fungi and plants (Zhang et al. 2014), thereby improving the biomass of soil fungi (Liu et al. 2012). This suggests that P deficiency might be used directly to predict the positive impacts of P addition on soil fungal richness.
Influences of N and P additions on the soil bacterial community composition
Our results showed that N addition had no impact on the community composition of soil bacteria, which agrees with N enrichment impacts on the bacterial community in soil across the globe (Leff et al. 2015). This may be because N addition did not decrease soil pH in the present study, in contrast to many previous studies that reported declines in pH, which is a crucial factor that changes the bacterial community (Wang et al. 2018; Guo et al. 2019). Moreover, the db-RDA results indicated that the changes in the bacterial community were mediated mainly by the soil available P concentration, suggesting that the small changes in soil N addition caused by N input did not directly affect the soil bacterial community composition by decreasing the pH and affecting the soil C/N ratio. Although the addition of N significantly affected the structure of the plant community and productivity in this studied ecosystem (Zhao et al. 2019), the changes in plant community structure and ANPP had no significant impact on the soil bacterial community, suggesting that the responses of soil bacteria to N addition might be lower than those of the plant community (Fig. S5).
In the current study, the addition of P significantly increased Actinobacteria and Acidobacteria abundances and decreased the abundances of Proteobacteria and Gemmatimonadetes. This result is consistent with previous results from a tropical forest (Wang et al. 2018). Several studies have shown that changes in pH caused by P addition play a vital role in influencing the community composition of soil bacteria (Ling et al. 2017; Wang et al. 2018). In the current study, P addition had little effect on soil pH (Fig. S3); however, the soil bacterial community was strongly determined by the soil P concentration with the addition of P and NP, suggesting that an increase in soil P availability and nutrient balance might play a critical role in shaping the soil bacterial community composition. In addition, the increase in Acidobacteria and Actinobacteria might improve plant growth because Acidobacteria have high metabolic activities in rhizosphere soil (Lee et al. 2008), and Actinobacteria may improve plant growth by alleviating soil disease suppression (Palaniyandi et al. 2013); this would increase C input from plants to the soil with the enhancement of soil bacterial activities (Li et al. 2015). However, the influence of varying C/N/P ratios caused by the addition of N and P on the soil bacteria community requires further study.
Influences of N and P additions on the community composition of soil fungi
In the present study, we found that N addition highly increased Ascomycota abundance, but decreased Basidiomycota abundances, which is consistent with previous studies showing that the addition of N can alter the community structure of soil fungi (Leff et al. 2015; Yan et al. 2018; Wu et al. 2021). One reason for this might be related to the increase in soil total N concentration caused by N addition as the soil total N concentration had a positive impact on soil fungal richness (Fig. 4). Ascomycota are saprotrophic fungi that can accelerate soil C decomposition (Xiong et al. 2014), and the increase in Ascomycota induced by N addition increases soil C decomposition and speed up the C cycle. This might explain why N addition led to the increased litter decomposition of Leymus chinensis in the temperate meadow (Gong et al. 2015). Nitrogen addition decreased Basidiomycota abundances, which may have reduced the competition pressures on Ascomycota for resources (Weber et al. 2013). Furthermore, Glomeromycota abundances remarkably declined under N addition suggesting that N addition reduces plant species diversity because most of the species are mycorrhizal plants.
In the present study, P addition significantly affected the community composition of fungi in the soil, which is in agreement with earlier studies (Liu et al. 2012; Nielsen et al. 2015; He et al. 2016). The abundances of Ascomycota and Mortierellomycota significantly increased, but Basidiomycota and Chytridiomycota abundances declined significantly upon P addition. Moreover, the db-RDA results showed that the N and P addition effects on the soil fungal community composition were possibly determined by the soil P concentration (Liu et al. 2018). These results support the previous studies, and changes in the soil fungal community structure alters the ecological function of the soil. For instance, Mortierellomycota can help prevent soil degradation (Li et al. 2019). Additionally, the changes in fungal community structure also supported previous studies that P addition can mitigate the negative impacts of N addition on the plant community structure (Limpens et al. 2004; Pilkingtona et al. 2007; Ceulemans et al. 2014). The current study indicates that changes in the community composition of soil fungi might be a good indicator to explain the impact of P addition on the plant community structure because of the tight association between plant roots and soil fungi. However, how these increased or decreased abundances of soil fungi affect the growth of different functional groups needs further study.
Conclusion
In this study, soil bacterial and fungal diversities responded differently to the addition of N and P in a salinized meadow. The addition of N had little influence on the richness of soil bacteria but had a positive impact on soil fungal richness. Phosphorus addition reduced the soil bacterial richness and increased the soil fungal richness. Nitrogen addition did not influence the bacterial community composition but affected the fungi in the soil. Additionally, both the communities of soil bacteria and fungi shifted due to P addition. The changes in soil P availability induced by N and P additions mainly determined the β-diversity of soil bacteria and fungi. Our findings suggest that the responses of the soil bacterial and fungal communities to 7-year N and P additions were not consistent in the studied ecosystem. Our results highlight that the soil fungal community might be better used to indicate the response of the soil microbial community to N and P enrichment in temperate meadow ecosystems. Moreover, the response of plant community composition to N and P additions was not consistent with the soil bacterial and fungal community composition. These results indicate that P addition might play a key role in affecting the soil microbial community composition in N-limited meadow ecosystems, and that the plant community is more flexible than the soil microbial community in tracking environmental changes.
References
Ahn H, James RT (2001) Variability, uncertainty, and sensitivity of phosphorus deposition load estimates in South Florida. Water Air Soil Poll 126:37–51. https://doi.org/10.1023/A:1005235118716
Bai Y, Wu J, Clark CM, Naeem S, Pan Q, Huang J, Zhang L, Han X (2010) Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from inner Mongolia grasslands. Glob Chang Biol 16:358–372. https://doi.org/10.1111/j.1365-2486.2009.01950.x
Bender SF, Plantenga F, Neftel A, Jocher M, Oberholzer HR, Kohl L, Giles M, Daniell TJ, van der Heijden MGA (2014) Symbiotic relationships between soil fungi and plants reduce N2O emissions from soil. ISME J 8:1336–1345. https://doi.org/10.1038/ismej.2013.224
Bender SF, Conen F, van der Heijden MGA (2015) Mycorrhizal effects on nutrient cycling, nutrient leaching and N2O production in experimental grassland. Soil Biol Biochem 80:283–292. https://doi.org/10.1016/j.soilbio.2014.10.016
Bennett JA, Maherali H, Reinhart KO, Lekberg Y, Hart MM, Klironomos J (2017) Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 355:181–184. https://doi.org/10.1126/science.aai8212
Campbell BJ, Polson SW, Hanson TE, Mack MC, Schuur EA (2010) The effect of nutrient deposition on bacterial communities in Arctic tundra soil. Environ Microbiol 12:1842–1854. https://doi.org/10.1111/j.1462-2920.2010.02189.x
Ceulemans T, Stevens CJ, Duchateau L, Jacquemyn H, Gowing DJG, Merckx R, Wallace H, van Rooijen N, Goethem T, Bobbink R, Dorland E, Gaudnik C, Alard D, Corcket E, Muller S, Dise NB, Dupré C, Diekmann M, Honnay O (2014) Soil phosphorus constrains biodiversity across European grasslands. Glob Chang Biol 20:3814–3822. https://doi.org/10.1111/gcb.12650
Cline LC, Zak DR (2015) Soil microbial communities are shaped by plant-driven changes in resource availability during secondary succession. Ecology 96:3374–3385. https://doi.org/10.1890/15-0184.1
Cui N, Shi L, Guo J, Zhang T (2021) Arbuscular mycorrhizal fungi alleviate elevated temperature and nitrogen deposition- induced warming potential by reducing soil N2O emissions in a temperate meadow. Ecol Indic 131:108193. https://doi.org/10.1016/j.ecolind.2021.108193
Delgado-Baquerizo M, Maestre FT, Reich PB, Jeffries TC, Gaitan JJ, Encinar D, Berdugo M, Campbell CD, Singh BK (2016) Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat Commun 7. https://doi.org/10.1038/ncomms10541
Eo J, Park KC (2016) Long-term effects of imbalanced fertilization on the composition and diversity of soil bacterial community. Agri Eco Environ 231:176–182. https://doi.org/10.1016/j.agee.2016.06.039
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. P Natl Acad Sci USA 103:626–631. https://doi.org/10.1073/pnas.0507535103
Fierer N, Lauber CL, Ramirez KS, Zaneveld J, Bradford MA, Knight R (2012) Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J 6:1007–1017. https://doi.org/10.1038/ismej.2011.159
Freedman Z, Eisenlord SD, Zak DR, Xue K, He ZL, Zhou JZ (2013) Towards a molecular understanding of N cycling in northern hardwood forests under future rates of N deposition. Soil Biol Biochem 66:130–138. https://doi.org/10.1016/j.soilbio.2013.07.010
Freedman ZB, Romanowicz KJ, Upchurch RA, Zak DR (2015) Differential responses of total and active soil microbial communities to long-term experimental N deposition. Soil Biol Biochem 90:275–282. https://doi.org/10.1016/j.soilbio.2015.08.014
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x
Gong S, Guo R, Zhang T, Guo J (2015) Warming and nitrogen addition increase litter decomposition in a temperate meadow ecosystem. PLoS One 10:e0116013. https://doi.org/10.1371/journal.pone.0116013
Guo QX, Yan LJ, Korpelainen H, Niinemets U, Li CY (2019) Plant-plant interactions and N fertilization shape soil bacterial and fungal communities. Soil Biol Biochem 128:127–138. https://doi.org/10.1016/j.soilbio.2018.10.018
Harrison S (2020) Plant community diversity will decline more than increase under climatic warming. Philos T Roy Soc B 375(1794):20190106
He D, Xiang XJ, He JS, Wang C, Cao GM, Adams J, Chu HY (2016) Composition of the soil fungal community is more sensitive to phosphorus than nitrogen addition in the alpine meadow on the Qinghai-Tibetan plateau. Biol Fert Soil 52:1059–1072. https://doi.org/10.1007/s00374-016-1142-4
Huang J, Hu B, Qi K, Chen W, Pang X, Bao W, Tian G (2016) Effects of phosphorus addition on soil microbial biomass and community composition in a subalpine spruce plantation. Eur J Soil Biol 72:35–41. https://doi.org/10.1016/j.ejsobi.2015.12.007
Jiang FY, Zhang L, Zhou JC, George TS, Feng G (2021) Arbuscular mycorrhizal fungi enhance mineralisation of organic phosphorus by carrying bacteria along their extraradical hyphae. New Phytol 230:304–315. https://doi.org/10.1111/nph.17081
Kang F, Yang B, Wujisiguleng, Yang X, Wang L, Guo JX, Sun W, Zhang Q, Zhang T (2020) Arbuscular mycorrhizal fungi alleviate the negative effect of nitrogen deposition on ecosystem functions in meadow grassland. Land Degrad Dev 31:748–759. https://doi.org/10.1002/ldr.3491
Lee SH, Ka JO, Cho JC (2008) Members of the phylum Acidobacteria are dominant and metabolically active in rhizosphere soil. FEMS Microbiol Lett 285:263–269. https://doi.org/10.1111/j.1574-6968.2008.01232.x
Leff JW, Jones SE, Prober SM, Barberan A, Borer ET, Firn JL, Harpole WS, Hobbie SE, Hofmockel KS, Knops JMH, McCulley RL, La Pierre K, Risch AC, Seabloom EW, Schutz M, Steenbock C, Stevens CJ, Fierer N (2015) Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. P Natl Acad Sci USA 112:10967–10972. https://doi.org/10.1073/pnas.1508382112
Li J, Li ZA, Wang FM, Zou B, Chen Y, Zhao J, Mo QF, Li YW, Li XB, Xia HP (2015) Effects of nitrogen and phosphorus addition on soil microbial community in a secondary tropical forest of China. Biol Fert Soil 51:207–215. https://doi.org/10.1007/s00374-014-0964-1
Li PD, Jeewon R, Aruna B, Li HY, Lin FC, Wang HK (2019) Metabarcoding reveals differences in fungal communities between unflooded versus tidal flat soil in coastal saline ecosystem. Sci Total Environ 690:911–922. https://doi.org/10.1016/j.scitotenv.2019.06.473
Li J, Li C, Kou Y, Yao M, He Z, Li X (2020) Distinct mechanisms shape soil bacterial and fungal co-occurrence networks in a mountain ecosystem. FEMS Microbiol Ecol 96(4):fiaa030. https://doi.org/10.1093/femsec/fiaa030
Li J, Sang C, Yang J, Qu L, Xia Z, Sun H, Jiang P, Wang X, He H, Wang C (2021) Stoichiometric imbalance and microbial community regulate microbial elements use efficiencies under nitrogen addition. Soil Biol Biochem 156. https://doi.org/10.1016/j.soilbio.2021.108207
Limpens J, Berendse F, Klees H (2004) How phosphorus availability affects the impact of nitrogen deposition on sphagnum and vascular plants in bogs. Ecosystems 7:793–804. https://doi.org/10.1007/s10021-004-0274-9
Ling N, Chen D, Guo H, Wei J, Bai Y, Shen Q, Hu S (2017) Differential responses of soil bacterial communities to long-term N and P inputs in a semi-arid steppe. Geoderma 292:25–33. https://doi.org/10.1016/j.geoderma.2017.01.013
Liu L, Gundersen P, Zhang T, Mo J (2012) Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol Biochem 44:31–38. https://doi.org/10.1016/j.soilbio.2011.08.017
Liu M, Liu J, Chen X, Jiang C, Wu M, Li Z (2018) Shifts in bacterial and fungal diversity in a paddy soil faced with phosphorus surplus. Biol Fert Soil 54:259–267. https://doi.org/10.1007/s00374-017-1258-1
Mei LL, Yang X, Zhang SQ, Zhang T, Guo JX (2019) Arbuscular mycorrhizal fungi alleviate phosphorus limitation by reducing plant N:P ratios under warming and nitrogen addition in a temperate meadow ecosystem. Sci Total Environ 686:1129–1139. https://doi.org/10.1016/j.scitotenv.2019.06.035
Mori H, Maruyama F, Kato H, Toyoda A, Dozono A, Ohtsubo Y, Nagata Y, Fujiyama A, Tsuda M, Kurokawa K (2014) Design and experimental application of a novel non-degenerate universal primer set that amplifies prokaryotic 16S rRNA genes with a low possibility to amplify eukaryotic rRNA genes. DNA Res 21:217–227. https://doi.org/10.1093/dnares/dst052
Mueller RC, Balasch MM, Kuske CR (2014) Contrasting soil fungal community responses to experimental nitrogen addition using the large subunit rRNA taxonomic marker and cellobiohydrolase I functional marker. Mol Ecol 23:4406–4417. https://doi.org/10.1111/mec.12858
Nielsen UN, Prior S, Delroy B, Walker JKM, Ellsworth DS, Powell JR (2015) Response of belowground communities to short-term phosphorus addition in a phosphorus-limited woodland. Plant Soil 391:321–331. https://doi.org/10.1007/s11104-015-2432-6
Palaniyandi SA, Yang SH, Zhang L, Suh JW (2013) Effects of actinobacteria on plant disease suppression and growth promotion. Appl Microbiol Biotech 97:9621–9636. https://doi.org/10.1007/s00253-013-5206-1
Pan Y, Cassman N, de Hollander M, Mendes LW, Korevaar H, Geerts RH, van Veen JA, Kuramae EE (2014) Impact of long-term N, P, K, and NPK fertilization on the composition and potential functions of the bacterial community in grassland soil. FEMS Microbiol Ecol 90:195–205. https://doi.org/10.1111/1574-6941.12384
Peñuelas J, Poulter B, Sardans J, Ciais P, van der Velde M, Bopp L, Boucher O, Godderis Y, Hinsinger P, Llusia J, Nardin E, Vicca S, Obersteiner M, Janssens IA (2013) Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat Commun 4: 1–10. https://doi/org/https://doi.org/10.1038/ncomms3934
Pilkingtona MG, Capornb SJM, Carrollb JA, Cresswellc N, Leed JA, Emmette BA, Bagchid R (2007) Phosphorus supply influences heathland responses to atmospheric nitrogen deposition. Environ Pollut 148:191–200. https://doi.org/10.1016/j.envpol.2006.10.034
R Core Team (2019) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna https://www.R-project.org/
Ramirez KS, Craine JM, Fierer N (2012) Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob Chang Biol 18:1918–1927. https://doi.org/10.1111/j.1365-2486.2012.02639.x
Rooney DC, Clipson NJW (2009) Phosphate addition and plant species alters microbial community structure in acidic upland grassland. Soil Microbial Ecol 57:4–13
Storer K, Coggan A, Ineson P, Hodge A (2018) Arbuscular mycorrhizal fungi reduce nitrous oxide emissions from N2O hotspots. New Phytol 220:1285–1295. https://doi.org/10.1111/nph.14931
Su JQ, Ding LJ, Xue K, Yao HY, Quensen J, Bai SJ, Wei WX, Wu JS, Zhou J, Tiedje JM (2015) Long-term balanced fertilization increases the soil microbial functional diversity in a phosphorus-limited paddy soil. Mol Ecol 24:136–150. https://doi.org/10.1111/mec.13010
Tan H, Barret M, Mooij MJ, Rice O, Morrissey JP, Dobson A, Griffiths B, O’Gara F (2013) Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol Fert Soil 49:661–672. https://doi.org/10.1007/s00374-012-0755-5
Thompson K, Bent E, Abalos D, Wagner-Riddle C, Dunfield K (2016) Soil microbial communities as potential regulators of in situ N2O fluxes in annual and perennial cropping systems. Soil Biol Biochem 103:262–273. https://doi.org/10.1016/j.soilbio.2016.08.030
van der Heijden MGA, Bardgett RD, van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310. https://doi.org/10.1111/j.1461-0248.2007.01139.x
Vellend M, Baeten L, Becker-Scarpitta A, Boucher-Lalonde V, McCune JL, Messier J, Myers-Smith IH, Sax DF (2017) Plant biodiversity change across scales during the anthropocene. Annu Rev Plant Biol 68:563–586. https://doi.org/10.1146/annurev-arplant-042916-040949
Vicars WC, Sickman JO, Ziemann PJ (2010) Atmospheric phosphorus deposition at a montane site: size distribution, effects of wildfire, and ecological implications. Atmos Environ 44:2813–2821. https://doi.org/10.1016/j.atmosenv.2010.04.055
Wan XH, Huang ZQ, He ZM, Yu ZP, Wang MH, Davis MR, Yang YS (2015) Soil C:N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil 387:103–116. https://doi.org/10.1007/s11104-014-2277-4
Wang H, Liu SR, Zhang X, Mao QG, Li XZ, You YM, Wang JX, Zheng MH, Zhang W, Lu XK, Mo JM (2018) Nitrogen addition reduces soil bacterial richness, while phosphorus addition alters community composition in an old-growth N-rich tropical forest in southern China. Soil Biol Biochem 127:22–30. https://doi.org/10.1016/j.soilbio.2018.08.022
Weber CF, Vilgalys R, Kuske CR (2013) Changes in fungal community composition in response to elevated atmospheric CO2 and nitrogen fertilization varies with soil horizon. Front Microbiol 4:78. https://doi.org/10.3389/fmicb.2013.00078
Wen X, Xu W, Li Q, Han MJ, Tang AH, Zhang Y, Luo XS, Shen JL, Wang W, Li KH, Pan YP, Zhang L, Li WQ, Collett JL, Zhong BQ, Wang XM, Goulding K, Zhang FS (2020) Changes of nitrogen deposition in China from 1980 to 2018. Environ Int 144:106022. https://doi.org/10.1016/j.envint.2020.106022
White T, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. PCR protocols: a guide to methods and applications 315–322
Wu L, Wang Y, Zhang S, Wei W, Kuzyakov Y, Ding X (2021) Fertilization effects on microbial community composition and aggregate formation in saline-alkaline soil. Plant Soil. https://doi.org/10.1007/s11104-021-04909-w
Xiong J, Peng F, Sun H, Xue X, Chu H (2014) Divergent responses of soil fungi functional groups to short-term warming. Microbial Ecol 68:708–715. https://doi.org/10.1007/s00248-014-0385-6
Yan G, Xing Y, Wang J, Zhang Z, Xu L, Han S, Zhang J, Dai G, Wang Q (2018) Effects of winter snowpack and nitrogen addition on the soil microbial community in a temperate forest in northeastern China. Ecol Indic 93:602–611. https://doi.org/10.1016/j.ecolind.2018.05.048
Yang Y, Li T, Wang YQ, Cheng H, Chang SX, Liang C, An SS (2021a) Negative effects of multiple global change factors on soil microbial diversity. Soil Biol Biochem 156. https://doi.org/10.1016/j.soilbio.2021.108229
Yang X, Mariotte P, Guo J, Hautier Y, Zhang T (2021b) Suppression of arbuscular mycorrhizal fungi decreases the temporal stability of community productivity under elevated temperature and nitrogen addition in a temperate meadow. Sci Total Environ 762:143137. https://doi.org/10.1016/j.scitotenv.2020.143137
Zeng J, Liu X, Song L, Lin X, Zhang H, Shen C, Chu H (2016) Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol Biochem 92:41–49. https://doi.org/10.1016/j.soilbio.2015.09.018
Zhang L, Fan JQ, Ding XD, He XH, Zhang FS, Feng G (2014) Hyphosphere interactions between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium promote phytate mineralization in soil. Soil Biol Biochem 74:177–183. https://doi.org/10.1016/j.soilbio.2014.03.004
Zhang T, Guo R, Gao S, Guo JX, Sun W (2015) Responses of plant community composition and biomass production to warming and nitrogen deposition in a temperate meadow ecosystem. PLoS One 10. https://doi.org/10.1371/journal.pone.0123160
Zhang T, Yang X, Guo R, Guo J (2016) Response of AM fungi spore population to elevated temperature and nitrogen addition and their influence on the plant community composition and productivity. Sci Rep 6. https://doi.org/10.1038/srep24749
Zhao Y, Yang B, Li M, Xiao R, Rao K, Wang J, Zhang T, Guo J (2019) Community composition, structure and productivity in response to nitrogen and phosphorus additions in a temperate meadow. Sci Total Environ 654:863–871. https://doi.org/10.1016/j.scitotenv.2018.11.155
Zhou J, Jiang X, Zhou B, Zhao B, Ma M, Guan D, Li J, Chen S, Cao F, Shen D (2016) Thirty four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in Northeast China. Soil Biol Biochem 95:135–143. https://doi.org/10.1016/j.soilbio.2015.12.012
Zhu JX, Wang QF, He NP, Smith MD, Elser JJ, Du JQ, Yuan GF, Yu GR, Yu Q (2016) Imbalanced atmospheric nitrogen and phosphorus depositions in China: implications for nutrient limitation. J Geophys Res-Biogeo 121:1605–1616. https://doi.org/10.1002/2016JG003393
Acknowledgements
This work was funded by National Natural Science Foundation of China (31770359, 32171645), Foundation of Science and Technology Commission of Jilin Province (20200201115JC) and the Fundamental Research Funds for the Central Universities (2412020ZD010). We would like to thank the anonymous reviewers for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Responsible Editor: Raúl Ochoa-Hueso
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 950 kb)
Rights and permissions
About this article
Cite this article
Yan, Y., Sun, X., Sun, F. et al. Sensitivity of soil fungal and bacterial community compositions to nitrogen and phosphorus additions in a temperate meadow. Plant Soil 471, 477–490 (2022). https://doi.org/10.1007/s11104-021-05237-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-05237-9