Abstract
Salinity is one of the major environmental factor that can greatly impact the growth, development, and productivity of barley. Our study aims to detect the natural phenotypic variation of morphological and physiological traits under both salinity and potassium nanoparticles (n-K) treatment. In addition to understanding the genetic basis of salt tolerance in barley is a critical aspect of plant breeding for stress resilience. Therefore, a foliar application of n-K was applied at the vegetative stage for 138 barley accessions to enhance salt stress resilience. Interestingly, barley accessions showed high significant increment under n-K treatment compared to saline soil. Based on genome-wide association studies (GWAS) analysis, causative alleles /reliable genomic regions were discovered underlying improved salt resilience through the application of potassium nanoparticles. On chromosome 2H, a highly significant QTN marker (A:C) was located at position 36,665,559 bp which is associated with APX, AsA, GSH, GS, WGS, and TKW under n-K treatment. Inside this region, our candidate gene is HORVU.MOREX.r3.2HG0111480 that annotated as NAC domain protein. Allelic variation detected that the accessions carrying C allele showed higher antioxidants (APX, AsA, and GSH) and barley yield traits (GS, WGS, and TKW) than the accessions carrying A allele, suggesting a positive selection of the accessions carrying C allele that could be used to develop barley varieties with improved salt stress resilience.
Key message
Highlighting the importance of the role of potassium nanoparticles in plant tolerance to abiotic stresses, including salinity is the potential for genetic improvement of barley crop resilience through the enhancement of antioxidant defense systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salinity is a significant environmental stressor that can profoundly affect the growth, development, and yield of barley (Hordeum vulgare L.), one of the world's oldest and most important cereal crops (Atta et al. 2023). The impact of salinity on barley, as with other crops, involves complex physiological, biochemical, and molecular responses (Al-Tardeh et al. 2023). Understanding these effects is crucial for developing strategies to enhance crop resilience and ensure food security in saline-affected areas (Thabet et al. 2022). High salinity in the soil creates an osmotic stress condition, making it difficult for plants to absorb water (Thabet et al. 2023). This leads to a reduction in plant turgor pressure, which is essential for cell expansion and growth. Salinity can lead to an imbalance of essential nutrients, as high concentrations of sodium (Na+) and chloride (Cl-) ions interfere with the uptake of potassium (K+), calcium (Ca2+), and magnesium (Mg2+) (Hussain et al. 2022). This imbalance can inhibit key physiological processes, including photosynthesis, enzyme activities, and protein synthesis. Furthermore, the accumulation of toxic ions, particularly Na+ and Cl-, can damage cellular structures and functions (Bagues et al. 2024). The combined effects of osmotic stress, nutrient imbalance, and ion toxicity can significantly reduce the growth rate of barley plants (Thabet et al. 2021a). This is observed in diminished root and shoot length, reduced leaf area, and overall stunted plant development. Ultimately, the physiological stresses of salinity can lead to significant reductions in barley yield (Thabet et al. 2021a). This is manifested in fewer and smaller grains per plant, lower seed germination rates, and reduced biomass production (Xue et al. 2023). Salinity stress produces reactive oxygen species (ROS), which can cause oxidative damage to cells. Barley plants respond by enhancing their antioxidant defense systems, including enzymes like superoxide dismutase (SOD), catalase (CAT), and peroxidases (POD), which detoxify ROS and mitigate cellular damage (Kesawat et al. 2023). Salinity stress triggers a complex network of gene expression changes in barley, involving genes related to ion transport, osmolyte biosynthesis, stress signaling, and transcription factors (Shah et al. 2021). These genetic responses play a critical role in plant adaptation to saline conditions by regulating physiological and biochemical pathways (Singh et al. 2022). Understanding the effects of salinity on barley has led to the development of several strategies to improve plant resilience. These include conventional breeding for salt-tolerant varieties, genetic engineering to introduce or enhance traits associated with salinity tolerance, and agronomic practices such as seed priming with potassium nanoparticles, as mentioned earlier, to bolster the plant's ability to cope with saline conditions (Singh et al. 2021). Therefore, studying and mitigating the effects of salinity on barley is a multi-disciplinary challenge that involves integrating plant physiology, molecular biology, genetics, and agronomy (Ahmad et al. 2023). Advances in these areas are critical for enhancing barley's resilience to salinity, ensuring stable yields, and contributing to global food security in the face of increasing soil salinization (Tarolli et al. 2024).
Potassium nanoparticles represent a cutting-edge approach in agricultural practices, offering numerous benefits over traditional potassium fertilizers (Yadav et al. 2023). These benefits stem from the unique properties of nanoparticles, including their small size, high surface area-to-volume ratio, and increased reactivity (Wang et al. 2023). Potassium nanoparticles can significantly increase the efficiency of potassium used in plants due to their nano size, which allows for easier uptake and assimilation by plant roots (Tarolli et al. 2024). This enhanced efficiency means that lower amounts of potassium are needed to achieve the desired effects on plant growth and yield, reducing fertilizer costs and environmental impact (El-tanahy et al. 2022). Research has shown that potassium nanoparticles can promote plant growth more effectively than conventional potassium fertilizers (Abd-Elrahman et al. 2023). This is due to their ability to improve water retention, enhance nutrient uptake, and stimulate growth-related enzymes and hormones (Hussain et al. 2022). As a result, plants treated with potassium nanoparticles often exhibit increased biomass, higher yield, and improved quality of agricultural products. Potassium is vital for plants' resistance to abiotic stresses such as drought, salinity, and extreme temperatures (Halaji et al. 2023). Potassium nanoparticles can enhance this stress tolerance more effectively than traditional potassium sources. They help maintain cellular osmotic balance, protect against oxidative damage by enhancing antioxidant enzyme activity, and support the opening and closing of stomata, which is crucial for water use efficiency and gas exchange under stress conditions (Bagues et al. 2024). The integration of nanotechnology in agriculture, particularly through the use of potassium nanoparticles, offers a novel approach to enhancing plant resilience to abiotic stresses (Ijaz et al. 2023). Future research will likely focus on the development of tailored nanoparticle formulations for specific crops and stress conditions, the elucidation of the mechanisms of action of nanoparticles at the cellular and molecular levels, and the assessment of long-term environmental impacts (Noman et al. 2023).
Genome-wide association scan (GWAS) play a crucial role in understanding the genetic basis of salt tolerance in barley, a critical aspect of plant breeding for stress resilience (Elakhdar et al. 2023). GWAS enables the identification of genes or genomic regions that contribute to salt tolerance (Javid et al. 2022). This discovery is vital for understanding the underlying genetic mechanisms that enable barley to withstand salinity stress (Chaurasia et al. 2021). The SNPs and QTLs identified through GWAS can be used as markers in breeding programs (Thabet and Alqudah 2023). Marker-assisted selection accelerates the development of salt-tolerant barley varieties by specifically selecting for desirable genetic traits (Thabet et al. 2020). Understanding the genetic basis of salt tolerance provides insights into the complex interactions between genetic factors and environmental conditions (Thabet et al. 2021b). This knowledge can guide the development of crop management strategies and breeding programs aimed at enhancing yield stability under saline stress (Alqudah et al. 2020). Therefore, this study aims to detect the natural phenotypic variation of morphological and physiological traits under both salinity and n-K treatment. A foliar application of n-K was applied at the vegetative stage for 138 barley accessions to enhance salt stress resilience. GWAS analysis is used to detect the genomic regions and potential candidate genes underlying improved salt resilience through the application of potassium nanoparticles.
Material and methods
Plant material
In the present investigation, a total of 138 spring barley accessions from various geographical origins were examined, encompassing 54 cultivars and 84 landraces. The collection was divided into 63 two-rowed and 75 six-rowed. The genotyping-by-sequencing (GBS) method was employed to genotype all the accessions, resulting in the identification of 19,276 single nucleotide polymorphisms (SNPs), as outlined by Milner et al. (2019). The initial sample utilized in this investigation was obtained from the German ex-situ IPK-Gatersleben GeneBank (Table S1).
Experimental design
Over two years (2022 and 2023), a total of 138 spring barley accessions with multiple geographical origins, including two- and six-row genotypes, and with different winter and spring growth habits, were cultivated at the Experimental Station of the University of Fayoum (29°11′20.36″N, 30°10′06.45″E). Soil samples were collected to assess the physical and chemical characteristics (Table S1), following the methodology outlined in the study of Jackson and Delhi (1967). The experimental saline soil where both trials were performed contained topsoil (0–90 cm depth) and according to Dahnke (1975), it is classified as salt-affected (ECe = 9.38 dS m−1, SAR = 12.2, and pH = 7.82; Table S2). The texture of saline soil was loamy sand with an average bulk density of 1.61 and 1.67 g cm−3 and the average percentage of available water was 12.4%/60 and 13.1%/60 cm depth, respectively (Table S2). The soil organic matter contents were 1.02 and 1.08% and total N of 0.05 and 0.06%, respectively. The physicochemical properties of both soils were analyzed following the procedures of Klute (1986). A completely randomized experimental design was used with two treatments, including control (saline soil) and a dosage of 100 ppm PHC® n-K. Each treatment was repeated four times. The nano fertilizer applied in this study was the commercially available product PHC® n-K. This suspension contains potassium (K) in an ionic state, with nanoparticles ranging in size from 500 to 2000 nanometers. This characteristic enables efficient absorption by plants. The plants were sprayed three times; 3, 4, and 5 weeks after sowing. According to Thabet and Alqudah (2023), mineral nitrogen (N), phosphorus (P), and potassium (K) fertilizers were used at the rates traditionally suggested for barley production in the study area: 150, 60, and 120 kg N, P2O5, and K2O ha−1, respectively. A local trade guide for barley crop production was developed to address cultural practices, pest and disease management, and other related issues. When the crop was harvested on April 20, several parameters were measured. These included spike length (SL), which is the measurement in centimeters from the base of the spike to the tip (excluding awns), spikelet per spike (SS), which is the count of developed spikelets per spike, and grains per spike (GS), which is the count of grains per spike. weight of grains per spike (WGS), measured in grams (g). A digital weight scale was used to count 1000 seeds from each genotype's bulk seed, which is known as a thousand-kernel weight (TKW).
Activities of enzymatic antioxidants assays
The subsequent procedures were executed at a temperature of 4 °C. To make enzyme extracts, 200 mg of freeze-dried leaves were homogenized in 2 ml of 0.1 M K-P buffer (pH 7.0) using a cold mortar. A 0.1 mM solution of ethylenediaminetetraacetic acid (EDTA) was introduced into the extraction buffer. To evaluate the activity of Ascorbate Peroxidase (APX), a solution containing 2 mM ascorbate was introduced into the extraction buffer. The homogenates underwent filtration via nylon material. Subsequently, the filtrate solution underwent centrifugation for 15 min at a force of 12,000 × g. The enzyme extracts were either immediately utilized or kept at a temperature of −25 °C until they were needed. The assessment of Superoxide Dismutase (SOD) activity, measured in Unit g−1 protein, was conducted by evaluating the SOD potential to prevent the reduction of NBT photochemical, as described by Beauchamp and Fridovich (1971). The quantity of enzyme necessary to inhibit 50% NBT photoreduction is equivalent to one unit of SOD activity. The enzyme activity that followed was measured as µmol H2O2 min−1 g −1 protein. The Catalase (CAT) activity was evaluated by observing the reduction in absorbance measurements at 240 nm due to the decomposition of H2O2 (Havir and McHale 1987). The assessment of APX activity was conducted using the methodologies outlined by Nakano and Asada (1981). This involved observing the oxidation of AsA, which was evident by a reduction in absorbance values at 290 nm. The measurement of Glutathione Reductase (GR) activity was conducted using Foster and Hess (1980) techniques. The activity was measured by monitoring the change in absorbance of a mixture consisting of 0.1 mL EE, 100 mmol K-P buffer (pH 7.0), 0.1 mmol EDTA, 0.5 mmol NADPH, and 0.1 mmol GSSG. The monitoring was performed at a wavelength of 340 nm for 3 min.
Statistical analysis
An analysis of variance (ANOVA) was used to examine variations in phenotypic values among all barley accessions in both circumstances. The significance level was determined at p <0.05. This analysis was performed using the R program (Allaire and MA 2012). Using the lme4 program, we calculated the best linear unbiased estimates (BLUEs) for each trait (Bates et al. 2015). A Pearson correlation analysis was conducted to examine the degree to which the relationship between the parameters. The study employed genotypic mean values and a significance level of p 0.05 for each treatment (Horn 1998). The broad-sense heritability for each variable was determined using the formula H2=VG/[VG+(Ge/nE)], where VG represents the genotype variance, Ge is the residual variance, and nE is the number of years.
Determinations of antioxidant contents
The leaf tissue was homogenized to extract AsA (L-Ascorbic acid) using a 5% solution of HPO3 (ice-cold) containing 1 mM EDTA. The concentration of AsA (µM g−1 FW) was measured by subjecting the homogenates to a 20 min centrifugation operation at a speed of 4.00 × g. The supernatants that were generated were employed for the assessment of AsA content (Huang et al. 2005). The methodologies employed by Yu et al. (2003), with a slight adaptation from Paradiso et al. (2008), were used to ascertain the glutathione (GSH) concentration (µM g−1 FW). The content of GSH was calculated using GSH standard curves. The quantification of α-tocopherol (aToC) was conducted using the methodologies described in the studies conducted by Ching and Mohamed (2001) and Konings et al. (1996).
genome-wide association scan (GWAS)
GWAS are a powerful method used to identify genetic variations associated with specific traits within populations. GWAS takes an unbiased approach, scanning the entire genome to find genetic markers linked to particular phenotypes. Among these advancements, the Fixed and random model Circulating Probability Unification (FarmCPU) model, implemented within the GAPIT (Genome Association and Prediction Integrated Tool) framework in R, represents a significant leap forward (Wang and Zhang 2021). This approach addresses some of the key challenges faced in traditional GWAS, particularly in controlling for false positives due to population structure and kinship while maintaining high power to detect true associations. Initially, FarmCPU uses a fixed effects model to test each genetic marker's association with the trait of interest, ignoring the confounding effects of population structure and kinship (Liu et al. 2016). This step is designed to identify a preliminary set of significantly associated markers. It then employs a random effects model, using the markers identified in the first step as covariates. This model accounts for population structure and kinship, adjusting the initial associations accordingly. FarmCPU iterates between the fixed and random effects models, updating the set of markers used in the random effects model based on the results from the fixed effects model. This iteration continues until the set of significantly associated markers stabilizes, ensuring that the final associations are not confounded by population structure or kinship. This method offers researchers a robust tool for unraveling the genetic basis of complex traits.
SNP-gene-based association and haplotype analysis
Linkage disequilibrium (LD, r2) was estimated for each chromosome, specifically within the strongly related genomic region. The results were then shown as a heatmap visual. This enabled us to establish the predominant physical location that has been exploited for candidate gene identification. The physical locations of SNPs that surpass the false discovery rate (FDR) threshold within the linkage disequilibrium interval were utilized for annotating high-confidence (HC) candidate genes, along with other relevant information, using the barley genome explorer web-based tool with the latest barley genome dataset (BARLEX; http://apex.ipk-gatersleben.de). Moreover, using the OPEN-ACCESS version of the Genevestigator software, expression patterns for our possible candidates were assessed using the RNA-seq expression database at various developmental stages in barley. The program includes the transcriptome and expression of barley genes from different tissues and organs under controlled and different stress conditions.
Results
Natural variation for morpho-physiological attributes
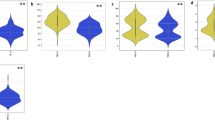
In this study, barley accessions showed significant natural phenotypic variation under both salinity and n-K treatment (Fig. 1 and 2). Under saline soil (control), the mean genotypic values for enzymatic and non-enzymatic antioxidants were 2.29 Unit g−1 protein for SOD, 0.44 µmol H2O2 min−1 g −1 protein for CAT, 1.10 µmol H2O2 min−1 g −1 protein for APX, 0.30 µmol H2O2 min−1 g −1 protein for GR, 21.71 µM g−1 FW for AsA, and 36.59 µM g−1 FW for GSH (Table S3). Under n-K treatment, the genotypic mean values were 5.60 Unit g−1 protein for SOD, 0.95 µmol H2O2 min−1 g −1 protein for CAT, 2.37 µmol H2O2 min−1 g −1 protein for APX, 0.85 µmol H2O2 min−1 g −1 protein for GR, 62 µM g−1 FW for AsA.78, and 75.22 µM g−1 FW for GSH (Table S4). Interestingly, all barley genotypes showed high increment for all antioxidants after foliar application of potassium nanoparticles by 57.59%, 47.75%, 49.68%, 64.77%, 65.24%, and 49.36% for SOD, CAT, APX, GR, AsA, and GSH, respectively (Fig. 1). For agronomical attributes, the mean values were 8.29 cm for SL, 9.51 for SS, 43.41 for GS, 1.37 gm for WGS, and 39.52 gm for TKW under salt stress (control) (Table S3). In parallel, the genotypic mean values under the n-K treatment were 11.03 cm for SL, 12.76 for SS, 50.83 for GS, 2.54 gm for WGS, and 55.48 gm for TKW (Table S4). Similar to all antioxidant components, the measured agronomic characteristics showed a significant increase after the foliar application of n-K by 24.92%, 25.69%, 14.85%, 48.08%, and 28.36% for SL, SS, GS, WGS, and TKW, respectively, when compared to saline soil (control) (Fig. 2). Heritability values (H2) were greater than 85% for all antioxidants, meanwhile, it was more than 90 for all morphological traits under salt stress (Tables S5, S6, S7). Under potassium nanoparticle treatment, H2 values were more than 90 for all of the studied morpho-physiological traits except for AsA content (86), indicating that the factors controlling the genotypic variations were genetically controlled rather than environmental factors.
Box plot analysis reveals the natural variation of enzymatic and non-enzymatic antioxidants, including (a) superoxide dismutase (SOD), b catalase (CAT), c ascorbate peroxidase (APX), d glutathione reductase (GR), e glutathione (GSH), f and ascorbic acid (AsA) under both control (C) and potassium nanoparticles (n-K). The degree of significance is indicated as **p, 0.01; ***p, 0.001
Box plot analysis reveals the natural variation of agronomical traits, including (a) spike length (SL), b spikelets per spike (SS), c grains per spike (GS), d weight of grains per spike (WGS), and e thousand kernel weight (TKW) under both control (C) and potassium nanoparticles (n-K). The degree of significance is indicated as *p, 0.05; **p, 0.01; ***p, 0.001
Correlation analysis
Pearson's correlation was detected between all of the studied traits, including antioxidants and agronomical characteristics (Fig. 3). Under saline soil, SOD showed a highly significant positive correlation with CAT, APX, and GR by r= 0.55**, 0.99***, and 0.89***, respectively (Fig. 3), indicating the positive role of all enzymatic antioxidants via enhancing the antioxidant system to respond to salt stress conditions. Markedly, SOD revealed a significant negative correlation with SG and WGS by r= −0.37** and −0.36 **, respectively (Fig. 3). In response to potassium nanoparticles application, SOD detected a high positive correlation with CAT, APX, and GR by r= 0.41**, 0.86***, and 0.75***, respectively (Fig. 3). In parallel, SOD revealed a significant negative correlation with SL, SS, and TKW by r= −0.2**, −0.2**, and −0.05 **, respectively (Fig.3). However, WGS and GS showed a positive correlation with enzymatic antioxidants such as CAT, APX, and GR, as well as non-enzymatic antioxidants, including AsA and GSH, suggesting the positive role of potassium nanoparticles in improving barley salt tolerance via strengthening the antioxidant defense system (Fig. 3).
Genome-wide association scan
GWAS analysis revealed a total of 160 QTNs that mapped on all barley chromosomes and were significantly associated with all morpho-physiological attributes under both salinity and potassium nanoparticles (Table S8). Under saline soil (control), the number of significant QTNs underpinning enzymatic and non-enzymatic antioxidants was 9 (CAT), 4 (SOD), 3 (APX), 3 (GR), 14 (AsA), and 1 (GSH) (Table S8 and Fig. 4). The highest significant QTN was detected for CAT and AsA on chromosomes 7H and 6H at position 28,227,628 bp and 3,489,250 bp with p-value = 9.17E-06 and 8.01E-06, respectively (Table S8and Fig. S1). Under potassium nanoparticles, the number of significant QTNs was 11 (CAT), 10 (SOD), 8 (APX), 4 (GR), 7 (AsA), and 7 (GSH) (Table S8 and Fig. 4). The most significant QTN was detected for CAT and GSH on chromosomes 6H and 1H at position 388,418,487 bp and 9,594,783 bp with p-value = 8.47E-14and 8.70E-06, respectively (Table S8). For all agronomical traits, the number of markers under saline soil was 23, 11, 10, and 6 QTNs for SL, TKW, GS, and WGS, respectively, (Table S8 and Fig S2). High significant QTNs were revealed to be associated with GS, TKW, and WGS at positions 572,438,008, 452,737,219, and 59,662,475 bp at p-value = 9.09E-13, 9.89E-10, and 6.18E-07, respectively (Table S8 and Fig. S2). Under the foliar n-K application, the number of markers under saline soil was 11, 7, 9, and 1 QTNs for SL, GS, TKW, and WGS, respectively, (Table S8 and Fig. S2). Interestingly, significant QTN (A:G) was shown to be associated with TKW that mapped on chromosome 2H at position 571,931,094 bp with p-value = 1.01E-11in response to n-K treatment (Table S8 and Fig. S2).
a Locus zoom in on regional plot colocalization of highly associated QTN (A:C). The x-axis shows the chromosomes and the QTN order. The y-axis shows the -log10 (p-value) for each QTN marker, b Structure of the candidate gene with sequence variation (HORVU.MOREX.r3.2HG0111480) in the third exon with LD heat map, and c Allele effect on the accessions at the natural genetic variant (A:C) of single gene-based
Candidate genes identification
Interestingly, ten genomic regions were detected within the LD region that harbor important candidate genes associated with plant stress resilience under salinity conditions (Table S9). Several potential candidate genes were detected based on GWAS analysis within the LD region that mapped on chromosomes 1H, 2H, 5H, 6H, and 7H (Table S9 and Table S10). For example, a highly significant QTN (C:T) at position 423,411,121 bp was found inside the gene HORVU.MOREX.r3.1HG0064420 annotated the F-box family protein and controlled the phenotypic variation of APX and SOD under salt stress (Table S10). On chromosome 2H, a highly significant QTN marker (A:C) was located at position 36,665,559 bp which is associated with APX, AsA, GSH, GS, WGS, and TKW under n-K treatment. Inside this region, our candidate gene is HORVU.MOREX.r3.2HG0111480 that annotated as NAC domain protein (Table S9 and Table S10). NAC (NAM, ATAF1/2, CUC2) domain proteins are a family of plant-specific transcription factors that play a crucial role in the response of plants to various environmental stresses, including salt stress. Allelic variation detected that the accessions carrying C allele showed higher antioxidants (APX, AsA, and GSH) and barley grain yield (GS, WGS, and TKW) than the accessions carrying A allele, suggesting a positive selection of the accessions carrying C allele that could be used to develop barley varieties with improved salt stress resilience (Table S10 and Fig. 4). Interestingly, an important QTN (A:G) located at position 489,069,485 bp on chromosome 5H was found near the candidate gene HORVU.MOREX.r3.5HG0494660 annotated as Superoxide dismutase [Cu-Zn] controls the variation of all enzymatic and non-enzymatic antioxidants under salt stress (Table S10). Superoxide dismutase [Cu-Zn] (Cu/Zn SOD) is a crucial antioxidant enzyme in plants, including barley that plays a significant role in the plant's tolerance to salt stress. A significant QTN (A:G) located at position 388,418,487 bp on chromosome 6H was found near the candidate gene HORVU.MOREX.r3.6HG0597850 annotated as GRF zinc finger/zinc knuckle protein controls the variation of CAT, AsA, and GSH under n-K conditions (Table S9 and Table S10). GRF (Growth-Regulating Factor) zinc finger/zinc knuckle proteins represent a class of plant-specific transcription factors that play significant roles in various aspects of plant growth, development, and stress responses, including salt tolerance in barley and other crops. Moreover, expression patterns for our potential candidate gene namely, HORVU.MOREX.r3.2HG0111480 annotated as NAC domain protein was assessed using the RNA-seq expression database at various developmental stages in barley (Fig. 5). Our candidate showed upregulation at the vegetative stage under salt stress conditions, suggesting the crucial role of this gene through modulating the antioxidant defense system, mitigating oxidative stress and improving plant resilience.
Differential gene expression of the HORVU.MOREX.r3.2HG0111480 gene that encodes peroxidase at different developmental stages in barley. a Up or down-regulation underlying the natural phenotypic variation in several organs of barley. b Up or down-regulation underlying the natural phenotypic variation under abiotic stress conditions, including salt stress
Discussion
Salinity is a substantial environmental stressful event that can induce profound alterations in barley growth, development, and yield (Atta et al. 2023). Understanding these effects is crucial for developing strategies to enhance crop resilience and ensure food security in saline-affected areas (Thabet et al. 2022). Salinity stress leads to the production of ROS, which can cause oxidative damage to cells. In our study, all barley genotypes showed high increments for all antioxidants after foliar application of potassium nanoparticles compared to saline conditions. Kesawat et al. (2023) reported that barley plants respond by enhancing their antioxidant defense systems, including enzymes like SOD, CAT, and POD, which detoxify ROS and mitigate cellular damage. Potassium is a cofactor for various enzymes involved in photosynthesis, protein synthesis, and other metabolic processes (Kumari et al. 2022). Nano potassium can enhance enzyme activity, promoting better growth and development of barley under salinity stress (Zhang et al. 2024). Salinity stress induces the production of ROS, causing oxidative damage to plant cells (Abeed et al. 2023). Nano potassium has been shown to boost the antioxidant defense system in plants, mitigating oxidative stress and improving plant resilience (Hasanuzzaman et al. 2018). Interestingly, our study reported a significant increase in all agronomic attributes after the application of the n-K under salt stress. Studies reported by (Hasanuzzaman et al. 2018) have demonstrated that nano potassium can significantly improve barley growth, biomass, and grain yield under salinity stress conditions. Nano potassium represents a promising approach to mitigating salinity stress in barley. Therefore, by improving nutrient uptake, enhancing osmotic adjustment, boosting enzyme activity, and strengthening antioxidant defenses, nano potassium helps barley plants thrive under saline conditions. As research in this field progresses, nano potassium could become a key component of sustainable agricultural practices, ensuring food security in regions affected by salinity stress.
GWAS and candidate gene discovery
GWAS is essential for comprehending the genetic foundation of salt tolerance in barley, which is a vital component of plant breeding aimed at enhancing stress resilience (Elakhdar et al. 2023). This finding is crucial for comprehending the fundamental genetic pathways that allow barley to endure salt stress. (Chaurasia et al. 2021). The SNPs and QTLs identified through GWAS can be used as markers in breeding programs (Thabet and Alqudah 2023). Marker-assisted selection accelerates the development of salt-tolerant barley varieties by specifically selecting for desirable genetic traits (Thabet et al. 2020). Understanding the genetic basis of salt tolerance provides insights into the complex interactions between genetic factors and environmental conditions (Thabet et al. 2021b). This knowledge can guide the development of crop management strategies and breeding programs aimed at enhancing yield stability under saline stress (Alqudah et al. 2020).
Several potential candidate genes were detected based on GWAS analysis within the LD region that mapped on chromosomes 1H, 2H, 5H, 6H, and 7H. For example, a highly significant QTN (C:T) at position 423,411,121 bp was found inside the gene HORVU.MOREX.r3.1HG0064420 annotated the F-box family protein and controlled the phenotypic variation of APX and SOD under salt stress. F-box proteins are one of the largest families of regulatory proteins in plants and are key components of the ubiquitin-proteasome pathway, a cellular mechanism for targeting proteins for degradation (Liu and Xue 2011). This pathway is crucial for regulating a wide range of cellular processes, including signal transduction, cell cycle control, and stress responses (Zhang et al. 2019). Under salt stress conditions, plants must modulate their internal processes to mitigate the effects of high salinity, which can cause osmotic stress, ion toxicity, and oxidative stress (Zhao et al. 2020). F-box proteins contribute to salt stress resilience in wheat through several mechanisms (Zhou et al. 2015). F-box proteins are involved in the regulation of ion channels and transporters that control the uptake and compartmentalization of sodium (Na+) and potassium (K+) ions (Zhou et al. 2015). This regulation is crucial for maintaining ion homeostasis under salt stress, preventing the accumulation of toxic levels of Na+ in the cytoplasm, and ensuring sufficient K+ for cellular functions (Balasubramaniam et al. 2023). F-box proteins can act as sensors or mediators that modulate the activity of other proteins involved in signaling pathways, such as those mediated by plant hormones like abscisic acid (ABA), which plays a pivotal role in stress responses (Emenecker and Strader 2020). The F-box protein AtFBA1 enhances the plant's ability to withstand salt and osmotic stress by initiating plant responses mediated by ABA (Gao et al. 2022). The F-box protein AtFOF2 in Arabidopsis has an important negative impact on seed germination and early seedling development by suppressing the expression of ABA signaling genes ABI3 and ABI5. However, higher levels of ABA are observed when AtFOF2 is overexpressed, leading to improved plant resilience to drought (Qu et al. 2020). As an example, the wheat F-box protein TaFBA-2A boosts transgenic rice's salt tolerance and adversely controls JA production (Gao et al. 2022); in Arabidopsis, the F-box protein MAX2 helps plants withstand bacterial and plant diseases (Piisilä et al. 2015). Interactions between GhSKP1A and the F-box protein GhTULP34 are responsible for the negative control of water stress in cotton (Li et al. 2021). Our candidate gene-encoded F-Box proteins were shown to regulate the variation of enzymatic antioxidants, including APX and SOD under saline conditions. These results agree with Zhou et al. (2015) who reported that the transgenic plants exhibited elevated levels of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and peroxidase (POD), compared to the wild-type (WT) plants. This could be attributed to the increased expression of certain antioxidant genes through the overexpression of TaFBA1. Altogether, F-box proteins may play a role in the degradation of negative regulators of these genes, thus facilitating the plant's response to oxidative stress caused by high salinity.
On chromosome 2H, a highly significant QTN marker (A:C) was located at position 36,665,559 bp which is associated with APX, AsA, GSH, GS, WGS, and TKW under n-K treatment. Inside this region, our candidate gene is HORVU.MOREX.r3.2HG0111480 that annotated as NAC domain protein. NAC (NAM, ATAF1/2, CUC2) domain proteins are a family of plant-specific transcription factors that play a crucial role in the response of plants to various environmental stresses, including salt stress (Bian et al. 2020). These proteins are characterized by a highly conserved NAC domain responsible for DNA binding and a variable transcriptional activation region that can interact with other proteins (Xia et al. 2023). According to Chen et al. (2013), the HvNAC6 gene in barley has been identified as a regulator of basal resistance to the biotrophic fungus Blumeria graminis f. sp. hordei. This gene has been found to have a role in mediating defense responses in barley that are dependent on ABA. The study conducted by Al Abdallat et al. (2014) demonstrated that the overexpression of the isoform HvSNAC1 in barley resulted in enhanced drought tolerance. This finding implies that this gene has the potential to enhance barley productivity in drought situations. In a recent study conducted by McGrann et al. (2015), it was found that the overexpression of a certain gene can augment the resistance of barley against Ramularia leaf spot. In rice, the SNAC1 gene was found to enhance drought and salt tolerance. Additionally, transgenic plants that overexpressed SNAC1 exhibited reduced water loss rates and increased sensitivity to ABA stress compared to wild-type plants (Xiong et al. 2001). The study conducted by Mao et al. (2012) showed that the upregulation of the wheat genes TaNAC2 and TaNAC67 in A. thaliana resulted in a notable enhancement in tolerance towards salt, drought, and cold stress. In this study, allelic variation detected that the accessions carrying C allele showed higher antioxidants (APX, AsA, and GSH) and barley yield traits (GS, WGS, and TKW) than the accessions carrying A allele, suggesting a positive selection of the accessions carrying C allele that could be used to develop barley varieties with improved salt stress resilience. The aforementioned research has provided evidence that NAC genes can be activated by both biotic and abiotic stresses across diverse circumstances. Interestingly, expression patterns for our potential candidate gene using the RNA-seq expression database showed upregulation of NAC gene at vegetative stage under salt stress in barley, suggesting the crucial role of this gene through modulating the antioxidant defense system, mitigating oxidative stress and improving plant resilience. Consequently, these genes probably play essential roles in enhancing stress tolerance in plants, particularly salt stress conditions.
Interestingly, an important QTN (A:G) located at position 489,069,485 bp on chromosome 5H was found near the candidate gene HORVU.MOREX.r3.5HG0494660 annotated as Superoxide dismutase [Cu-Zn] controls the variation of all enzymatic and non-enzymatic antioxidants under salt stress. Superoxide dismutase [Cu-Zn] (Cu/Zn SOD) is a crucial antioxidant enzyme in plants, including barley that plays a significant role in the plant's tolerance to salt stress (Huo et al. 2022). Salt stress, like other abiotic stresses, leads to the overproduction of reactive oxygen species (ROS), including superoxide radicals (O2−), which can cause oxidative damage to lipids, proteins, and nucleic acids, ultimately affecting plant growth and productivity (Mishra et al. 2023). Cu/Zn SOD is pivotal in the plant's antioxidant defense system (Kayihan et al. 2012). It catalyzes the dismutation of superoxide radicals into oxygen and hydrogen peroxide (H2O2), a less reactive ROS (Hasanuzzaman et al. 2020). This activity is essential for the detoxification of superoxide radicals generated in various cellular compartments, including chloroplasts, mitochondria, and cytosol, during salt stress (Hasanuzzaman et al. 2020). Hydrogen peroxide, while still a reactive molecule, is less damaging and can be further detoxified by other antioxidant enzymes such as catalase (CAT) and peroxidases (POX) (Wang et al. 2018). This sequential detoxification process is crucial for maintaining cellular redox homeostasis under salt stress conditions (Ighodaro and Akinloye 2018). Beyond its direct role in detoxifying ROS, hydrogen peroxide generated by the action of Cu/Zn SOD can serve as a signaling molecule, modulating the expression of genes involved in stress responses, including genes encoding for other antioxidant enzymes, osmoprotectants, and stress-protective proteins (Mishra et al. 2023). Thus, Cu/Zn SOD indirectly influences the plant's adaptive responses to salt stress. Genetic studies and transgenic approaches have shown that overexpression of Cu/Zn SOD in barley and other plants enhances tolerance to salt stress. Plants with higher levels of Cu/Zn SOD exhibit better growth, lower levels of oxidative damage, and improved physiological parameters under salt stress conditions compared to wild-type plants. This improved tolerance is attributed to the enhanced capacity to scavenge ROS and maintain cellular redox homeostasis. (Nefissi Ouertani et al. 2021) reported that the Cu/Zn-SOD gene expression level was the most prominent feature of barley development under salt stress. This suggests that the primary focus should be on mitigating oxidative stress and maintaining cell homeostasis. In recent studies, it has been observed that the augmentation of antioxidative defense capacity is commonly employed as a means to promote stress tolerance in plants. As an illustration, the upregulation of the Cu/Zn SOD gene in Arachis hypogaea, Kandelia candel, and wheat has been observed to enhance the ability to withstand salt stress, leading to an augmented capacity for antioxidative defense (Jing et al. 2015; Negi et al. 2015; Wang et al. 2016). The protective role of Cu/Zn SOD under salt stress is part of a coordinated network of antioxidant defenses. The activity of Cu/Zn SOD is complemented by other antioxidant enzymes and molecules, forming an integrated defense system against oxidative stress. The balance and interaction among these components are critical for effective stress tolerance. In conclusion, Cu/Zn SOD is a key player in barley's defense mechanism against salt-induced oxidative stress, contributing to the detoxification of ROS, protection of cellular components, and modulation of stress-responsive pathways. Its role underscores the importance of antioxidant defenses in plant tolerance to abiotic stresses, including salinity, and highlights the potential for genetic improvement of crop resilience through the manipulation of antioxidant enzyme activities.
A significant QTN (A:G) located at position 388,418,487 bp on chromosome 6H was found near the candidate gene HORVU.MOREX.r3.6HG0597850 annotated as GRF zinc finger/zinc knuckle protein controls the variation of CAT, AsA, and GSH under n-K conditions. GRF (Growth-Regulating Factor) zinc finger/zinc knuckle proteins represent a class of plant-specific transcription factors that play significant roles in various aspects of plant growth, development, and stress responses, including salt tolerance in barley and other crops (Liu et al. 2022). These proteins are characterized by their unique DNA-binding GRF zinc finger domains, which allow them to regulate the expression of genes critical for plant development and stress adaptation (Han et al. 2021). Markedly, the regulation of plant tolerance under abiotic stress is mediated by the CCCH zinc-finger proteins, which directly stimulate the expression of target genes associated with tolerance (Han et al. 2022). An illustration of this can be seen in the enhancement of salt tolerance in plants through the activation of downstream genes associated with salt tolerance, including SOS1, AtGSTU5, and AtP5CS1 (Han et al. 2014). The regulation of ABA-dependent response genes RAB18, COR15A, and RD22 by AtC3H17 has been found to enhance the salt and oxidative tolerance of plants (Seok et al. 2018). Certain zinc-finger proteins of the CCCH type are activated by salt stress and are strongly linked to the ability of plants to withstand salt stress (Han et al. 2014). For instance, when rice is exposed to different salt treatments, the genes OsC3H33, OsC3H37, and OsC3H50 are all activated (Jamil et al. 2010). Similarly, the presence of NaCl triggers the expression of GhZFP1 in cotton, suggesting that these zinc-finger proteins of the CCCH family may play a role in controlling the plant's ability to withstand salt stress (Guo et al. 2009). The adaptation of plants to salt stress is regulated by various genes, including AtSZF1 and AtSZF2 in Arabidopsis (Sun et al. 2007), OSC3H33, OSC3H37, OSC3H47, and OSC3H50 in rice (Jamil et al. 2010; Wang et al. 2015), and GhTZF1 in cotton (Zhou et al. 2014). Moreover, the salt tolerance of transgenic tobacco plants is greatly enhanced through the overexpression of GhZFP1, which exerts its effects on Na+ homeostasis and K+ acquisition (Guo et al. 2009). Zinc-finger proteins from CCCH can also help plants cope with ROS. In the event of elevated salt stress, OsTZF1 can mitigate the detrimental effects of ROS by modulating the expression of genes associated with redox homeostasis, including those responsible for ferritin and metallothionin, as well as those encoding antioxidant enzymes such as peroxidase (POD) and glutathione S-transferase (GST) (Jan et al. 2013). The salt tolerance of transgenic Brassica oleracea is enhanced through the overexpression of BoC3H (Jiang et al. 2017). The potential enhancement of salt tolerance in broccoli with the application of BoC3H mechanisms involves the reduction of relative conductivity, hydrogen peroxide (H2O2), and malondialdehyde (MDA), as well as the elevation of CAT, POD, and SOD levels. In general, GRF zinc finger proteins are key regulatory molecules in barley that modulate plant growth, development, and stress responses. Their role in regulating gene expression relevant to salt stress adaptation makes them potential targets for genetic manipulation aimed at improving salt tolerance in barley and other crop species.
Conclusions
Understanding the genetic basis of salt tolerance provides insights into the complex interactions between genetic factors and environmental conditions. Barley accessions exhibited a considerable increase with n-K treatment in comparison to saline soil. The application of potassium nanoparticles led to the discovery of causative alleles and trustworthy chromosomal areas that are responsible for better salt resilience, as determined by GWAS analysis. A QTN marker (A:C) of high significance was identified at location 36,665,559 bp on chromosome 2H. This marker is related to APX, AsA, GSH, GS, WGS, and TKW in the presence of n-K treatment. The candidate gene within this area is HORVU.MOREX.r3.2HG0111480, which has been encoded as NAC domain protein. Allelic variation was observed, with accessions carrying the C allele exhibiting higher levels of antioxidants (APX, AsA, and GSH) and barley yield traits (GS, WGS, and TKW) than accessions carrying the A allele. This suggests that there is a positive selection for accessions carrying the C allele, which could be utilized in the development of barley varieties with enhanced resilience to salt stress. This knowledge can guide the development of crop management strategies and breeding programs aimed at enhancing yield stability under saline stress
Data availability
All data supporting the findings of this study are available within the paper and its supplementary materials published online.
References
Abd-Elrahman SH, El-Gabry YAE-G, Hashem FA, Ibrahim MFM, El-Hallous EI, Abbas ZK, Darwish DBE, Al-Harbi NA, Al-Qahtani SM, Taha NM (2023) Influence of nano-chitosan loaded with potassium on potassium fractionation in sandy soil and strawberry productivity and quality. Agronomy 13:1126
Abeed AHA, Saleem MH, Asghar MA, Mumtaz S, Ameer A, Ali B, Alwahibi MS, Elshikh MS, Ercisli S, Elsharkawy MM, Ali S, Soudy FA (2023) Ameliorative effects of exogenous potassium nitrate on antioxidant defense system and mineral nutrient uptake in radish (Raphanus sativus L.) under salinity stress. ACS Omega 8:22575–22588
Ahmad I, Zhu G, Zhou G, Younas MU, Suliman MSE, Liu J, Zhu YM, Salih EGI (2023) Integrated approaches for increasing plant yield under salt stress. Front Plant Sci 14:1215343
Al Abdallat AM, Ayad JY, Abu Elenein JM, Al Ajlouni Z, Harwood WA (2014) Overexpression of the transcription factor HvSNAC1 improves drought tolerance in barley (Hordeum vulgare L.). Mol Breed 33:401–414
Allaire JJB, MA, (2012) RStudio: integrated development environment for R. Boston, MA 770:165–171
Alqudah AM, Sallam A, Stephen Baenziger P, Börner A (2020) GWAS: fast-forwarding gene identification and characterization in temperate cereals: lessons from Barley–A review. J Adv Res 22:119–135
Al-Tardeh SM, Alqam HN, Kuhn AJ, Kuchendorf CM (2023) In vitro assessment of salinity stress impact on early growth in ten certified palestinian barley cultivars (Hordeum vulgare L.) potentially suitable for cultivation on former quarry substrates. Water 15:1065
Atta K, Mondal S, Gorai S, Singh AP, Kumari A, Ghosh T, Roy A, Hembram S, Gaikwad DJ, Mondal S, Bhattacharya S, Jha UC, Jespersen D (2023) Impacts of salinity stress on crop plants: improving salt tolerance through genetic and molecular dissection. Front Plant Sci 14:1241736
Bagues M, Neji M, Karbout N, Boussora F, Triki T, Guasmi F, Nagaz K (2024) Mitigating salinity stress in barley (Hordeum vulgare L.) through biochar and NPK fertilizers: impacts on physio-biochemical behavior and grain yield. Agronomy 14:317
Balasubramaniam T, Shen G, Esmaeili N, Zhang H (2023) Plants’ response mechanisms to salinity stress. Plants 12:2253
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bian Z, Gao H, Wang C (2020) NAC Transcription factors as positive or negative regulators during ongoing battle between pathogens and our food crops. Int j mol sci 22:81
Chaurasia S, Singh AK, Kumar A, Songachan LS, Yadav MC, Kumar S, Kumari J, Bansal R, Sharma PC, Singh K (2021) Genome-wide association mapping reveals key genomic regions for physiological and yield-related traits under salinity stress in wheat (Triticum aestivum L.). Genomics 113:3198–3215
Chen YJ, Perera V, Christiansen MW, Holme IB, Gregersen PL, Grant MR, Collinge DB, Lyngkjær MF (2013) The barley HvNAC6 transcription factor affects ABA accumulation and promotes basal resistance against powdery mildew. Plant Mol Biol 83:577–590
Ching LS, Mohamed S (2001) Alpha-tocopherol content in 62 edible tropical plants. J Agric Food Chem 49:3101–3105
Dahnke W (1975) Recommended chemical soil test procedures for the North Central Region
Elakhdar A, Slaski JJ, Kubo T, Hamwieh A, Hernandez Ramirez G, Beattie AD, Capo-Chichi LJA (2023) Genome-wide association analysis provides insights into the genetic basis of photosynthetic responses to low-temperature stress in spring barley. Front plant sci 14:1159016
El-tanahy AMM, Marzouk NM, Soliman MS, Mounir AM (2022) The impact of previously prepared potassium and iron in nano forms using gamma radiation on growth and productivity of green bean %J. Egypt J Chem 65:707–725
Emenecker RJ, Strader LC (2020) Auxin-abscisic acid interactions in plant growth and development. Biomolecules 10:281
Foster JG, Hess JL (1980) Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol 66:482–487
Gao L, Jia S, Cao L, Ma Y, Wang J, Lan D, Guo G, Chai J, Bi C (2022) An F-box protein from wheat, TaFBA-2A, negatively regulates JA biosynthesis and confers improved salt tolerance and increased JA responsiveness to transgenic rice plants. Plant Physiol Biochem 182:227–239
Guo YH, Yu YP, Wang D, Wu CA, Yang GD, Huang JG, Zheng CC (2009) GhZFP1, a novel CCCH-type zinc finger protein from cotton, enhances salt stress tolerance and fungal disease resistance in transgenic tobacco by interacting with GZIRD21A and GZIPR5. The New phytol 183:62–75
Halaji B, Haghighi M, Amiri A, Kappel N (2023) Effects of potassium and nanocapsule of potassium on pepper growth and physiological changes in high-temperature stress. J Soil Sci Plant Nutr 23:6317–6330
Han G, Wang M, Yuan F, Sui N, Song J, Wang B (2014) The CCCH zinc finger protein gene AtZFP1 improves salt resistance in Arabidopsis thaliana. Plant Mol Biol 86:237–253
Han G, Qiao Z, Li Y, Wang C, Wang B (2021) The roles of CCCH zinc-finger proteins in plant abiotic stress tolerance. Int j mol sci 22:8327
Han G, Qiao Z, Li Y, Yang Z, Wang C, Zhang Y, Liu L, Wang B (2022) RING zinc finger proteins in plant abiotic stress tolerance. Front Plant Sci 13:877011
Hasanuzzaman M, Bhuyan MHMB, Nahar K, Hossain MS, Mahmud JA, Hossen MS, Masud AAC, Moumita FM (2018) Potassium: a vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 8:31
Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:681
Havir EA, McHale NA (1987) Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant physiol 84:450–455
Horn PS (1998) Introduction to robust estimation and hypothesis testing. Taylor & Francis, New York
Huang C, He W, Guo J, Chang X, Su P, Zhang L (2005) Increased sensitivity to salt stress in an ascorbate-deficient arabidopsis mutant. J exp bot 56:3041–3049
Huo C, He L, Yu T, Ji X, Li R, Zhu S, Zhang F, Xie H, Liu W (2022) The superoxide dismutase gene family in Nicotiana tabacum: genome-wide identification, characterization, expression profiling and functional analysis in response to heavy metal stress. Front Plant Sci 13:904105
Hussain MI, Al-Dakheel AJ, Chaudhry UK, Khan MI, Alhaithloul HAS, Alghanem SM, Alaklabi A (2022) Morpho-physiological response of barley to assess genotypic differences of salinity tolerance under hyper arid climate. Agric Water Manag 272:107832
Ighodaro OM, Akinloye OA (2018) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J Med 54:287–293
Ijaz M, Khan F, Ahmed T, Noman M, Zulfiqar F, Rizwan M, Chen J, K HMS, Li B, (2023) Nanobiotechnology to advance stress resilience in plants: current opportunities and challenges. Mater today Bio 22:100759
Jackson MJPL, New Delhi (1967) Prentice Hall of India 498
Jamil M, Iqbal W, Bangash A, Rehman SU, Imran QM, Rha ESJPJB (2010) Constitutive expression of OSC3H33, OSC3H50 and OSC3H37 genes in rice under salt stress. Pak J Bot 42:4003–4009
Jan A, Maruyama K, Todaka D, Kidokoro S, Abo M, Yoshimura E, Shinozaki K, Nakashima K, Yamaguchi-Shinozaki K (2013) OsTZF1, a CCCH-tandem zinc finger protein, confers delayed senescence and stress tolerance in rice by regulating stress-related genes. Plant Physiol 161:1202–1216
Javid S, Bihamta MR, Omidi M, Abbasi AR, Alipour H, Ingvarsson PK (2022) Genome-wide association study (GWAS) and genome prediction of seedling salt tolerance in bread wheat (Triticum aestivum L.). BMC Plant Biol 22:581
Jiang M, Jiang J-J, Miao L-X, He C-MJPC, Tissue Culture O (2017) Over-expression of a C3H-type zinc finger gene contributes to salt stress tolerance in transgenic broccoli plants. Plant cell. Tissue Organ C (PCTOC) 130:239–254
Jing X, Hou P, Lu Y, Deng S, Li N, Zhao R, Sun J, Wang Y, Han Y, Lang T, Ding M, Shen X, Chen S (2015) Overexpression of copper/zinc superoxide dismutase from mangrove Kandelia candel in tobacco enhances salinity tolerance by the reduction of reactive oxygen species in chloroplast. Front Plant Sci 6:23
Kayihan C, Eyidogan F, Afsar N, Oktem HA, Yucel M (2012) Cu/Zn superoxide dismutase activity and respective gene expression during cold acclimation and freezing stress in barley cultivars. Biol Plantarum 56:693–698
Kesawat MS, Satheesh N, Kherawat BS, Kumar A, Kim HU, Chung SM, Kumar M (2023) Regulation of reactive oxygen species during salt stress in plants and their crosstalk with other signaling molecules-current perspectives and future directions. Plants 12:864
Klute AJA (1986) Methods of soil analysis. Part 1:9
Konings EJ, Roomans HH, Beljaars PR (1996) Liquid chromatographic determination of tocopherols and tocotrienols in margarine, infant foods, and vegetables. J AOAC Int 79:902–906
Kumari VV, Banerjee P, Verma VC, Sukumaran S, Chandran MAS, Gopinath KA, Venkatesh G, Yadav SK, Singh VK, Awasthi NK (2022) Plant nutrition: an effective way to alleviate abiotic stress in agricultural crops. Int J Mol Sci 23:15
Li Z, Wang X, Cao X, Chen B, Ma C, Lv J, Sun Z, Qiao K, Zhu L, Zhang C, Fan S, Ma Q (2021) GhTULP34, a member of tubby-like proteins, interacts with GhSKP1A to negatively regulate plant osmotic stress. Genomics 113:462–474
Liu TB, Xue C (2011) The Ubiquitin-proteasome system and F-box proteins in pathogenic fungi. Mycobiology 39:243–248
Liu X, Huang M, Fan B, Buckler ES, Zhang Z (2016) Iterative usage of fixed and random effect models for powerful and efficient genome-wide association studies. PLOS Genetics 12:e1005767
Liu Y, Khan AR, Gan Y (2022) C2H2 zinc finger proteins response to abiotic stress in plants. Int J Mol Sci 23:2730
Mao X, Zhang H, Qian X, Li A, Zhao G, Jing R (2012) TaNAC2, a NAC-type wheat transcription factor conferring enhanced multiple abiotic stress tolerances in Arabidopsis. J Exp Bot 63:2933–2946
McGrann GR, Steed A, Burt C, Goddard R, Lachaux C, Bansal A, Corbitt M, Gorniak K, Nicholson P, Brown JK (2015) Contribution of the drought tolerance-related stress-responsive NAC1 transcription factor to resistance of barley to Ramularia leaf spot. Mol Pathol 16:201–209
Milner SG, Jost M, Taketa S, Mazón ER, Himmelbach A, Oppermann M, Weise S, Knüpffer H, Basterrechea M, König P, Schüler D, Sharma R, Pasam RK, Rutten T, Guo G, Xu D, Zhang J, Herren G, Müller T, Krattinger SG, Keller B, Jiang Y, González MY, Zhao Y, Habekuß A, Färber S, Ordon F, Lange M, Börner A, Graner A, Reif JC, Scholz U, Mascher M, Stein N (2019) Genebank genomics highlights the diversity of a global barley collection. Nature Genet 51:319–326
Mishra N, Jiang C, Chen L, Paul A, Chatterjee A, Shen G (2023) Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front Plant Sci 14:1110622
Nakano Y, Asada KJP, physiology c (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(867):880
NefissiOuertani R, Abid G, Karmous C, Ben Chikha M, Boudaya O, Mahmoudi H, Mejri S, Jansen RK, Ghorbel A (2021) Evaluating the contribution of osmotic and oxidative stress components on barley growth under salt stress. AoB Plants. https://doi.org/10.1093/aobpla/plab034
Negi NP, Shrivastava DC, Sharma V, Sarin NB (2015) Overexpression of CuZnSOD from Arachis hypogaea alleviates salinity and drought stress in tobacco. Plant Cell Rep 34:1109–1126
Noman M, Ahmed T, Wang J, Ijaz M, Shahid M, Islam MS, Azizullah Manzoor I, Li D, Song F (2023) Nano-enabled crop resilience against pathogens: potential, mechanisms and strategies. Crop Health 1:15
Paradiso A, Berardino R, de Pinto MC, Sanità di Toppi L, Storelli MM, Tommasi F, De Gara L (2008) Increase in ascorbate-glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant & Cell Physiol 49:362–374
Piisilä M, Keceli MA, Brader G, Jakobson L, Jõesaar I, Sipari N, Kollist H, Palva ET, Kariola T (2015) The F-box protein MAX2 contributes to resistance to bacterial phytopathogens in Arabidopsis thaliana. BMC Plant Biol 15:53
Qu L, Sun M, Li X, He R, Zhong M, Luo D, Liu X, Zhao X (2020) The Arabidopsis F-box protein FOF2 regulates ABA-mediated seed germination and drought tolerance. Plant Sci : Int Jo Exp Plant Biol 301:110643
Seok HY, Nguyen LV, Park HY, Tarte VN, Ha J, Lee SY, Moon YH (2018) Arabidopsis non-TZF gene AtC3H17 functions as a positive regulator in salt stress response. Biochem biophys Res Commun 498:954–959
Shah WH, Rasool A, Saleem S, Mushtaq NU, Tahir I, Hakeem KR, Rehman RU (2021) Understanding the Integrated pathways and mechanisms of transporters, protein kinases, and transcription factors in plants under salt stress. Int J Gen 2021:5578727
Singh M, Nara U, Kumar A, Choudhary A, Singh H, Thapa S (2021) Salinity tolerance mechanisms and their breeding implications. J, Genet Eng & Biotechnol 19:173
Singh P, Choudhary KK, Chaudhary N, Gupta S, Sahu M, Tejaswini B, Sarkar S (2022) Salt stress resilience in plants mediated through osmolyte accumulation and its crosstalk mechanism with phytohormones. Front Plant Sci 13:1006617
Sun J, Jiang H, Xu Y, Li H, Wu X, Xie Q, Li C (2007) The CCCH-type zinc finger proteins AtSZF1 and AtSZF2 regulate salt stress responses in Arabidopsis. Plant & Cell Physiol 48:1148–1158
Tarolli P, Luo J, Park E, Barcaccia G, Masin R (2024) Soil salinization in agriculture: mitigation and adaptation strategies combining nature-based solutions and bioengineering. iScience. https://doi.org/10.1016/j.isci.2024.108830
Thabet SG, Alqudah AM (2023) New genetic insights into improving barley cope with salt stress via regulating mineral accumulation, cellular ion homeostasis, and membrane trafficking. Environ Exp Bot 208:105252
Thabet SG, Moursi YS, Karam MA, Börner A, Alqudah AM (2020) Natural variation uncovers candidate genes for barley spikelet number and grain yield under drought. Stress 11:533
Thabet SG, Moursi YS, Sallam A, Karam MA, Alqudah AM (2021) Genetic associations uncover candidate SNP markers and genes associated with salt tolerance during seedling developmental phase in barley. Environ Exp Bot 188:104499
Thabet SG, Sallam A, Moursi YS, Karam MA, Alqudah AM (2021) Genetic factors controlling nTiO(2) nanoparticles stress tolerance in barley (Hordeum vulgare) during seed germination and seedling development. Functional Plant Biol : FPB 48:1288–1301
Thabet SG, Alomari DZ, Börner A, Brinch-Pedersen H, Alqudah AM (2022) Elucidating the genetic architecture controlling antioxidant status and ionic balance in barley under salt stress. Plant Mol Biol 110:287–300
Thabet SG, Alqahtani MD, Jabbour AA, Alqudah AM (2023) Genetic associations underpinning the metabolite-mediated salt stress tolerance in Barley. Plant molecular biology reporter
Wang J, Zhang Z (2021) GAPIT version 3: boosting power and accuracy for genomic association and prediction. Gen, Proteom & Bioinform 19:629–640
Wang W, Liu B, Xu M, Jamil M, Wang G (2015) ABA-induced CCCH tandem zinc finger protein OsC3H47 decreases ABA sensitivity and promotes drought tolerance in Oryza sativa. Biochem Biophys Res Commun 464:33–37
Wang M, Zhao X, Xiao Z, Yin X, Xing T, Xia G (2016) A wheat superoxide dismutase gene TaSOD2 enhances salt resistance through modulating redox homeostasis by promoting NADPH oxidase activity. Plant Mol Biol 91:115–130
Wang Y, Branicky R, Noë A, Hekimi S (2018) Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol 217:1915–1928
Wang X, Xie H, Wang P, Yin H (2023) Nanoparticles in plants: uptake, transport and physiological activity in leaf and root. Mater (Basel, Switzerland) 16:3097
Xia L, Sun S, Han B, Yang X (2023) NAC domain transcription factor gene GhNAC3 confers drought tolerance in plants. Plant Physiol Biochem 195:114–123
Xiong L, Ishitani M, Lee H, Zhu JK (2001) The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress-and osmotic stress-responsive gene expression. The Plant cell 13:2063–2083
Xue Z, Wang B, Qu C, Tao M, Wang Z, Zhang G, Zhao M, Zhao S (2023) Response of salt stress resistance in highland barley (Hordeum vulgare L. var. nudum) through phenylpropane metabolic pathway. PloS One 18:e0286957
Yadav A, Yadav K, Abd-Elsalam KA (2023) Nanofertilizers: types delivery and advantages in agricultural sustainability. Agrochemicals 2(296):336
Yu CW, Murphy TM, Lin CH (2003) Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Functional Plant Biol : FPB 30:955–963
Zhang X, Gonzalez-Carranza ZH, Zhang S, Miao Y, Liu C-J, Roberts JA (2019) F-Box Proteins in Plants. Annu Plant Rev Online 2:307–328
Zhang L, Jiang Q, Zong J, Guo H, Liu J, Chen J (2024) Effects of supplemental potassium on the growth, photosynthetic characteristics, and ion content of Zoysia matrella under salt. Stress 10:31
Zhao C, Zhang H, Song C, Zhu J-K, Shabala S (2020) Mechanisms of plant responses and adaptation to soil salinity. The Innovation 1:100017
Zhou T, Yang X, Wang L, Xu J, Zhang X (2014) GhTZF1 regulates drought stress responses and delays leaf senescence by inhibiting reactive oxygen species accumulation in transgenic Arabidopsis. Plant Mol Biol 85:163–177
Zhou S-M, Kong X-Z, Kang H-H, Sun X-D, Wang W (2015) The involvement of wheat f-box protein gene TaFBA1 in the oxidative stress tolerance of plants. PloS One 10:e0122117
Acknowledgments
The authors would like to thank Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R318), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
Funding
This study is supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R318), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
SGT designed the experiment and analyzed the data. SGT wrote the manuscript. SGT, FAS, AB, and AMA edited the manuscript. SGT, FAS, AB, and AMA performed the experiments. SGT conceived the idea and participated in the interpretation of the results.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests.
Ethical approval
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thabet, S.G., Safhi, F.A., Börner, A. et al. Genome-wide association scan reveals the reinforcing effect of nano-potassium in improving the yield and quality of salt-stressed barley via enhancing the antioxidant defense system. Plant Mol Biol 114, 97 (2024). https://doi.org/10.1007/s11103-024-01489-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11103-024-01489-y