Abstract
Abiotic stresses have been observed to cause alterations in the morphology, physiology, and biochemistry of plants. However, in recent years, the utilization of nanocompounds has emerged as a strategy to induce modifications in multiple facets of plant biology. These modifications include plant growth, nutrient absorption, the production of significant secondary metabolites, and the improvement of plants’ resistance against both abiotic and biotic stress factors. A completely randomized factorial experiment with 12 replications was created. Potassium sources including control, potassium (K), and nanocapsule-potassium (N-K) with concentration 1 µM and temperature treatments including control temperature (25 °C) and high-temperature stress (35 °C) were applied as treatments. In the control treatment, proline was increased at the high temperature, whereas proline was reduced at both treated temperatures by K and N-K. High temperature raised electrolyte leakage (EL), which peaked in the control treatment but was lowered by K and N-K. Temperature-dependent increase in glucose and fructose was observed in control and K treatments when the temperature was 35 °C, but no significant difference was observed between different levels of K at 35 °C. When K was not applied at high temperatures, the main stress indicators such as antioxidant activity (DPPH) and malondialdehyde (MDA) rose significantly, as did the water potential and linoleic acid. When high temperatures were applied, nanocapsule-potassium applied in high temperatures had the lowest stress indices. In conclusion, stress indices diminish when nanocapsule-potassium is applied under high temperatures. Additionally, nanocapsule-potassium applied at high temperatures was preferable to K applied at high temperatures in terms of pepper growth and resistance measures. Likewise, the application of nanocapsule-potassium at high temperatures alters the fatty acid composition of membranes and antioxidant enzymes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Abiotic stress is one of the limiting variables for plant growth and development, such as excessive temperature, drought, and salinity. Heat stress, which disturbs homeostasis, restricts plant growth and development, and even results in plant death, is one of the most severe types of abiotic stress experienced by plants as a result of the severity of global warming brought on by greenhouse gas emissions (Nazar et al. 2017). The chloroplast activity is the most heat-sensitive cell function, and high-temperature stress causes structural and functional damage (Zeng et al. 2021). To cope with the stress caused by high temperatures, plants use a variety of strategies, including long-term morphological and phenological changes as well as short-term acclimatization (Shenoda et al. 2021). At different developmental stages and depending on the tissue type, the method of response to heat stress varies (Ullah et al. 2022).
Pepper (Capsicum annuum L.) is a warm-season plant, but it is very susceptible to temperatures higher than 35 °C which mainly damages flower formation besides photosynthesis reduction (Ahmadi and Souri 2021). Decreased reproduction and yield may result from flowers falling due to excessive temperatures (Haghighi et al. 2019). Heat-stressed pepper transplants showed an increase in proline carbohydrates and chlorophyll degradation (Javanmardi et al. 2014).
It is widely known how potassium affects plant growth. Reactive oxygen species (ROS) are produced less by plants when the K status is elevated (Tohidloo et al. 2018; Pourranjbari Saghayesh and Souri 2018). Potassium protects the photosynthetic electron transport activity and prevents the activity of NADPH-oxidases, which helps to reduce reactive oxygen species (ROS). Lack of potassium can affect the assimilation, transport, and use of CO2 during photosynthetic processes (Waraich et al. 2012). In K-deficient plants, membrane and chlorophyll (chl) breakdown is preferred (Cakmak 2005). The activation of the adenosine triphosphate (ATP) synthase enzyme is triggered by potassium. The plasma membrane-bound H+-ATPase is affected by the potassium concentration (Hasanuzzaman et al. 2018). Potassium has been reported to reduce many plant stresses, including drought, freezing, and high light intensity (Waraich et al. 2012). In recent years, the relationship between phytohormones and K has been investigated (Wang et al. 2013); phytohormones interact with one another and other signaling molecules that regulate biochemical processes and metabolism, exerting physiological responses about nearly all aspects of plant growth and development and enhancing stress tolerance (Nadeem et al. 2016).
Nanotechnology offers an excellent solution to reduce the overuse of fertilizers and improve sustainable agriculture practices. High surface area, rapid diffusion, and the capacity to increase soil fertilization and boost plant development are all characteristics of nanofertilizers (Haleem 2020). In order to prevent unfavorable nutrient losses to the soil, water, and air through direct internalization by plants and to prevent the interaction of nutrients with soil, microorganisms, water, and air, nanofertilizers will combine nanodevices to synchronize the release of fertilizer N and K with their uptake by crops (DeRosa et al. 2010).
The improved adhesion of nanomaterials with surface coatings or nano-coated fertilizer particles to plants can be ascribed to their heightened surface tension in comparison to conventional surfaces. Furthermore, it has been observed that nanocoatings provide effective surface protection against larger particles, as demonstrated by Khot et al. (2012). A nanocapsule consists of an external shell that encloses an active compound, such as a chemical or biological agent. The shell is composed of diverse components, such as lipids, polymers, viral capsids, and nanoclays. According to Bamrungsap et al. (2012), these particles possess the ability to modify their morphology and dimensions in reaction to external stimuli, such as variations in pH. The principal function of nanocapsules is to serve as a protective enclosure for the active compound until its subsequent release. Furthermore, nanocapsules can improve the solubility of the compound and aid in its penetration into plant tissues. The process of beginning the release of nanocapsules can be achieved by modifying pH levels or by enzymatic degradation, as described by Chand Mali et al. (2020).
Ajirloo et al. (2015) reported the maximum plant height and stem diameter were obtained with a potassium nanofertilizer application of 400 kg per hectare. Results also indicated that the 300 kg per ha K nanofertilizer treatment had the maximum fruit production, fruit weight, fruit diameter, and fruit number per plant (Ajirloo et al. 2015).
According to the literature, we suppose that K and especially N-K can decrease the deleterious effect of stress so this research aimed to assess the impact of foliar potassium and nanocapsule-potassium on alleviating the deleterious impacts of heat stress, namely, on reproductive development and yield. This study sought to assess the influence of various potassium sources in relation to a previously mentioned study and the problem of high temperatures in the field and greenhouse during pepper cultivation. According to our knowledge, nanocapsule-potassium has never been used for this purpose.
2 Materials and Methods
2.1 Experimental Design and Plant Preparation
This study was conducted on pepper plants (Capsicum annuum L., cv. Aristotelian) in a research greenhouse of the Department of Horticulture, Isfahan University of Technology, Iran, with an average daily temperature of 25 ± 2 °C and night temperature of 17 ± 2 °C and the length of the light–dark period was 14 to 10 h. An experiment was designed as a factorial experiment based on a completely randomized block design (RCBD), with 12 replications for each treatment. The factorial experiment included 2 factors: (1) two temperature regimes, i.e., 25 ± 2 °C and 35 ± 2 °C, and (2) and foliar application with three substances, namely, distilled water (C), potassium (K), and nanocapsule-potassium (N-K) which were applied to pepper plant.
The Ghaem Isfahan Seedling Production Company purchased pepper seedlings with three leaves in April 2018. Seeds were sown in a plug filled with cocopeat/perlite (1:1 V/V). Pepper seedlings with 4–5 true leaves were transferred to the 5-L container filled with the same plug substrate. During the first week of the experiment, seedlings were exposed to a half-nutrient solution concentration and then to a complete-nutrient solution, each time with irrigation. The composition nutrient solutions (in mg L−1) was done as follows: N = 116, P = 21, K = 82, Ca = 125, Mg = 21, S = 28, Fe = 6.8, Mn = 1.97, Zn = 0.25, B = 0.70, Cu = 0.07, and Mo = 0.05, used KH2PO4, NH4NO3, Fe EDTA, CuSO4, MnSO4, ZnSO4, Ca (NO3)2, Na2MO4, and H3BO3. The pH of the nutrient solution was adjusted to 6.5 utilizing 1 M KOH or 1 M H2SO4 (Jones 1930). The vegetative stage was determined after the complete establishment of seedlings and before the emergence of the first flower bud. The reproductive stage was specified at the onset of the first flower opening in 50% of the plants. Distilled water (control), KCl as potassium (K), and nanocapsule-potassium (N-K) with a concentration 1 µM were sprayed on the sample one time a week from the 8-leaf stage of pepper to the end of the experiment. In order to provide temperature stress, the plants of pepper were transferred to the vegetation chambers (EYELA LTI-1000 SD) under a 14-h photoperiod at a photosynthetic photon-flux density of 270 μmol m−2 s−1, a temperature of 25 ± 2°C and 35 ± 2°C, and a humidity of 70% for 6 h, matching with the hot hours of a day.
This experiment lasted 2.5 months. Plant samples were collected to measure the essential indices after 56 days of treatment. During stress, relative water content, photosystem II quantum efficiency, leaf leakage percentage, water potential changes, and growth indices such as shoot and root fresh and dry weight, fruits, numbers number of flowers, number of flowers that aborted, and stem length were measured.
2.2 Investigation of Physicochemical Results of Nanoliposomes
By examining the morphology of nanoliposomes using a scanning electron microscope (SEM), images of lecithin nanoliposomes with aminolevulinic acid are shown in optimal conditions (Fig. 1 supplementary). These images were prepared after drying in a freeze-dryer. In all of these forms, nanoliposomes are generally irregularly shaped and have uneven surfaces. The presence of teeth and cavities on the surface is due to the use of a freeze-dryer to dry the microcoatings. Ice particle elimination by sublimation leads to the formation of open cavities (Fonte et al. 2012). The particles had an average size of 290 ± 65 nm. The results of encapsulation efficiency of nanoliposomes showed that its efficiency is equal to 81%. The percentage of encapsulation efficiency of lipophilic active ingredients in liposomes was reported to be higher than 90% in research. The researchers stated that the encapsulation efficiency of nano-encapsulated polyphenols in thin-layer ultrasonically prepared liposomes was 61.52% (Lu et al. 2011). The results of measuring particle size and dispersion index at a concentration of 4% lecithin show ranges of 78–94 nm and 0.285–0.355, respectively. The average particle size was 91.8 ± 9.5 nm (Fig. 2 , supplementary). The different sizes that were obtained by SEM and DLS (dynamic light scattering) were related to the aggregation of particles during drying which led to an increase in size detected by SEM. Most liposomes produced by this method are less than 100 nm in size. However, research (Rasti et al. 2012) has shown that heat-prepared liposomal particles require sonication techniques to reduce size. Smaller nanoliposomes are more stable due to their higher resistance to gravity (Fathi et al. 2012). Gibis et al. (2012) prepared liposomes (1% soy lecithin) containing grape seed polyphenolic extract (0.1 w/v) using high-pressure homogenization. Liposomes containing grape extract had average droplets of less than 100 nm (Gibis et al. 2012). It can be concluded that the use of ultrasonic method is more effective in preparing nanoliposomes of smaller sizes. Reducing the particle size increases the number of particles per unit weight of used fertilizer. Also, reducing the particle size increases the specific area of the fertilizer. This reduction increases the solubility of fertilizers despite the low solubility of fertilizers such as zinc oxide in water. Particle shrinkage and weight loss of each fertilizer particle increase the level of zinc uptake and ultimately increase the uptake of zinc by the plant (Prasad et al. 2012).
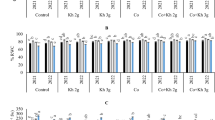
The interaction effect of different sources of potassium and temperature on some growth parameters of pepper. Treatments include control (C), potassium (K), and nanocapsule-potassium (N-K) and temperature treatment including 25 and 35 °C. Significant differences between treatments are shown by various letters (p < 0.05) according to the least significant difference test (LSD). Bars show the mean standard error
The interaction effect of different sources of potassium and temperature on flower, fruit, and leaf number of pepper. Treatments include control (C), potassium (K), and nanocapsule-potassium (N-K) and temperature treatment including 25 and 35 °C. Significant differences between treatments are shown by various letters (p < 0.05) according to the least significant difference test (LSD). Bars show the mean standard error
One of the key elements in the stability of nanoparticles is zeta potential. The surface charge of the nanoparticles increases with zeta potential, which also increases electrostatic repulsion and decreases particle adhesion. The low zeta potential of nanoparticles reduces their stability and particle adhesion during storage in solution. The zeta potential in the sample varied from − 82.2 mV to − 77.1 mV, and their mean was − 79.1 with a standard deviation of ± 2.72 mV. This information is presented in Fig. 3, supplementary. The main reason for the negative surface charge in nanoliposomes is the presence of lecithin as an anionic emulsifier. For electrostatically stabilized nanosuspensions, a minimum zeta potential of − 30 mV is required, whereas in electrostatically stabilized suspensions the minimum zeta potential of − 20 mV is sufficient. According to previous research by Rasti et al. (2012), it was observed that by decreasing the size of liposomes, the zeta potential increases.
The interaction effect of different sources of potassium and temperature on some physiological parameters in pepper. Treatments include control (C), potassium (K), and nanocapsule-potassium (N-K) and temperature treatment including 25 and 35 °C. Significant differences between treatments are shown by various letters (p < 0.05) according to the least significant difference test (LSD). Bars show the mean standard error
2.3 Fresh and Dry Weights (FW and DW)
During the experiment, growth indices such as shoot and root fresh and dry weight, the number of flowers, fruit, and leaves, the number of flowers that aborted, and stem and root length were measured. All the biochemical and physiological parameters were measured at the harvest time from 12 plants for each treatment that of them was measured and then averaged.
Shoot and root tissues were individually weighed (FW (fresh weight)) and afterward were kept in the oven at 70°C for 48 h (DW) to measure dry weight. Stem and root length were measured with a ruler.
2.4 Electrolyte Leakage (EL)
Three leaves from each treatment were washed three times with deionized water and subsequently immersed in 6 mL of deionized water. A preliminary assessment of electrical conductivity (ECi) was conducted at the onset of the rehydration phase. Subsequently, the tubes containing the segments were placed back into a dark environment at a temperature of 25 °C. Following this, measurements (ECf) were conducted at various intervals of rehydration time (0.5, 1.5, 3.5, 7.5, and 22.5 h). After conducting the aforementioned readings, the samples were subjected to autoclaving and subsequently cooled to a temperature of 25 °C. The total electrical conductivity (ECt) of the samples was then measured (Bajji et al. 2002). The expression of electrolyte leakage was calculated using the formula (ECf-ECi)/(ECt-ECi) × 100.
2.5 Proline Assay
The content of proline was measured based on the Bates assay. The leaf sample (0.1 g) was homogenized in 10 mL aqueous sulfosalicylic acid (3%). A mixed solution containing 2 mL of each supernatant, ninhydrin acid, and glacial acetic acid was incubated at 100 °C for 1 h. The ice bath was used for stopping the reaction; then, 4 mL toluene was mixed for 15–20 s. The mixture was read at 520 nm, and the content of proline was expressed as µmol L-proline equivalent g−1 FW (Bates et al. 1973).
2.6 Relative Water Content (RWC)
At the end of the experiment, relative water content (RWC) was calculated (Dhopte and Manuel 2002). Accordingly, 7-mm leaf discs were employed to specify RWC. The weight of the given discs was calculated (FW) in each treatment. The sample hydrated for 48 h at 5 °C in darkness (TW) to reach saturation (constant weight). The leaf discs were then oven-dried for 24 h at 105 °C (DW). RWC was obtained using the equation
2.7 Leaf Water Potential
Two to four mature and fully exposed leaves were collected for sampling for each treatment. The leaf samples are enclosed within a foil-laminate bag for a minimum duration of 10 min before their removal. The leaves remain inside the bag throughout the measurement process. The hydrostatic pressure necessary to expel water from the vascular tissue of a detached leaf is equivalent to the water potential and can be quantified using a pressure gauge. The measurement of leaf water potential (Ψ leaf) was conducted during the midday period (11:30–12:30 h) using a Scholander pressure chamber (3115 model, Santa Barbara, USA) (Rodriguez-Dominguez et al. 2022).
2.8 Chlorophyll Fluorescence
Chlorophyll fluorescence was measured with a chlorophyll fluorometer (RS232, Handy PEA, UK) between 8:00 and 9:00 am. Leaves were acclimated in the dark for 30 min. The maximum photochemical quenching of the photosystem II (Fv/Fm) was presented (Maxwell and Johnson 2000).
2.9 Antioxidant Activity (DPPH)
The determination of the antioxidant activity of pepper leaves was conducted by the methodology outlined by Koleva et al. (2002). Following the addition of 200 μL of an analytical sample solution and 800 μL of a Tris–HCl buffer (pH 7.4) into a test tube or sampling tube, 1 mL of the DPPH solution was subsequently introduced. The solution was promptly subjected to agitation using a test tube mixer for 10 s. Subsequently, it was placed at ambient temperature in a lightless environment. The absorbance of the solution at a wavelength of 517 nm was measured by the spectrophotometer (UV 160A, Shimadzu Corp., Kyoto, Japan) precisely 30 min following the addition of the DPPH solution. The blank consisted of a solution composed of 1.2 mL of ethanol and 800 μL of Tris–HCl buffer. The absorbance observed upon the introduction of the analytical sample was denoted as As, while the absorbance observed upon the introduction of ethanol in place of the sample was identified as Ac. The inhibition ratio (%) was calculated using the subsequent equation:
2.10 Antioxidant Enzymatic Activities
Leaf samples were homogenized with potassium phosphate buffer (pH 6.8, 100 mM) containing 1% polyvinylpyrrolidone (PVP) and EDTA (4 mM) to estimate total soluble protein. The catalase (CAT) enzyme activity was extracted and evaluated as described by (Tabatabaei and Ehsanzadeh 2016). CAT enzyme activity was assessed by measuring at 240 nm. The SOD activity was measured by the method described by (Khoshbakht et al. 2018). The changes in absorbance for SOD were recorded at 560 nm. APX enzymatic activity was estimated based on the absorbance changes at 290 nm expressed as umg−1 Pr (Nakano and Asada 1981).
2.11 K+ Content
A total of four uniform leaves were collected for each treatment. These leaves were carefully washed using tap water, followed by a final rinse with deionized water. Subsequently, the leaves were dried at a temperature of 65 °C until a constant weight was achieved. Finally, the dried leaves were ground to analyze their mineral composition. The extraction process involved the utilization of 1 N hydrochloric acid (HCl) following the dry ashing of the plant tissue material at a temperature of 550 °C for 5 h. The concentrations of potassium (K) were determined using an atomic absorption spectrophotometer after the process of digestion (Murillo‐Amador et al. 2007).
2.12 Lipid Peroxidation (MDA)
The quantity of MDA created by the thiobarbituric acid reaction, as reported by Jiang and Zhang (2001), was measured to estimate the level of lipid peroxidation. A 0.5% (w/v) thiobarbituric acid solution containing 20% (w/v) trichloroacetic acid was added to the crude extract in the same amount. The homogenate was cooked for 30 min at 95 °C before being instantly cooled. During the mixture’s centrifugation at 3000 g for 10 min, the supernatant’s absorbance was measured at 532 and 600 nm (U-2000, Hitachi Instruments, Tokyo, Japan). The MDA content was calculated by deducting the nonspecific absorbance (600 nm).
2.13 Glucose and Fructose Measurement
The leaf sample was mixed with 80% ethanol and homogenized for 10 min using an ultrasonic cleaner before being centrifuged at 4 °C for the same amount of time. The supernatant solution was delivered into the 20 µL loop using 5 mL syringe filters that each included two 0.2 mL membrane filters. A refractive index detector on a high-performance liquid chromatography (HPLC) system (Shimadzu, Japan). An SCR-101 N (30 cm 9.7 mm) column with an SCR (N) guard column was used as the HPLC column (5 cm 4 mm). Deionized water flowing at a rate of 0.7 mL min−1 and 60 °C served as the mobile phase (Adams et al. 2001).
2.14 Fatty Acid Assay
According to the procedure outlined by Motamedi et al. (2019), the fatty acid content of the leaf was also determined using a gas chromatograph (Agilent 6890 N) outfitted with a flame ionization detector (FID) and an HP-88 capillary column (100 m 250 m). By methylating with sodium methoxide, fatty acid methyl esters were produced (0.5 N). The split mode was used to inject (1 µL) at a split ratio of 1:30. The carrier gas, nitrogen, had a flow rate of 1.1 mL min−1. The oven’s temperature was set to rise from 150 °C (held for 1 min) to 190 °C (held for 2 min) and finally to 240 °C, in increments of 5 °C every minute (held for 8 min).
2.15 Statistical Analysis
The factorial experiment was set up using a completely randomized block design with 12 replications. All obtained data analysis was done with a two-way analysis of variance by SAS (V. 9.4, Cary, NC, USA), and the means of each treatment were compared by using the least significance difference (LSD) test at the 95% level of probability. Principal component analysis (PCA) was carried out using Statgraphics Centurion, Version XVI.
3 Results
The results of ANOVA and the interaction effect of different sources of potassium and temperature treatment on all measured parameters are presented in Supplementary Tables 1, 2, 3, 4, 5, 6, 7, and 8.
3.1 Morphological Characteristics
The interaction effect between heat stress and K on growth traits shows a statistically significant difference (p < 0.01). At a temperature of 25 °C, the stem exhibited the highest fresh weight in the absence of potassium supplementation. The fresh weight of the stem was observed to increase with rising temperature when potassium was applied, as compared to the application of N-K potassium and the absence of potassium (Fig. 1A).
The addition of potassium and N-K at elevated temperatures increased the dry weight of the stem, as compared to the stem weight at a temperature of 25 °C. Conversely, the lowest wet weight of the stem was observed at high temperatures in the absence of potassium supplementation (Fig. 1B). The fresh and dry weight of the roots exhibited an increase at a temperature of 25 °C upon the application of potassium. However, under high-temperature conditions, the absence of potassium and the treatment with N-K increased the fresh and dry weight of the root (Fig. 1C, D). The length of the stem did not change significantly (Fig. 1E). The root length observed at high temperatures in the absence of potassium and N-K treatment exhibited the greatest extent among all conditions tested. Despite the elevated temperature conditions, the application of N-K did not result in any discernible alteration in root length when compared to the control temperature of 25 °C (Fig. 1F). The fresh and dry root weights of all plants examined under 25 °C conditions were more significant for K-treated plants, although N-K was greater in two parameters in heat treatment at 35 °C.
The K treatment at 35 °C resulted in the most leaves, according to the mean comparison (Fig. 2A–D). Heat stress in the control treatment considerably changed flowers’ abscission but not when K from both sources was applied. With increasing temperature, the application of N-K could increase the number of flowers per plant compared optimum temperature (Fig. 2A). The highest number of leaves was at high temperature with the application of potassium, and when potassium was not used, there was no change in the number of leaves despite the high temperature (Fig. 2B). In general, the amount of flower shedding was higher at high temperature than at 25 °C, but with the application of potassium and N-K, the amount of shedding decreased compared to when potassium was not used (Fig. 2C). By adding potassium and N-K at both temperatures of 25 and 35 °C, the number of fruits increased compared to the control, and when potassium and N-K were used, the number of fruits per plant was less with increasing temperature (Fig. 2D).
3.2 Physiological Characteristics
Based on the findings of the analysis of the mutual effects of heat stress and potassium source on the physiological characteristics studied in this experiment, it showed a significant difference. The addition of potassium and N-K at temperatures of 25 and 35 °C resulted in a decrease in water potential. The control treatment exhibited the highest water potential, while the use of N-K at high temperatures resulted in the lowest water potential (Fig. 3A). With the addition of potassium and N-K at both temperatures of 25 and 35 °C, the chlorophyll fluorescence did not change significantly compared to the control (Fig. 3B). Overall, the application of potassium and N-K at elevated temperatures resulted in increased RWC and chlorophyll fluorescence compared to the absence of potassium. However, it is important to note that there was no statistically significant difference observed between the treatment groups (Fig. 3C). In general, it was observed that the protein content showed an upward trend as the temperature increased, in contrast to the conditions at 25 °C. Conversely, the addition of potassium and N-K resulted in a decrease in protein levels when compared to the absence of potassium (Fig. 3D). The application of potassium and N-K at temperatures of 25 °C and 35 °C resulted in an increase in potassium levels compared to the control. Conversely, in the absence of potassium and N-K, the temperature rise led to a decrease in potassium content in the leaves (Fig. 3E).
3.3 Antioxidant Enzyme Activities, EL, MDA Content, and Proline
The control group exhibited an elevation in antioxidant enzyme activity, proline levels, MDA levels, and EL as a result of heat stress. In general, it was observed that the quantity of EL increased in response to rising temperatures when compared to the temperature of 25 °C. Conversely, the introduction of K and N-K resulted in a decrease EL when compared to the control (Fig. 4A–F). The application of K increased catalase (CAT) activity under high-temperature conditions. The CAT enzyme exhibited its highest level of activity at a temperature of 35 °C in the presence of K. Conversely, in the absence of K, the activity of SOD decreased (Fig. 4B). The highest levels of superoxide dismutase (SOD) activity were observed in the control and K treatments under high-temperature conditions, while the addition of N-K resulted in a decrease in SOD activity. The application of N-K resulted in a significant decrease in El, SOD, POD, and MDA levels at both treatment temperatures (Fig. 4A–F). The maximum level of peroxidase (POD) activity was observed at elevated temperatures in the absence of K. Conversely, the addition of K or exposure to high temperatures did not result in any significant alteration in POD levels when compared to the control condition at 25 °C (Fig. 4C). The levels of superoxide dismutase (SOD) exhibited an upward trend as the temperature increased in both the control and K treatment groups, in contrast to the 25 °C condition. However, when N-K was utilized, no statistically significant alteration in SOD levels was observed at elevated temperatures compared to the 25 °C condition (Fig. 4D). The lowest concentration of MDA was observed under conditions of elevated temperature in conjunction with the application of K, while the highest concentration of MDA was observed in the absence of K (Fig. 4E). The proline content was found to be the highest under conditions of high temperature in the absence of K. Conversely, when K was present or when the high temperature was combined with K, there was no significant alteration in the proline levels compared to those observed at a temperature of 25 °C (Fig. 4F).
The interaction effect of different sources of potassium and temperature on some stress indices in pepper. Treatments include control (C), potassium (K), and nanocapsule-potassium (N-K) and temperature treatment including 25 and 35 °C. Significant differences between treatments are shown by various letters (p < 0.05) according to the least significant difference test (LSD). Bars show the mean standard error
3.4 Glucose and Fructose Content
Heat stress increased the content of glucose and fructose, but these levels did not change much when K and N-K were added. When N-K was applied, the glucose and fructose content decreased (Fig. 5A, B).
The interaction effect of different sources of potassium and temperature on glucose and fructose in pepper. Treatments include control (C), potassium (K), and nanocapsule-potassium (N-K) and temperature treatment including 25 and 35 °C. Significant differences between treatments are shown by various letters (p < 0.05) according to the least significant difference test (LSD). Bars show the mean standard error
3.5 Saturated and Unsaturated Fatty Acids
When K and N-K were applied, the saturated/unsaturated ratio rose, with N-K having the biggest impact. Linoleic acid was the most unsaturated fatty acid across all treatments, while palmitic acid was the most saturated. In the presence of K, the saturated/unsaturated ratio increased at 35 °C while it dropped in the control and N-K (Table 1).
3.6 Principal Component Analysis
The principal component analysis (PCA) method was utilized to assess the most important effects of K treatment on pepper growth and antioxidant enzyme (Fig. 6). The RWC and potassium concentrations in the N-K-T (nanocapsule-potassium applied at high temperature) treatment were positively connected with linoleic acid, according to the PCA. Furthermore, there was a substantial association between fatty acid levels and K-T treatment; it has the strongest antioxidant activity in both K and N-K and vegetative features and secondary metabolites differ significantly. The antioxidant enzyme was shown to be marginally connected to DPPH and MDA.
The principal component analysis of the interaction between the various sources of K application and the high temperature of pepper in all of the assessed parameters. T: high temperature, K-T: K application in high temperature, N-K-T: nanocapsule-potassium applied in high temperature, N-K nanocapsule-potassium in optimum temperature, FWS: shoot fresh weight, DWS: shoot dry weight, FWR: root fresh weight, DWR: root dry weight, SH-L: stem length, R-L: root length, F-N: number of flowers per plant, ABSi: flower abscission per plant, F-N: number of fruits per plant, EL: EL, PB: water pressure potential, Fv/Fm: chlorophyll fluorescence, RWC: RWC, K: K, Prl: proline, CAT: CAT, POD: POD, PRt: protein, MDA: MDA, DPPH: DPPH, SOD: SOD, PAL: palmitic acid, STA: stearic acid, OLI: oleic acid, LIN: linolelaidic acid, TLN: α-linoleic acid, LINO: linoleic acid, Glu: glucose, Fru: fructose
3.7 Spider Diagram
When K was not applied at high temperatures, the stress indices, such as DPPH and MDA, significantly rose, followed by a rise in water potential and linoleic acid. N-K-T showed the lowest stress indices when the high temperature was applied (nanocapsule-potassium applied at high temperature) (Fig. 7).
A spider graph is used to illustrate the effect that various sources of K application and high temperatures in fatty acids, glucose, and fructose have on pepper. T: high temperature, K-T: K application in high temperature, N-K-T: nanocapsule K applied in high temperature, N-K: nanocapsule-potassium in optimum temperature, EL: EL, PB: water pressure potential, Fv/Fm: chlorophyll fluorescence, K: K, Prl: proline, CAT: CAT, PRt: protein, MDA: MDA, DPPH: DPPH, PAL: palmitic acid, STA: stearic acid
4 Discussion
High temperature reduces the cell’s relative humidity, which in turn reduces the size of the cell and inhibits plant development (Hasanuzzaman et al. 2013). Another factor influencing the reduction in relative growth of stressed plants is the slower rate of assimilate formation caused by reduced photosynthesis and improved respiration (Hasanuzzaman et al. 2013). Shareef et al. (2020) observed a reduction in plant growth when exposed to temperatures of 49/31 °C. This decrease in growth was found to be linked to the occurrence of stress-induced damages, as evidenced by a decline in membrane stability, which serves as an indicator of membrane impairment. The observed phenomenon can likely be attributed to a decline in the capacity to swiftly and comprehensively restructure cellular membranes (Shareef et al. 2020).
In plant tissues, fertilizer potassium increases cell volume, strong roots, and water absorption (Hasanuzzaman et al. 2018). A lack of potassium can hinder the transport, use, and photosynthetic CO2 fixation of assimilates (Waraich et al. 2012). Previous research has recorded and explained changes in stress indices under heat stress; the effects of potassium deficiency on Ocimum basilicum L. indicated a decrease in root and leaf biomass production, as well as an inhibition of root extension and a reduction in leaf growth (Attia et al. 2022). According to Hasanuzzaman et al. (2018), the administration of potassium has been found to enhance growth parameters by modulating hormonal balance. This is achieved through an increase in growth-promoting hormones and a decrease in growth-inhibiting hormones.
Better plant height and wider stem diameter were obtained when K fertilizer was applied to the tomato crop at a ratio of 300 kg ha−1. The best yield and yield components were obtained when K and N fertilizers were used together (Ajirloo et al. 2015). Ajirloo et al. (2015) used a factorial field experiment to treat Lycopersicum esculentum L. with varying ratios of N fertilizer and K-nano fertilizer. Bulk N fertilizer and K-nano fertilizer were shown to have a synergistic effect on plant growth and yield of tomatoes, with statistically significant favorable impacts on several yield components such as plant height, number of fruits per plant, and fruit weight. Metabolic activity decreases dramatically with rising temperatures over 25–30 °C (Rayle and Cleland 1992). The results of our research also showed that at high temperatures, the N-K treatment was more effective than the K treatment in improving the fresh weight of the stem.
By increasing the number of leaves and their surface area, the plant can generate adequate light, utilize nutrients, and increase photosynthesis (Wang et al. 2019). Fertilization promotes the development of materials, the beginning point for producing cultured sap, as well as the nutrients necessary to accelerate and increase the number of leaves (Hasanuzzaman et al. 2013). According to Shareef (2019), the observed increase in leaf area can be attributed to the higher potassium levels. This finding suggests that an adequate supply of this nutrient is crucial for plant growth, particularly in relation to its involvement in cell division. Additionally, potassium plays a significant role in enhancing the functionality of plant hormones like auxins and gibberellins, which directly contribute to cell expansion and elongation, ultimately leading to an increase in leaf area (Shareef 2019). The findings of our study indicate that the application of potassium (K) under heat stress conditions resulted in a significant increase in leaf count when compared to the control treatment.
The principal component analysis (PCA) method was utilized to assess the most important effects of K treatment on pepper growth and antioxidant enzyme (Fig. 6). The RWC and potassium concentrations in the N-K-T (nanocapsule-potassium applied at high temperature) treatment were positively connected with linoleic acid, according to the PCA. The antioxidant enzyme was shown to be marginally connected to DPPH and MDA.
It was revealed that chlorophyll fluorescence diminished at high-temperature circumstances. The deterioration was particularly severe at very high temperatures and in temperature-sensitive genotypes (Wahid et al. 2007; Camejo et al. 2005). The fluorescence of chlorophyll varies as a response to high-temperature stressors. In other words, whereas measurements of chlorophyll fluorescence decreased, Fo values increased. The Fv/Fm ratio may be used to calculate the maximal PS II photochemical efficiency of dark-adapted leaves (Camejo et al. 2005).
When a considerable quantity of K+ is lost from the chloroplast of wheat cells, photosynthesis is reduced; in such cases, the administration of K benefits plant cells in enduring heat stress by improving photosynthetic activity. Foliar K treatments increased the accumulation and translocation of photosynthates and dry materials (Dubey et al. 2020). The deprivation of potassium (K) resulted in a reduction in the process of photosynthesis by restricting the diffusion of carbon dioxide (CO2) into and through the leaves. This limitation affected various physiological factors such as stomatal conductance (gs), mesophyll conductance (gm), and biochemical processes. The decline in photosynthetic activity can be attributed to three primary factors: stomatal resistance, mesophyll resistance, and biochemical limitations (Imtiaz et al. 2023).
K+ protects plants by assisting in protein synthesis, various enzymatic processes, carbohydrate biosynthesis, and enhancing plant cell water-use efficiency. The heat tolerance of wheat is increased under heat stress circumstances by foliar spraying with potassium orthophosphate (KH2PO4), reducing leaf damage (Dias and Lidon 2010).
Increase the temperature, proline content, and electrolyte loss (Ghai et al. 2016) MDA, a byproduct of fatty acid oxidation, is recognized as a marker of cell membrane lipid peroxidation due to the formation of reactive oxygen species (Cai et al. 2015). The quantity of MDA is reduced, which reduces membrane damage and, as a consequence, reduces peroxidation of membrane lipids, conserving membrane integrity and reducing tissue damage (Ge et al. 2012). The findings of our study indicate that exposure of pepper plants to heat stress resulted in a significant increase in the content of malondialdehyde (MDA) in the leaves. The observed phenomenon can potentially be attributed to the heightened accumulation of reactive oxygen species (ROS) within the plant system, resulting in lipid peroxidation, nucleic acid impairment, and protein oxidation (Miller et al. 2010).
The study conducted by Abdollah et al. (2023) demonstrated that exposure to heat stress resulted in a reduction in membrane stability, as evidenced by an increase in electrolyte leakage. However, the application of potassium treatment significantly mitigated this effect, leading to enhanced membrane stability. The elevated membrane stability index observed in the presence of potassium is associated with the plant’s antioxidant responses, which serve to safeguard it against oxidative harm. This phenomenon can be attributed to the increased levels of ions and the subsequent activation of antioxidant enzymes (Da Silva et al. 2021).
Elevated temperatures have been found to have a direct impact on various biological processes. Specifically, high-temperature conditions have been observed to result in the denaturation and aggregation of proteins, damage to lipid membranes, inactivation of enzymes in chloroplasts and mitochondria, limitations in protein synthesis, degradation of proteins, and compromised integrity of cellular membranes (Waraich et al. 2012).
Furthermore, heat stress enhances ROS production and lipid peroxidation in pepper plants (Camejo et al. 2005). The presence of antioxidant enzymes such as SOD, POD, and increased K status reduces the generation of reactive oxygen species (ROS) in plants (Hasanuzzaman et al. 2018). According to Imtiaz et al. (2023), recent studies have revealed that potassium ions (K +) play a role in enhancing the antioxidant defense system and increasing the concentration of osmolytes. This mechanism effectively mitigates the oxidative stress induced by reactive oxygen species (ROS) and malondialdehyde (MDA).
According to Li et al. (2023), the enzyme activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) in plants treated with a 0.3% KH2PO4 solution exhibited a statistically significant increase compared to the control group that did not receive the spray treatment. The application of a 0.3% KH2PO4 solution has the potential to mitigate the accumulation of reactive oxygen species (ROS) (Xue et al. 2012). Additionally, the detrimental impact of ROS can be counteracted by augmenting the activities of antioxidant enzymes and safeguarding against oxidative damage in the presence of heat stress. Based on the results mentioned above, our study revealed that the administration of potassium resulted in an elevation in the activity levels of catalase (CAT) and superoxide dismutase (SOD). However, the intervention did not yield any discernible impact on the activity of POD.
Potassium supports photosynthetic electron transport activity while decreasing the activity of NADPH-oxidases, which aids in the reduction of ROS (Hasanuzzaman et al. 2018). K+ control is linked to the activity of enzymes involved in ROS detoxification (Cakmak 2005). Temperature influenced the distribution of pepper fruit and shoot dry matter as well as glucose levels. Heat exposure raised the levels of glucose and fructose in the fruit by 17–21%. In reactive oxygen species, soluble carbohydrates seem to serve a dual function (Motamedi et al. 2019). The findings of our study additionally demonstrated that elevated temperatures led to an increase in the levels of glucose and fructose. The activity of invertase, an enzyme responsible for breaking down sucrose, has the potential to regulate the allocation of carbon and signaling of sugars, thereby impacting the import and utilization of sucrose. A study conducted by Li et al. (2012) observed that, in high-temperature conditions, heat-tolerant tomatoes exhibited greater activities of cell wall and vascular invertases in young fruit compared to heat-sensitive tomatoes.
5 Conclusion
Based on the results, it can be concluded that heat stress exerts an inhibitory impact on pepper. The administration of nanocapsule-potassium at elevated temperatures leads to a mitigation of the adverse consequences associated with heat stress. In addition, the application of nanocapsule-potassium at elevated temperatures induces changes in the fatty acid composition, particularly in linoleic acid, within cell membranes. Moreover, the utilization of nanocapsule-potassium mitigates the adverse impact of high temperatures by reducing the levels of antioxidant enzymes, relative water content (RWC), antioxidant activity (DPPH), and malondialdehyde (MDA). The effectiveness of K and specifically N-K on vegetative development appears to have been somewhat ineffective. However, N-K and K were more effective on physiological changes, and enzyme antioxidant and K concentration interfere with the increasing resistance against heat stress. Therefore, it may be said that K and N-K are useful during the reproductive stage. The findings from the experiment indicate that the application of potassium, specifically in the form of nanocapsules, could potentially offer some level of protection to pepper plants against heat stress-induced damage.
Data Availability
The data presented in this study are available on request from the authors.
References
Adams SR, Cockshull KE, Cave CRJ (2001) Effect of temperature on the growth and development of tomato fruits. Annal Bot 88:869–877. https://doi.org/10.1006/anbo.2001.1524
Ahmadi M, Souri MK (2021) Importance of chemical sources in salt-induced salinity on plant growth characteristics in pepper. Acta Hort 1315:425–432. https://doi.org/10.17660/ActaHortic.2021.1315.63
Ajirloo AR, Shaaban M, Motlagh ZR (2015) Effect of K nano-fertilizer and N bio-fertilizer on yield and yield components of tomato (Lycopersicon esculentum L.). Int J Adv Biol Biom Res 3:138–143
Attia H, Rebah F, Ouhibi C, Saleh MA, Althobaiti AT, Alamer KH, Ben Nasri M, Lachaâl M (2022) Effect of potassium deficiency on physiological responses and anatomical structure of basil. Ocimum Basilicum l Biol 11:1557. https://doi.org/10.3390/biology11111557
Bajji M, Kinet JM, Lutts S (2002) The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul 36:61–70. https://doi.org/10.1023/A:1014732714549
Bamrungsap S, Zhao Z, Chen T, Wang L, Li C, Fu T, Tan W (2012) Nanotechnology in therapeutics: a focus on nanoparticles as a drug delivery system. Nanomed 7:1253–1271
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Cai Z, Yang R, Xiao H, Qin X, Si L (2015) Effect of preharvest application of Hanseniaspora uvarum on postharvest diseases in strawberries. Postharvest Biol Technol 100:52–58. https://doi.org/10.1016/j.postharvbio.2014.09.004
Cakmak I (2005) The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Soil Sci Plant Nutr 168:521–530. https://doi.org/10.1002/jpln.200420485
Camejo D, Rodríguez P, Morales MA, Dell’Amico JM, Torrecillas A, Alarcón JJ (2005) High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. Plant Physiol 162:281–289. https://doi.org/10.1016/j.jplph.2004.07.014
Chand Mali S, Raj S, Trivedi R (2020) Nanotechnology a novel approach to enhance crop productivity. Biochem Biophys Rep 24:100821. https://doi.org/10.1016/j.bbrep.2020.100821
Da Silva DL, De Mello PR, Tenesaca LFL, Da Silva JLF, Mattiuz BH (2021) Silicon attenuates calcium deficiency by increasing ascorbic acid content, growth and quality of cabbage leaves. Sci Rep 11:1–10. https://doi.org/10.1038/s41598-020-80934-6
DeRosa MC, Monreal C, Schnitzer M, Walsh R, Sultan Y (2010) Nanotechnology in fertilizers. Nat Nanotechnol 5:91–91. https://doi.org/10.1038/nnano.2010.2
Dhopte AM, Livera MM (2002) Principles and techniques for plant scientist. Phytochem Anal 13:8–17. Published by Agrobios, 2018
Dias A S, Lidon F C (2010) Bread and durum wheat tolerance under heat stress: a synoptical overview. Emir. J Food Agric 412–436. https://doi.org/10.9755/ejfa.v22i6.4660
Dubey R, Pathak H, Chakrabarti B, Singh S, Gupta DK, Harit RC (2020) Impact of terminal heat stress on wheat yield in India and options for adaptation. Agric Syst 181:102826. https://doi.org/10.1016/j.agsy.2020.102826
Fathi M, Mozafari MR, Mohebbi M (2012) Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci 23:13–27. https://doi.org/10.1016/j.tifs.2011.08.003
Fonte P, Soares S, Costa A, Andrade JC, Seabra V, Reis S, Sarmento B (2012) Effect of cryoprotectants on the porosity and stability of insulin-loaded PLGA nanoparticles after freeze-drying. Biomatter 2:329–339. https://doi.org/10.4161/biom.23246
Ge W, Jiao YQ, Sun BL, Qin R, Jiang WS, Liu DH (2012) Cadmium-mediated oxidative stress and ultrastructural changes in root cells of poplar cultivars. S Afr J Bot 83:98–108. https://doi.org/10.1016/j.sajb.2012.07.026
Ghai N, Kaur J, Jindal SK, Dhaliwal MS, Pahwa K (2016) Physiological and biochemical response to higher temperature stress in hot pepper (Capsicum annuum L.). J Appl Nat Sci 8:1133–1137. https://doi.org/10.31018/jans.v8i3.930
Gibis M, Vogt E, Weiss J (2012) Encapsulation of polyphenolic grape seed extract in polymer-coated liposomes. Food Func 3:246–254. https://doi.org/10.1039/c1fo10181a
Haghighi M, Ramezani MR, Rajaii N (2019) Improving oxidative damage, photosynthesis traits, growth and flower dropping of pepper under high temperature stress by selenium. Mol Biol Rep 46:497–503. https://doi.org/10.1007/s11033-018-4502-3
Haleem AM (2020) Fabrication and characterization for phosphate nanofertilizer through polymer coating technique. Eng Technol 38:34–40. https://doi.org/10.30684/etj.v38i1B.576
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int Mol Sci 14:9643–9684. https://doi.org/10.3390/ijms14059643
Hasanuzzaman M, Bhuyan MB, Nahar K, Hossain MS, Mahmud JA, Hossen MS, Masud AAC, Fujita M (2018) Potassium: a vital regulator of plant responses and tolerance to abiotic stresses. Agron 8:31. https://doi.org/10.3390/agronomy8030031
Imtiaz H, Mir AR, Corpas FJ, Hayat S (2023) Impact of potassium starvation on the uptake, transportation, photosynthesis, and abiotic stress tolerance. Plant Growth Regul 99:429–448. https://doi.org/10.1007/s10725-022-00925-7
Javanmardi J, Rahemi M, Nasirzadeh M (2014) Responses of tomato and pepper transplants to high-temperature conditioning. Int J Veg Sci 20:374–391. https://doi.org/10.1080/19315260.2013.816209
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42:1265–1273. https://doi.org/10.1093/pcp/pce162
Jones JB (1930) Hydroponics: a practical guide for the soilless grower. CRC Press, Boca Raton, FL
Khoshbakht D, Asghari MR, Haghighi M (2018) Effects of foliar applications of nitric oxide and spermidine on chlorophyll fluorescence, photosynthesis and antioxidant enzyme activities of citrus seedlings under salinity stress. Photosynthetica 56:1313–1325. https://doi.org/10.1007/s11099-018-0839-z
Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW (2012) Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot 35:64–70. https://doi.org/10.1016/j.cropro.2012.01.007
Koleva II, Van Beek TA, Linssen JP, Groot AD, Evstatieva LN (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal 13:8–17. https://doi.org/10.1002/pca.611
Li Z, Palmer WM, Martin AP, Wang R, Rainsford F, Jin Y, Patrick JW, Yang Y, Ruan YL (2012) High inver-tase activity in tomato reproductive organs correlates with enhanced sucrose import into, and heat tolerance of, young fruit. J Exp Bot 63:1155–1166
Li J, Li Z, Li X, Tang X, Liu H, Li J, Song Y (2023) Effects of spraying KH2PO4 on flag leaf physiological characteristics and grain yield and quality under heat stress during the filling period in winter wheat. Plants 12:1801. https://doi.org/10.3390/plants12091801
Lu Q, Li DC, Jiang JG (2011) Preparation of a tea polyphenol nanoliposome system and its physicochemical properties. J Agric Food Chem 59:13004–13011. https://doi.org/10.1021/jf203194w
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence a practical guide. J Exp Bot 51:659–668. https://doi.org/10.1093/jexbot/51.345.659
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
Motamedi M, Haghighi M, Goli A (2019) Physiological changes of sweet and hot peppers in vegetative and reproductive growth stages treated by Ca and H2O2 under unforeseen heat stresses. Sci Hortic 249:306–313. https://doi.org/10.1016/j.scienta.2019.01.040
Murillo-Amador B, Yamada S, Yamaguchi T, Rueda-Puente E, Ávila-Serrano N, García-Hernández JL, Nieto-Garibay A (2007) Influence of calcium silicate on growth, physiological parameters and mineral nutrition in two legume species under salt stress. J Agron Crop Sci 193:413–421. https://doi.org/10.1111/j.1439-037X.2007.00273.x
Nadeem SM, Ahmad M, Zahir ZA, Kharal MA (2016) Role of phytohormones in stress tolerance of plants. In: Hakeem, K. and Akhtar, M., (eds) Plant, Soil and Microbes. Springer, Cham. pp 385–421 https://doi.org/10.1007/978-3-319-29573-2_17
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nazar R, Iqbal N, Umar S (2017) Heat stress tolerance in plants: action of salicylic acid. Salicylic acid 145–161. https://doi.org/10.1007/978-981-10-6068-7_8
Pourranjbari Saghaiesh S, Souri MK (2018) Root growth characteristics of Khatouni melon seedlings as affected by potassium nutrition. Pol Hortorum Cultus 17:191–198. https://doi.org/10.24326/asphc.2018.5.17
Prasad TNVKV, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Reddy KR, Sreeprasad TS, Sajanlal PR, Pradeep T (2012) Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr 35:905–927. https://doi.org/10.1080/01904167.2012.663443
Rasti B, Jinap S, Mozafari MR, Yazid AM (2012) Comparative study of the oxidative and physical stability of liposomal and nanoliposomal polyunsaturated fatty acids prepared with conventional and Mozafari methods. Food Chem 135:2761–2770. https://doi.org/10.1016/j.foodchem.2012.07.016
Rayle DL, Cleland RE (1992) The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol 99:1271
Rodriguez-Dominguez CM, Forner A, Martorell S, Choat B, Lopez R, Peters JMR, Pfautsch S, Mayr S, Carins-Murphy MR, McAdam SAM, Richardson F, Diaz-Espejo A, Hernandez-Santana V, Menezes-Silva PE, Torres-Ruiz JM, Batz TA, Sack L (2022) Leaf water potential measurements using the pressure chamber: synthetic testing of assumptions towards best practices for precision and accuracy. Plant Cell Environment 45:2037–2061. https://doi.org/10.1111/pce.14330
Shareef HJ (2019) Salicylic acid and potassium nitrate promote flowering through modulating the hormonal levels and protein pattern of date palm Phoenix dactylifera “Sayer” offshoot. Acta Agric Slov 114:231–238. https://doi.org/10.14720/aas.2019.114.2.8
Shareef HJ, Abdi G, Fahad S (2020) Change in photosynthetic pigments of Date palm offshoots under abiotic stress factors. Folia Oecol 47:45–51. https://doi.org/10.2478/foecol-2020-0006
Shenoda JE, Sanad MNME, Rizkalla AA, El-Assal S, Ali RT, Hussein MH (2021) Effect of long-term heat stress on grain yield, pollen grain viability and germinability in bread wheat (Triticum aestivum L) under field conditions. Heliyon 7:e07096. https://doi.org/10.1016/j.heliyon.2021.e07096
Tabatabaei S, Ehsanzadeh P (2016) Photosynthetic pigments, ionic and antioxidative behaviour of hulled tetraploid wheat in response to NaCl. Photosynthetica 54:340–350. https://doi.org/10.1007/s11099-016-0083-3
Tohidloo G, Souri MK, Eskandarpour S (2018) Growth and fruit biochemical characteristics of three strawberry genotypes under different potassium concentrations of nutrient solution. Open Agric 3:356–362. https://doi.org/10.1515/opag-2018-0039
Ullah A, Nadeem F, Nawaz A, Siddique KH, Farooq M (2022) Heat stress effects on the reproductive physiology and yield of wheat. J Agron Crop Sci 208:1–17. https://doi.org/10.1111/jac.12572
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223. https://doi.org/10.1016/j.envexpbot.2007.05.011
Wang M, Zheng Q, Shen Q, Guo S (2013) The critical role of potassium in plant stress response. Int J Mol Sci 14:7370–7390. https://doi.org/10.3390/ijms14047370
Wang J, Lv J, Liu Z, Liu Y, Song J, Ma Y, Ou L, Zhang X, Liang C, Wang F, Juntawong N (2019) Integration of transcriptomics and metabolomics for pepper (Capsicum annuum L) in response to heat stress. Int J Mol Sci 20:5042. https://doi.org/10.3390/ijms20205042
Waraich EA, Ahmad R, Halim A, Aziz T (2012) Alleviation of temperature stress by nutrient management in crop plants: a review. J Soil Sci Plant Nutr 12:221–244. https://doi.org/10.4067/S0718-95162012000200003
Xue D, Jiang H, Hu J, Zhang X, Guo L, Zeng D, Dong G, Sun G, Qian Q (2012) Characterization of physiological response and identification of associated genes under heat stress in rice seedlings. Plant Physiol Biochem 61:46–53
Zeng C, Jia T, Gu T, Su J, Hu X (2021) Progress in research on the mechanisms underlying chloroplast-involved heat tolerance in plants. Genes (basel) 12:1343. https://doi.org/10.3390/genes12091343.PMID:34573325;PMCID:PMC8471720
Acknowledgements
The authors highly appreciate and thank the University of Isfahan and the Hungarian University of Agriculture and Life Sciences for their support.
Funding
Open access funding provided by Hungarian University of Agriculture and Life Sciences.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology B.H. and M.H.; investigation, validation, analysis, and visualization B.H. and A.A.; writing—original draft preparation B.H., A.A., M.H.; reviewing and editing N.K. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Halaji, B., Haghighi, M., Amiri, A. et al. Effects of Potassium and Nanocapsule of Potassium on Pepper Growth and Physiological Changes in High-Temperature Stress. J Soil Sci Plant Nutr 23, 6317–6330 (2023). https://doi.org/10.1007/s42729-023-01486-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01486-y