Abstract
Purpose
Nitrogen (N) and phosphorus (P) additions are the widely used restoration management for degraded grasslands. However, soil microbial community responses to N and P additions are still not well understood, especially multiple levels or/and combined N and P additions in high-altitude grasslands. In this study, the single and interactive effects of N and P additions on the soil microbes were explored.
Methods
A field experiment with two factors (N 0, 7.5, and 15 g N m−2 year−1; P 0, 3.27, and 6.55 g P m−2 year−1) was conducted by a completely randomized block design in a Tibetan plateau steppe. Total and specific microbial biomass (bacteria, fungi, G+/G− bacteria, saprotrophs, actinomycetes, and arbuscular mycorrhizal fungi) were analyzed by using phospholipid fatty acids (PLFAs). Additionally, based on their fixed weight composites, we used a composite variable (phenotype) to represent the microbial community.
Results
The results showed that N addition decreased the microbe phenotype, total microbial biomass, and specific microbial biomass (total bacteria, G+, and G− bacteria). P application and its interaction with N application all increased the microbial phenotype, total microbial biomass, and specific microbial biomass (total bacteria, G+, G−, saprotrophs, and actinomycetes). Besides, the microbial phenotype was positively correlated with P application rates and not correlated with N application rates.
Conclusion
In conclusion, P was the key limiting factor for soil microbes, and its interaction with N is also important for some specific microbes. In addition, long-term responses of soil microbes after N and P amendments need further investigation in the Tibetan alpine steppe.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
As the “Roof of the World” (Royden et al. 2008), the Tibetan alpine is an important water reservoir, playing a key role in regulating climate change (Chen et al. 2014). However, the Tibetan alpine ecosystem is also vulnerable (Yeh et al. 2014), some of which have gradually degraded due to anthropogenic activities and climate change (Lin et al. 2015).
In the Tibetan alpine, nitrogen (N) and phosphorus (P), the key elements that drive biological processes (Tischer et al. 2015), may be the limiting factors for plant growth; thus, fertilization may improve the quality of grassland. Soil microbes are the critical component of grassland ecosystems, and they are correlated with the plant community (Dong et al. 2019). Plant diversity can promote soil microbial diversity by increasing the diversity of food resources and plant hosts for symbiotic and pathogenic microbes, and soil microbes can promote plant diversity by increasing the diversity of available nutrient pools in return (Klironomos et al. 2011; Zeng et al. 2016). Generally, it is believed that microbial biomass depends directly on the inputs of carbon to the soil. Some studies have found that microbial biomass may also be limited by the availability of N or P (Wardle 1992; Liu et al. 2012). In our previous studies, we found a significant increasing tendency of biomass after fertilization (Dong et al. 2016), which was consistent with some findings that fertilization is an appropriate remedy and is one of the main approaches used to restore degraded grasslands (Wang et al. 2015b; Xu et al. 2015).
The effects of N additions on plant communities have been well studied in some grassland ecosystems (Nemergut et al. 2008; Ramirez et al. 2012; Mueller et al. 2015; Leff et al. 2015), while the responses of the microbial community remain poorly understood. Nelson et al. (2015) found that microorganisms have different abilities to utilize N through various assimilatory and dissimilatory pathways, and the community composition and microbial diversity are likely to influence N cycling in ecosystems. In natural ecosystems, fertilizer can have strong effects on the aboveground community as well as the belowground soil community (Bezemer et al. 2005). N and P additions affect aboveground biomass frequently in the Tibetan alpine steppe (Dong et al. 2016), and N addition also profoundly impacts the soil microbial communities across different terrestrial ecosystems (Zhou et al. 2012; Zhang et al. 2014; Yang et al. 2015). Furthermore, P addition also significantly stimulates the soil inorganic N pools (Wang et al. 2014), which results in more N being immobilized by microbial communities and plants.
Phosphorus is another macronutrient for both plant and microbial growth and can be broadly categorized into either mineral or organic P in soils. Plants obtain the majority of their P from the labile inorganic phosphate pool within soils, and the bioavailability of labile P is often very low (Curtin and Smillie 1984; Rooney and Clipson 2008). In particular, functionally significant groups, such as mycorrhizal fungi, are known to affect the P supply in higher plants (Stevenson 1986) and are likely to be prominent in P nutrition in unimproved grasslands (Rooney and Clipson 2008). Some studies have reported that there was no significant response of soil microbial biomass to P addition because of low bioavailability (Bünemann et al. 2004; Shi et al. 2012). To date, most studies reported the effect of single N or P addition, while fewer studies focused on the interaction between N and P, especially with multiple rates respect to the response of the microbial community in the Tibetan alpine steppe.
In this study, we conducted a field experiment with several rates of N and P addition to investigate the relationship between the soil-microbe-plant in a Tibetan alpine steppe. The objectives of our study were to determine the following: (1) the responses of different soil microbial community to N and P application; and (2) the main environmental factors that controlled the soil microbial community. Based on the previous findings, we hypothesized the following: (1) the main soil microbial community, including bacteria and fungi, was affected by N and P application, while other soil microbial community remained unaffected; and (2) the contents of available N and P were the controlled environmental factors for the soil microbial community. These investigations would help us figure out the limiting factors for soil microbial community and restore the degraded grassland.
2 Materials and methods
2.1 Study site
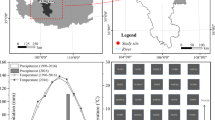
An in situ field experiment was established on the Tibetan alpine steppe, which was located in Baingoin County on the central Qinghai-Tibet alpine (N 31° 26′, E 90° 02′, 4678 m above sea level), in southwestern of China. Before its establishment, the steppe was grazed daily by yaks and sheep, and no fertilizer had been used. The location is a semiarid cold alpine steppe, and the area has a frigid, sub-humid, and semiarid alpine monsoon climate. Based on information from the local observatory, the annual sunshine duration is 3210.3 h, and the annual mean temperature is − 1.2 °C (the mean maximum temperature is 14.7 °C in July, and the mean minimum temperature is − 17.5 °C in January). The annual rainfall ranges from 289 to 390 mm, with a mean of 301.2 mm, 80% of which falls from June to September. The evaporation ranges from 1993.4 to 2104.3 mm. The soil at the study site is alpine steppe soil (according to Chinese Soil Taxonomy) and Gelic Cambisols (according to the Food and Agriculture Organization of the United Nations classification). Soil properties measured prior to fertilization are presented in the supplementary material (Supplementary Table 1). The field experiment was on a 5.5° gentle slope near the upper edge, which was dominated by Stipa purpurea, and was accompanied by Leontopodium leontopodioide and Heteropappus bowerii.
2.2 Experimental design
The two factors, N (three levels: N0, 0; N1, 7.5 g N m−2 year−1; N2, 15 g N m−2 year−1) and P (three levels: P0, 0; P1, 3.27 g P m−2 year−1; P2, 6.55 g P m−2 year−1), in a completely randomized block design formed 9 treatments with four replications. In July 2013, the fertilized experimental plots were established, and they were all fenced using wire netting to prevent disturbance caused by large animals (e.g., yaks and sheep). The 36 plots measured 5 × 5 m2 in size and were separated by 2-m buffer zones to avoid cross-contamination. The distance to the nearest pen exceeded 3 m. Nitrogen was added as urea, of which the effective component is CO(NH2)2 (containing 46.4% N), and P was applied as a triple super-phosphate, of which the effective component is Ca(H2PO4)2·H2O (containing 46% P2O5). The pelletized fertilizer was evenly applied twice annually, and usually at dusk.
2.3 Soil sampling and analysis
Soil sampling was conducted in September 2014, which is two growing seasons after the first fertilization. Seven soil cores (3.5 cm diameter) of top 10 cm were randomly collected and then mixed each plot. Visible plant roots and stones were manually removed before passing the soil through a sieve (2-mm-square aperture mesh). The soil was divided into two subsamples. One of the subsamples was immediately transported to the laboratory and stored at − 80 °C for subsequent phospholipid fatty acid (PLFA) analysis. The other was air-dried for the analysis of pH, soil organic matter (SOM), soil total N (TN), total soil P (TP), soil available N (AN), soil available potassium (AK), and soil available P (AP).
The pH was measured using water (soil:water 1:2.5); the SOM was determined by potassium dichromate oxidation (Nelson and Sommers 1982); the TN was determined by the Kjeldahl method (Bremner and Mulvaney 1982); and AN was measured by the alkali-hydrolyzed reduction diffusing method. The soil available P was measured by the NaHCO3 extraction–molybdenum antimony anti-colorimetric method as described by Olsen (Olsen 1954). The soil total P was determined by the H2SO4-HClO4 digestion method, and the absorbance of AP/TP was measured at 880 nm using a UV-visible spectrophotometer after coloring with the molybdenum antimony reagent (UV2700, SHIMADZU Corporation, Japan). The soil available K, which was extracted by CH3COONH4 (1 M), was determined by atomic absorption spectrophotometry using a spectrophotometer (ZEEnit700P, Analytik Jena AG, Germany). The soil moisture content (SMC) was determined by using the gravimetric method, with a percent of the water occupying the oven-dried soil.
2.4 Plant sampling and analysis
In accordance with earlier studies, the total species were divided into 5 communities: Gramineae (including S. purpurea, Poa annual, and Festuca coelestis), Compositae (including L. leontopodioide and Heteropappus poppy flower), Cyperaceae (including C. oxyleuca V. Krecz, Carex moorcroftii, and Kobresia pygmaea), Rosaceae (including Potentilla bifurca Linn. and Potentilla multifida), and forbs (including sickle pod beans jujube, Rhodiola rosea L., and Androsace mariae).
One 1 × 1-m2 plot was chosen randomly within each 5 × 5-m2 plot to investigate the plant community structure (height, coverage, and biomass). In the 1 × 1-m2 plots, all of the aboveground plants were clipped to the surface, sorted into species, and saved in separate marked paper bags after the survey. The plant root was excavated from the soil sample (0–10-cm depth) using a 2-mm-square aperture mesh and was washed with water. Then, the above and below plant were oven-dried at 65 °C until constant weight. Afterwards, the plant samples were digested by H2O2-H2SO4, then by using indophenol blue colorimetry for the plant total N and using the Mo-Sb colorimetric method for the plant total P (Dudenhoeffer et al. 2013).
The plant Shannon diversity (H′) and Pielou’s evenness were calculated to determine the changes in the plant community. The formulas are showed as below:
where IV is an important values index, b is the species’ relative biomass, h is the species’ relative height, c is the species’ relative coverage, Pi is the species’ IV, and S is the plant richness.
2.5 Analysis of the microbial community structure
The soil microbial community was characterized using PLFA as described by Bossio and Scow (Bossio and Scow 1998). For each sample, the abundance of individual fatty acid methyl esters was expressed as nmol PLFA g−1 dry soil.
The following biomarkers were used: gram-positive bacteria (i14:0, i15:0, a15:0, i16:0, a17:0, and i17:0) and gram-negative bacteria (16:1ω7c, cy17:0, 18:1ω7c, cy19:0) (Liu et al. 2015; Tischer et al. 2015). The sum of the gram-positive bacteria (G+), gram-negative bacteria (G−) and non-specific bacteria (17:0) was used as a measure of the bacterial biomass. Saprotrophic fungal biomarkers (18:1ω9c and 18:2ω6, 9c) and arbuscular mycorrhizal fungi (AMF) (16:1ω5c and 20:1ω9c) were summed and included in the fungal microbial group (Muruganandam et al. 2010; Gray et al. 2011). The ratio of fungi to bacterial PLFAs (F:B) was used to analyze the changes in the microbial community structure. The degree of physiological stress experienced by the gram-negative bacterial community was estimated by using the bacterial stress index (BSI) for each sample: BSI = cy17:0/16:1ω7c (Guckert et al. 1986; Bossio and Scow 1998). The following were used as indicators for actinomycetes: 16:0 10-methyl, 17:0 10-methyl, and 18:0 10-methyl, TBSA (Gray et al. 2011; Liu et al. 2015). The total PLFAs also included 14:0, 16:0, 16:1 2OH, 16:1ω9c, and 17:1ω8c, which were also used to analyze the composition of the microbial community (Liu et al. 2015), while there was no group that they represented. The microbial Shannon diversity was calculated according to formula (2), where Pi is the individual PLFAs’ relative concentration, and S is the number of PLFAs.
2.6 Statistical analysis
We used the “phenotype” as a composite variable to represent the microbes, soil, or plant properties, including the plant-biomass phenotype and plant-nutrient phenotype of different communities (Roger et al. 2013). Briefly, each variable from the original measurements was divided by its highest value for the same property, and then the fixed weight composite variable was produced, which ranged from 0 to 1, with the no detection value equal to 0 and the highest value equal to 1 (Grace and Bollen 2008). All the fixed weight composites of each treatment were taken together to combine the (microbe/soil/plant) “phenotype.” The properties used for each phenotype are presented in the supplementary material (Supplementary Table 2). To compare the differences between the treatments, the biomasses of the total PLFAs, bacteria, and fungi, G+, G− and AMF were calculated to μmol per g of dry soil. A one-way analysis of variance (ANOVA) was performed to determine the significance of each treatment, and two-way ANOVAs were performed to examine the significance of N and P and their interactions. Statistical significance was determined at P < 0.05. All statistical analyses were conducted using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Duncan’s multiple range test (DMRT) was used to test the differences among treatments. Tamhan’s T2 was used to test the differences among treatments when homogeneity of variances was not equal.
The concentration of the individual PLFAs was standardized to the unit variance (scaling) before being subjected to principal component analysis (PCA) by R using the vegan package. Detrended correspondence analysis (DCA) was performed between soil properties and the microbial community composition, and then, a redundancy analysis (RDA) was chosen to analyze the relationship, because the length of the gradient was 0.534 (< 3.0). PCA was also used for the soil and plant properties using the vegan package. The mutual Pearson correlation coefficient among the microbe phenotype, soil phenotype, or plant phenotype was calculated, and their individual Pearson correlations with N or P addition levels were also produced. The mutual correlation of the microbial community (indicated by total PLFAs biomass, microbial diversity), plant community (indicated by plant community diversity and evenness, plant biomass, N, or P content of plant), and the PCA of soil were also performed by using the R “PerformanceAnalytics” package.
3 Results
The plant-biomass phenotype, plant-nutrient phenotype, and aboveground biomass were significantly influenced by N and P application, and there were interactive effects between N and P application on the plant-nutrient phenotype (Table 1). We named PC1 based on the plant-nutrient properties as plant-nutrient N and PC2 based on the plant-nutrient phosphorus (Supplementary Table 3). The results of ANOVA indicated that both single and interactive effects of N and P addition had a significant impact on the plant-nutrient nitrogen and plant-nutrient phosphorus. The PC1 of soil properties was named for the soil nitrogen, and PC2 was named for the soil phosphorus (Supplementary Table 4). Compared to single N addition, the soil phenotype and soil N were significantly affected by sole P addition, and the pH was significantly influenced by N addition, while the interactive effects between N and P on soil properties were not significant (Table 1).
A principal component analysis was used for the soil microbes by the mole percent of the individual PLFAs, and PC1 could explain 62.11% of the variance, while PC2 could explain 13.04% of the variance. Compared to the control, with P or N amendments, the microbial community had a changed tendency (Fig. 1).
Principal component analysis (PCA) of all sites using PLFA concentrations (nmol g−1 dry soil). Green indicates 0 g P m−2 year−1, black indicates 3.27 g P m−2 year−1, red indicates 6.55 g P m−2 year−1. White square indicates 0 g N m−2 year−1, white up-pointing triangle indicates 7.5 g N m−2 year−1, White square with X indicates 15 g N m−2 year−1
When comparing the microbial responses at the specific community level, we found that G+, G−, and bacteria were significantly influenced by N addition; furthermore, these microbial communities all had highly significant responses after P addition or the interaction between N and P addition. The sole N or P addition had non-significant impacts on fungi, while their interaction was highly significant. Saprotrophs and actinomycetes were significantly influenced by P addition and the interaction between N and P addition (Table 2). However, some specific microbes, such as i14:0 (G+), a15:0 (G+), 10Me16:0 (actinomycetes), i17:0 (G+), 10Me17:0 (actinomycetes), 18:2ω6, 9c (saprotroph), 18:1ω9c (saprotroph), and 18:1ω7c (G−), were also affected by the P addition and the interaction between N and P addition (Supplementary Table 5).
Treatments of N0P1, N0P2, and N1P2 significantly improved the microbial phenotype and total microbial biomass compared to that of the control (Fig. 2a). There was a definite tendency for the microbe phenotype and the total microbial biomass to increase slightly with the increasing P application, while the total microbial biomass was decreased by the N addition (Fig. 2b, c). The interactive effects of NP were also significant for the microbe phenotype (P < 0.001, F = 4) and the total microbial biomass (P < 0.001, F = 4).
The significance of microbe phenotype, total PLFAs biomass, and microbial Shannon diversity at different treatment (a), nitrogen application level (b), and phosphorus application level (c). The significant difference was indicated by different letter. N0, 0; N1, 7.5 g N m−2 year−1; N2, 15 g N m−2 year−1; P0, 0; P1, 7.5 g P2O5 m−2 year−1; P2, 15 g P2O5 m−2 year−1
The correlation for all possible pairs of soil phenotype, microbial phenotype, and plant phenotype was non-significant, and there were no correlations of the microbial phenotype to soil phenotype or plant phenotype. The microbial phenotype was significantly positively correlated with the P application rates, while it had no correlation with N addition rates (Fig. 3a). We could not find a significant relationship between the microbial phenotype and soil phosphorus (P = 0.8021), soil nitrogen (P = 0.3841), plant-nutrient phosphorus (P = 0.1940), or plant-nutrient nitrogen (P = 0.1197) (Fig. 3b). However, there was no correlation between the total microbial biomass and microbial diversity to the principal components of soil or plant properties (Fig. 3b).
The correlation between soil, plant, and microbes. Panel a expressed the relationship between phenotype and N, P application, and panel b showed the correlation between the principal components of soil, plant properties, and microbes. The number in panel a indicated the correlations coefficients. In panel b, positive correlations are displayed in blue and negative correlations in red color; color intensity and the size of the circle are proportional to the correlation coefficients; in the right side of the correlogram, the legend color shows the correlation coefficients and the corresponding colors. P, plant community; PN, plant nutrient; S, soil; SOM, soil organic matter content. **P < 0.01 and *P < 0.05
There were significant correlations between G− bacteria to soil AP and soil TP, while soil TN was negatively correlated with the G− bacteria (Fig. 4a). The i16:0 (G+), 18:1ω9c (saprotrophic fungus), and 20:1ω9c (AMF) indicated the statuses of the soil AP, AN, and TN, respectively, which were shown by the definite correlation between them (Fig. 4b). The 16:1ω7c (G−) was correlated with the soil AP and TP (Fig. 4b).
The redundancy analysis of microbial community or specific PLFA to soil properties. AP, soil available phosphorus content; TP, soil total phosphorus content; SOM, soil organic matter content; AN, soil available nitrogen content; TN, soil total nitrogen content; AK, soil available potassium content; SMC, soil moisture; Act indicates the Actinomycetes; SaF, saprotrophic fungi; AMF, arbuscular mycorrhizal fungus; G−, Gram-negative bacteria; G+, Gram-positive bacteria; F/B, ratio of fungi to bacteria
4 Discussion
4.1 Effects of N and P addition on the microbes
In addition to the plant biomass, the microbial communities were also very sensitive to N and/or P addition (Leff et al. 2015; Dong et al. 2019; Zhou et al. 2020). It is well known that bacteria and fungi are the main components of the microbial community (Che et al. 2019). Past studies suggested that high N addition led to reduction in microbial biomass in Tibetan alpine steppe and other grasslands (Geisseler et al. 2016). Generally, bacteria were more sensitive to N addition than fungi (Zhang et al. 2016). These changes may be because of the reduced C-allocation to the belowground after N amendment (Demoling et al. 2008). N application tended to increase the G+:G− ratio, while opposite results were found in other grasslands (Geisseler et al. 2016). This was because N fertilizer reduced the synthesis of various energy-consuming oxidative enzymes, such as phenol oxidase and peroxidase, thereby leading to a limitation of substrate resources (Cusack et al. 2011; Li et al. 2015). The negative impact of adding N fertilizer was temporary, as the combined N and P addition could still enhance the microbial biomass. These results highlighted that P was the limiting nutrient factor for the soil microbes and the combined application of N and P had positive effects on the microbes in the Tibetan alpine steppe. Additionally, soil microbes can interact with plant by several relationships, including pathogenesis, parasitism, and mutualism, to affect them (Newton et al. 2010; Zhang et al. 2019). In some cases, plant species richness was the main factor controlling the soil microbial biomass (Zeng et al. 2016; Geisseler et al. 2016). However, the plant Shannon diversity was not affected by the N and P or their combined effects, and there was a non-significant correlation between the plant Shannon diversity and the total PLFAs biomass in this study. One potential reason is that the short duration of treatments could not trigger obvious changes in plant community composition. (Kulmatiski and Beard 2011; Zong and Shi 2019).
At the specific microbe level, most bacteria, e.g., i14:0 and 16:1ω7c, were negatively correlated with the content of soil total N and available N, while others were positive, such as i17:0 (G+) and 18:1ω7c (G−). These different correlations were due to the different growth strategies of the microbes, and their varied communicated relationships among them (Redman et al. 2001; Schimel et al. 2007). With the increasing N input, the biomass of plant root showed a decreased tendency (4.23 g, 3.25 g, and 3.87 g of dried root at 0, 7.5, and 15 g N m−2 year−1, respectively) and the microbial biomass also showed the same trend in this field study. The plant litterfall is an important source of soil carbon (C) for bacteria and increased plant litter can enhance the abundance of bacteria (Wang et al. 2015a). With the N application, the aboveground C of plant was significantly enhanced, while belowground C of plant was decreased and the soil C remained unaffected (Lu et al. 2011; Li et al. 2015). The negative effects of N enrichment on the microbial biomass also can be explained by the reduced C to soil induced by N (Smith et al. 2010; Chen et al. 2015). The contrasting responses of the microbial biomass and aboveground biomass also indicated that 15 g N m−2 year−1 was suitable for plants but not for the soil microbes. As another composition of soil microbial community, soil fungi showed different responses facing N and P addition compared to the soil microbes (Table 1). This was because soil fungi have different functional roles and patterns of resource utilization related to decomposition compared to the soil bacteria (Schneider et al. 2012), and their shifts in the relative abundance altered the biogeochemical cycles (Mueller et al. 2015), thus affected the responses of bacteria and fungi to N and P addition.
Compared to N addition, P application had larger effects on soil microbes in this study. The soil microbe phenotype and the microbial biomass were significantly enhanced by P application. This was because with the increasing of the content of the soil available P after P addition, the belowground biomass of plant was improved, hence enhanced the plant C distribution to soil (Huang et al. 2018). Another reason was that the increased soil P availability enhanced the rate of C cycles, especially the fresh labile C (Nottingham et al. 2015). The positive correlations between the content of soil available P and the soil microbe phenotype (R2 was 0.226 and P value was 0.185) and the microbial biomass (R2 was 0.226 and P value was 0.256) also supported these points.
The different effects of N and P addition were attributed to their impacts on the soil pH. In the former study, we found that there was a slightly decreasing tendency of the soil pH after N addition (Dong et al. 2016). In contrast, the P addition had no significant influence on the soil pH in this study. The decrease of soil pH induced by N addition constrained the superiority of the soil microbes (Rousk et al. 2010), which resulted in the negative effects of N addition and positive effects of P addition to the soil microbes. The changes in soil available N also explained the effects of N and P addition on the soil microbes. The plant community is a superior competitor for N absorption compared to soil microbes, and the increased content of soil available N lessened its limitation to plant growth, while still limited soil microbes (Hodge et al. 2000; Leff et al. 2015). In this study, P addition had negative effects on the content of soil available N, contrasted with the positive effects of N addition; otherwise, the content of soil available N had a negative correlation with the microbial biomass (correlation coefficient was − 0.123, and P value was 0.477) and positive correlation with the aboveground biomass (the correlation coefficient was 0.312, and the P value was 0.064), proving the varied effects of N and P addition on the soil microbial biomass.
4.2 The relationship between microbes and edaphic properties and plant community
The relationships between soil microbes and edaphic properties were diverse because soil microbes had different growth strategies and environmental tolerance (Schimel et al. 2007; Allison and Martiny 2008). The differences in the microbial community structure, such as the changes in bacteria, G+, G−, and saprotrophic fungi, were attributed to the changes of the substrate availability (Marschner et al. 2003).
The saprotrophic fungus was tightly positively correlated with the content of soil available N and SOM in this study. These results were because saprotrophic fungi are abundant in the condition with high SOM content, then stimulated the N retain, and enhanced the content of available N in the soil (Blaško et al. 2015). Actinomycetes biomass were increased with the improvement of the content of soil available N in this study, while this result was contrary to the finding in a semiarid grassland (Wang et al. 2017). Different from saprotrophic fungi and actinomycetes, the biomass of bacteria, including the total bacteria community, G+ bacteria and G− bacteria, presented decreased tendency with the increasing content of soil available N. These were because the high urea rate can cause the ammonia toxicity to soil microbes (Geisseler and Scow 2014). Furthermore, the high rate application of chemical fertilizer also decreased the bacterial biomass by decreasing soil pH and increasing the ionic strength (Omar and Ismail 1999; Aldén et al. 2001; Demoling et al. 2007; Geisseler and Scow 2014). For the AMF, they had positive relationships with the content of total soil N and available N, while negative relationships with the total plant N content of each functional group in this study. AMF transported N to their host plant (Hodge and Storer 2015), enhancing the plant growth (Goomaral et al. 2013; Yang et al. 2015). The cost-benefit balance between the AMF and the host plant was destroyed by N application, and the mutualism converted to competitive or parasitic at high N rate (Johnson et al. 1997; Blanke et al. 2005).
The soil pH was an important factor that affected relationships between the soil microbial community and plant community (Geisseler and Scow 2014), and there were several reasons to explain its impacts. First, the decreases in the soil pH directly affected the microbial community component (Högberg et al. 2007; Chen et al. 2013; Chen et al. 2015). Second, the decreasing pH caused the loss of base cations, such as the soil available potassium, then increased susceptibility of the microorganisms to various stresses and decreased the soil microbial biomass (Grayston et al. 2001; Högberg et al. 2007; Chen et al. 2015). Third, reduction in soil pH, also decreased the extracellular enzyme activity and the decomposition rate. As a result, soil microbial community and soil biological processes were changed (Kaneko and Kofuji 2000; Marschner et al. 2003; Geisseler et al. 2009; Chen et al. 2015).
In the investigated field, soil P was a limited factor for soil microbes. P application released this limitation, especially for the total bacteria, G−, and G+ (Dong et al. 2019). In the arctic/alpine, some studies found that the soil microbial biomass showed decreased or no changed trends (Jonasson et al. 1996; Gao et al. 2016), indicated that the P limitation was not universal in the Tibetan. The positive correlations between the bacterial biomass and the P content of total plant also indicated that the bacteria could enhance the plant assimilation of P. The correlation between the content of soil available P and i16:0 (G+) biomass is worth noting and the i16:0 was a good indicator of the soil available P and important in soil P cycles.
4.3 The varied responses of plant and microbial community after fertilization in the Tibetan alpine steppe
In the Tibetan plateau steppe, plant and soil microbes had different N-P demand. The former investigation found that the plant community, such as plant richness, evenness and biomass, was significantly improved after N addition but not for P addition (Dong et al. 2016). In contrary, P application was beneficial for the microbial community, indicated by microbe phenotype and total microbial biomass, but N application decreased the total microbial biomass. At the level of microbial community, most microbial communities, including total bacteria, G+, G−, fungi, AMF, saprotrophs, and actinomycetes, were all significantly affected by single P or combined with N application, and the main effect of single N addition was non-significant for saprotrophs and actinomycetes. The different responses in the facing of N and P addition were because of their different physiological profiles and N-P demand (Garland 1997; Treseder 2008). In consistent with the content of soil available P, the content of soil total N and available N were also good indicators for the 20:1ω9c and 18:1ω9c, respectively. Otherwise, the i16:0 biomass was affected by N and P addition and can be as an indicator of the content of soil available P. Each individual PLFAs were affected by varied surroundings factors, indicating that different microbial community were constructed by different soil factors and that fungal and bacterial populations had different physiological and functional characteristics (Rooney and Clipson 2008).
Anthropogenic activities and climate change have caused severe degradation in the Tibetan alpine steppe. N and P application is a good way to restore the degraded steppe (Klimkowska et al. 2007; Kiehl et al. 2010). Plant community and soil microbial community can affect each other. After N application, plant biomass was increased but microbial abundance had no significant responses. However, microbial abundant was increased while plant biomass had no significant responses under P addition in this study. These contrasting phenomena between the plant ecosystem and the microbial ecosystem indicated they had different responses to N and P addition, and ecological values of N and P addition should be measured from the whole ecosystem view need further investigation.
5 Conclusion
In this study, we found that P addition had positive effects on microbial biomass and microbe phenotype, while N addition decreased the total microbial biomass. Specifically, G+, G−, and bacteria were significantly influenced by N and P additions; saprotroph and actinomycetes were significantly affected by P and its combination with N application; fungal community had non-significant responses to sole N and P amendments, while their interaction was significant. The content of soil available P, total P, and total N were the main environmental factors in constructing the microbial communities, and the biomass of 20:1ω9c, 18:1ω9c, and i16:0 were good indicators for the content of soil total N, available N, and available P, respectively. These microbial changes were also correlated with plants N and P absorption after the N and P application. In the investigated Tibetan alpine steppe, P was the limited factor for soil microbes, and the treatments of N0P1 (N, 0 g m−2 year−1; P, 3.27 g m−2 year−1) and N0P2 (N, 0 g m−2 year−1; P, 6.55 g m−2 year−1) were better application rates for the microbial community. In the future, the plant-soil-microbe ecosystem should be taken together and the long-term responses of soil microbial community need to be explored after N and P addition under climate changes.
References
Aldén L, Demoling F, Bååth E (2001) Rapid method of determining factors limiting bacterial growth in soil. Appl Environ Microbiol 67:1830–1838
Allison SD, Martiny JBH (2008) Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci U S A 105:11512–11519
Bezemer TM, De Deyn GB, Bossinga TM, Van Dam NM, Harvey JA, Van der Putten WH (2005) Soil community composition drives aboveground plant–herbivore–parasitoid interactions. Ecol Lett 8:652–661
Blanke V, Renker C, Wagner M, Füllner K, Held M, Kuhn AJ, Buscot F (2005) Nitrogen supply affects arbuscular mycorrhizal colonization of Artemisia vulgaris in a phosphate-polluted field site. New Phytol 166:981–992
Blaško R, Holm Bach L, Yarwood SA, Trumbore SE, Högberg P, Högberg MN (2015) Shifts in soil microbial community structure, nitrogen cycling and the concomitant declining N availability in ageing primary boreal forest ecosystems. Soil Biol Biochem 91:200–211
Bossio DA, Scow KM (1998) Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microb Ecol 35:265–278
Bremner JM, Mulvaney C (1982) Nitrogen—total. Methods of soil analysis. Part 2. Chemical and microbiological properties, (methodsofsoilan2), 595-624
Bünemann EK, Bossio DA, Smithson PC, Frossard E, Oberson A (2004) Microbial community composition and substrate use in a highly weathered soil as affected by crop rotation and P fertilization. Soil Biol Biochem 36:889–901
Che R, Wang Y, Li K, Xu Z, Hu J, Wang F, Rui Y, Li L, Pang Z, Cui X (2019) Degraded patch formation significantly changed microbial community composition in alpine meadow soils. Soil Tillage Res 195:104426
Chen D, Lan Z, Bai X, Grace JB, Bai Y (2013) Evidence that acidification-induced declines in plant diversity and productivity are mediated by changes in below-ground communities and soil properties in a semi-arid steppe. J Ecol 101:1322–1334
Chen B, Zhang X, Tao J, Wu J, Wang J, Shi P, Zhang Y, Yu C (2014) The impact of climate change and anthropogenic activities on alpine grassland over the Qinghai-Tibet Plateau. Agric For Meteorol 189-190:11–18
Chen D, Lan Z, Hu S, Bai Y (2015) Effects of nitrogen enrichment on belowground communities in grassland: relative role of soil nitrogen availability vs. soil acidification. Soil Biol Biochem 89:99–108
Curtin D, Smillie GW (1984) Influence of liming on soluble and labile P in fertilized soil. Commun Soil Sci Plant 15:177–188
Cusack DF, Silver WL, Torn MS, Burton SD, Firestone MK (2011) Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 92:621–632
Demoling F, Figueroa D, Bååth E (2007) Comparison of factors limiting bacterial growth in different soils. Soil Biol Biochem 39:2485–2495
Demoling F, Ola Nilsson L, Bååth E (2008) Bacterial and fungal response to nitrogen fertilization in three coniferous forest soils. Soil Biol Biochem 40:370–379
Dong J, Cui X, Wang S, Wang F, Pang Z, Xu N, Zhao G, Wang S (2016) Changes in biomass and quality of Alpine steppe in response to N & P Fertilization in the Tibetan Plateau. PLoS One 11:e0156146
Dong J, Che R, Jia S, Wang F, Zhang B, Cui X, Wang S, Wang S (2019) Responses of ammonia-oxidizing archaea and bacteria to nitrogen and phosphorus amendments in an alpine steppe. Eur J Soil Sci n/a
Dudenhoeffer C, Nelson K, Motavalli P, Burdick B, Dunn D, Goyne K (2013) Utility of phosphorus enhancers and strip-tillage for corn production. J Agric Sci 5:37–46
Gao Y, Cooper DJ, Ma X (2016) Phosphorus additions have no impact on plant biomass or soil nitrogen in an alpine meadow on the Qinghai-Tibetan Plateau, China. Appl Soil Ecol 106:18–23
Garland JL (1997) Analysis and interpretation of community-level physiological profiles in microbial ecology. FEMS Microbiol Ecol 24:289–300
Geisseler D, Scow KM (2014) Long-term effects of mineral fertilizers on soil microorganisms – a review. Soil Biol Biochem 75:54–63
Geisseler D, Horwath WR, Doane TA (2009) Significance of organic nitrogen uptake from plant residues by soil microorganisms as affected by carbon and nitrogen availability. Soil Biol Biochem 41:1281–1288
Geisseler D, Lazicki PA, Scow KM (2016) Mineral nitrogen input decreases microbial biomass in soils under grasslands but not annual crops. Appl Soil Ecol 106:1–10
Goomaral A, Iwase K, Undarmaa J, Matsumoto T, Yamato M (2013) Communities of arbuscular mycorrhizal fungi in Stipa krylovii (Poaceae) in the Mongolian steppe. Mycoscience 54:122–129
Grace JB, Bollen KA (2008) Representing general theoretical concepts in structural equation models: the role of composite variables. Environ Ecol Stat 15:191–213
Gray SB, Classen AT, Kardol P, Yermakov Z, Michael Mille R (2011) Multiple climate change factors interact to alter soil microbial community structure in an old-field ecosystem. Soil Sci Soc Am J 75:2217–2226
Grayston SJ, Griffith GS, Mawdsley JL, Campbell CD, Bardgett RD (2001) Accounting for variability in soil microbial communities of temperate upland grassland ecosystems. Soil Biol Biochem 33:533–551
Guckert JB, Hood MA, White DC (1986) Phospholipid ester-linked fatty acid profile changes during nutrient deprivation of Vibrio cholerae: increases in the trans/cis ratio and proportions of cyclopropyl fatty acids. Appl Environ Microbiol 52:794–801
Hodge A, Storer K (2015) Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil 386:1–19
Hodge A, Robinson D, Fitter A (2000) Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci 5:304–308
Högberg MN, Högberg P, Myrold DD (2007) Is microbial community composition in boreal forest soils determined by pH, C-to-N ratio, the trees, or all three? Oecologia 150:590–601
Huang J, Yu H, Liu J, Luo C, Sun Z, Ma K, Kang Y, Du Y (2018) Phosphorus addition changes belowground biomass and C:N:P stoichiometry of two desert steppe plants under simulated N deposition. Sci Rep 8:3400
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol 135:575–585
Jonasson S, Michelsen A, Schmidt IK, Nielsen EV, Callaghan TV (1996) Microbial biomass C, N and P in two arctic soils and responses to addition of NPK fertilizer and sugar: implications for plant nutrient uptake. Oecologia 106:507–515
Kaneko N, Kofuji R-i (2000) Effects of soil pH gradient caused by stemflow acidification on soil microarthropod community structure in a Japanese red cedar plantation: an evaluation of ecological risk on decomposition. J For Res Jpn 5:157–162
Kiehl K, Kirmer A, Donath TW, Rasran L, Hölzel N (2010) Species introduction in restoration projects – evaluation of different techniques for the establishment of semi-natural grasslands in Central and Northwestern Europe. Basic Appl Ecol 11:285–299
Klimkowska A, Van Diggelen R, Bakker JP, Grootjans AP (2007) Wet meadow restoration in Western Europe: a quantitative assessment of the effectiveness of several techniques. Biol Conserv 140:318–328
Klironomos J, Zobel M, Tibbett M, Stock WD, Rillig MC, Parrent JL, Moora M, Koch AM, Facelli JM, Facelli E, Dickie IA, Bever JD (2011) Forces that structure plant communities: quantifying the importance of the mycorrhizal symbiosis. New Phytol 189:366–370
Kulmatiski A, Beard KH (2011) Long-term plant growth legacies overwhelm short-term plant growth effects on soil microbial community structure. Soil Biol Biochem 43:823–830
Leff JW, Jones SE, Prober SM, Barberán A, Borer ET, Firn JL, Harpole WS, Hobbie SE, Hofmockel KS, Knops JMH, McCulley RL, La Pierre K, Risch AC, Seabloom EW, Schütz M, Steenbock C, Stevens CJ, Fierer N (2015) Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc Natl Acad Sci U S A 112:10967–10972
Li Y, Liu Y, Wu S, Niu L, Tian Y (2015) Microbial properties explain temporal variation in soil respiration in a grassland subjected to nitrogen addition. Sci Rep UK 5:18496
Lin L, Li YK, Xu XL, Zhang FW, Du YG, Liu SL, Guo XW, Cao GM (2015) Predicting parameters of degradation succession processes of Tibetan Kobresia grasslands. Solid Earth 6:1237–1246
Liu L, Gundersen P, Zhang T, Mo J (2012) Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol Biochem 44:31–38
Liu L, Gundersen P, Zhang W, Zhang T, Chen H, Mo J (2015) Effects of nitrogen and phosphorus additions on soil microbial biomass and community structure in two reforested tropical forests. Sci Rep UK 5:14378
Lu M, Zhou X, Luo Y, Yang Y, Fang C, Chen J, Li B (2011) Minor stimulation of soil carbon storage by nitrogen addition: a meta-analysis. Agric Ecosyst Environ 140:234–244
Marschner P, Kandeler E, Marschner B (2003) Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol Biochem 35:453–461
Mueller R, Belnap J, Kuske C (2015) Soil bacterial and fungal community responses to nitrogen addition across soil depth and microhabitat in an arid shrubland. Front Microbiol 6:891
Muruganandam S, Israel DW, Robarge WP (2010) Nitrogen transformations and microbial communities in soil aggregates from three tillage systems. Soil Sci Soc Am J 74:120–129
Nelson D, Sommers LE (1982) Total carbon, organic carbon, and organic matter. Methods of soil analysis. Part 2. Chemical and microbiological properties, (methodsofsoilan3), 539-579
Nelson MB, Berlemont R, Martiny AC, Martiny JBH (2015) Nitrogen cycling potential of a grassland litter microbial community. Appl Environ Microbiol 81:7012–7022
Nemergut DR, Townsend AR, Sattin SR, Freeman KR, Fierer N, Neff JC, Bowman WD, Schadt CW, Weintraub MN, Schmidt SK (2008) The effects of chronic nitrogen fertilization on alpine tundra soil microbial communities: implications for carbon and nitrogen cycling. Environ Microbiol 10:3093–3105
Newton AC, Fitt BDL, Atkins SD, Walters DR, Daniell TJ (2010) Pathogenesis, parasitism and mutualism in the trophic space of microbe–plant interactions. Trends Microbiol 18:365–373
Nottingham AT, Turner BL, Stott AW, Tanner EVJ (2015) Nitrogen and phosphorus constrain labile and stable carbon turnover in lowland tropical forest soils. Soil Biol Biochem 80:26–33
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate, 939. United States Department Of Agriculture, Washington, pp 1–19
Omar SA, Ismail MA (1999) Microbial populations, ammonification and nitrification in soil treated with urea and inorganic salts. Folia Microbiol 44:205–212
Ramirez KS, Craine JM, Fierer N (2012) Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob Chang Biol 18:1918–1927
Redman RS, Dunigan DD, Rodriguez RJ (2001) Fungal symbiosis from mutualism to parasitism: who controls the outcome, host or invader? New Phytol 151:705–716
Roger A, Colard A, Angelard C, Sanders IR (2013) Relatedness among arbuscular mycorrhizal fungi drives plant growth and intraspecific fungal coexistence. ISME J 7:2137–2146
Rooney DC, Clipson NJW (2008) Phosphate addition and plant species alters microbial community structure in acidic upland grassland soil. Microb Ecol 57:4
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351
Royden LH, Burchfiel BC, van der Hilst RD (2008) The geological evolution of the Tibetan Plateau. Science 321:1054–1058
Schimel J, Balser TC, Wallenstein M (2007) Microbial stress-response physiology and its implications for ecosystem function. Ecology 88:1386–1394
Schneider T, Keiblinger KM, Schmid E, Sterflinger-Gleixner K, Ellersdorfer G, Roschitzki B, Richter A, Eberl L, Zechmeister-Boltenstern S, Riedel K (2012) Who is who in litter decomposition? Metaproteomics reveals major microbial players and their biogeochemical functions. ISME J 6:1749–1762
Shi Y, Lalande R, Ziadi N, Sheng M, Hu Z (2012) An assessment of the soil microbial status after 17 years of tillage and mineral P fertilization management. Appl Soil Ecol 62:14–23
Smith R, Funk A, Wahlstrand J, Mirin R, Cundiff S, Steiner J, Schafer M, Kira M, Koch S (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nature Geoscience 3:315–322
Stevenson FJ (1986) The internal cycle of nitrogen of soil: carbon, nitrogen, phosphorus, sulphur, micronutrients. John Wiley & Sons, New York
Tischer A, Werisch M, Döbbelin F, Camenzind T, Rillig MC, Potthast K, Hamer U (2015) Above- and belowground linkages of a nitrogen and phosphorus co-limited tropical mountain pasture system – responses to nutrient enrichment. Plant Soil 391:333–352
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Wang C, Zhu F, Zhao X, Dong K (2014) The effects of N and P additions on microbial N transformations and biomass on saline-alkaline grassland of Loess Plateau of Northern China. Geoderma 213:419–425
Wang C, Wang G, Wang Y, Rafique R, Ma L, Hu L, Luo Y (2015a) Urea addition and litter manipulation alter plant community and soil microbial community composition in a Kobresia humilis meadow. Eur J Soil Biol 70:7–14
Wang J, Wang Z, Zhang X, Zhang Y, Ran C, Zhang J, Chen B, Zhang B (2015b) Response of Kobresia pygmaea and Stipa purpurea grassland communities in northern Tibet to nitrogen and phosphate addition. Mt Res Dev 35:78–86
Wang R, Dorodnikov M, Dijkstra FA, Yang S, Xu Z, Li H, Jiang Y (2017) Sensitivities to nitrogen and water addition vary among microbial groups within soil aggregates in a semiarid grassland. Biol Fertil Soils 53:129–140
Wardle DA (1992) A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol Rev 67:321–358
Xu X, Liu H, Song Z, Wang W, Hu G, Qi Z (2015) Response of aboveground biomass and diversity to nitrogen addition along a degradation gradient in the Inner Mongolian steppe, China. Sci Rep UK 5:10284
Yang A, Liu N, Tian Q, Bai W, Williams M, Wang Q, Li L, Zhang W-H (2015) Rhizosphere bacterial communities of dominant steppe plants shift in response to a gradient of simulated nitrogen deposition. Front Microbiol 6:789
Yeh ET, Nyima Y, Hopping KA, Klein JA (2014) Tibetan pastoralists’ vulnerability to climate change: a political ecology analysis of snowstorm coping capacity. Hum Ecol 42:61–74
Zeng J, Liu X, Song L, Lin X, Zhang H, Shen C, Chu H (2016) Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol Biochem 92:41–49
Zhang X, Wei H, Chen Q, Han X (2014) The counteractive effects of nitrogen addition and watering on soil bacterial communities in a steppe ecosystem. Soil Biol Biochem 72:26–34
Zhang W, Cui Y, Lu X, Bai E, He H, Xie H, Liang C, Zhang X (2016) High nitrogen deposition decreases the contribution of fungal residues to soil carbon pools in a tropical forest ecosystem. Soil Biol Biochem 97:211–214
Zhang P, Li B, Wu J, Hu S (2019) Invasive plants differentially affect soil biota through litter and rhizosphere pathways: a meta-analysis. Ecol Lett 22:200–210
Zhou F, Ding J, Li T, Zhang X (2020) Plant communities are more sensitive than soil microbial communities to multiple environmental changes in the Eurasian steppe. Glob Ecol Conserv 21:e00779
Zhou X, Zhang Y, Downing A (2012) Non-linear response of microbial activity across a gradient of nitrogen addition to a soil from the Gurbantunggut Desert, northwestern China. Soil Biology and Biochemistry 47:67–77
Zong N, Shi P (2019) Enhanced community production rather than structure improvement under nitrogen and phosphorus addition in severely degraded alpine meadows. Sustainability 11
Funding
The project was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (No: XDA20050104), the National Natural Science Foundation of China (No. 41230750), and the National Key Research and Development Program (No. 2016YFC0501802).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 242 kb)
Rights and permissions
About this article
Cite this article
Dong, J., Wang, S., Niu, H. et al. Responses of soil microbes and their interactions with plant community after nitrogen and phosphorus addition in a Tibetan alpine steppe. J Soils Sediments 20, 2236–2247 (2020). https://doi.org/10.1007/s11368-020-02586-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-020-02586-3